Abstract
The critical closing pressure (CrCP) of the cerebral circulation depends on both tissue intracranial pressure and vasomotor tone. CrCP defines the arterial blood pressure (ABP) at which cerebral blood flow approaches zero, and their difference (ABP − CrCP) is an accurate estimate of cerebral perfusion pressure. Here we demonstrate a novel non-invasive technique for continuous monitoring of CrCP at the bedside. The methodology combines optical diffuse correlation spectroscopy (DCS) measurements of pulsatile cerebral blood flow in arterioles with concurrent ABP data during the cardiac cycle. Together, the two waveforms permit calculation of CrCP via the two-compartment Windkessel model for flow in the cerebral arterioles. Measurements of CrCP by optics (DCS) and transcranial Doppler ultrasound (TCD) were carried out in 18 healthy adults; they demonstrated good agreement (R = 0.66, slope = 1.14 ± 0.23) with means of 11.1 ± 5.0 and 13.0 ± 7.5 mmHg, respectively. Additionally, a potentially useful and rarely measured arteriole compliance parameter was derived from the phase difference between ABP and DCS arteriole blood flow waveforms. The measurements provide evidence that DCS signals originate predominantly from arteriole blood flow and are well suited for long-term continuous monitoring of CrCP and assessment of arteriole compliance in the clinic.
Keywords: Arterioles, cerebral blood flow measurement, intrinsic optical imaging, near infrared spectroscopy, neurocritical care
Introduction
A key management strategy for acute brain injury is to maintain cerebral blood flow (CBF), so that oxygen and glucose delivery match metabolic demands.1–3 While insufficient CBF leads to ischemic cell damage, excessive CBF can lead to parenchymal damage via vasogenic edema and/or intracranial hemorrhage.4 Accordingly, a major therapeutic goal is to maintain the pressure gradient across the cerebral vascular bed—termed the cerebral perfusion pressure (CPP)—at target levels that ensure adequate blood flow for metabolism.5–7
Clearly, optimal management of CPP would benefit from accurate and continuous measurement of this parameter. CPP is often clinically defined as the difference between two measurable quantities: the arterial blood pressure (ABP) and the intracranial pressure (ICP), i.e. ABP − ICP. ABP is typically measured by an arterial cannula connected to a pressure transducer, and ICP is typically measured invasively with a ventricular catheter or an intraparenchymal monitor.2,8 While this definition of CPP is useful, it neglects the effects of active wall tension or vasomotor tone.9–12 As a result, the true pressure gradient driving cerebral blood flow is not ABP − ICP; rather, it is a parameter called “actual” CPP, i.e. aCPP = ABP − CrCP.9,12–14 CrCP is the critical closing pressure of the cerebral circulation. Cerebral blood flow ceases when ABP drops below CrCP, which in turn depends on both ICP and active wall tension in the small arterioles and pre-capillary sphincters.9,12,15
Noninvasive assessment of aCPP using CrCP has been proposed by several investigators16–23 as a more appropriate measure of the cerebral bed pressure gradient. Unlike other factors, CrCP provides access to the vasomotor tone, which may be helpful for assessment of cerebral vascular reserve, i.e. the capacity to increase flow.24–27 In addition, noninvasive determination of CrCP is valuable in its own right. Invasive ICP monitors carry risks, including intracranial hemorrhage and infection, and these risks may be too great in some brain injured patients; these at-risk populations include patients with coagulopathies, as well as infants and children.
To date, one method, transcranial Doppler (TCD) ultrasound, has been employed as a means to estimate CrCP and to noninvasively assess cerebral perfusion pressure.16–19,28–30 TCD measures blood velocity in major feeder arteries such as the middle cerebral artery (MCA), which is an accurate measure of CBF if the artery diameter remains constant. CrCP measured with TCD has been validated in patients with induced ventricular fibrillation.13 However, while TCD is a powerful technique, it has limitations. Stable fixation of TCD probes is challenging, and consequently, continuous long-term monitoring with TCD is difficult.18,31 Some patients (∼8%) do not have adequate acoustic bone windows for measurement of satisfactory TCD waveforms.30 Further, because TCD interrogates large proximal arteries, TCD measurements may be relatively insensitive to localized/heterogeneous brain diseases/injuries. Finally, high MCA blood flow velocities, for example, during cerebral vasospasm, can produce turbulent flow. In this case, the assumed linear relationship between pressure and flow velocity is suspect and could result in an underestimation of CrCP.32
Here we demonstrate a novel method for continuous noninvasive monitoring of CrCP using near-infrared light.21–23 The method leverages the diffuse correlation spectroscopy (DCS) technique to noninvasively measure microvascular cerebral blood flow through the arterioles with high temporal resolution (20 Hz).33–36 The DCS instrumentation is well-suited for continuous long-term monitoring of CrCP at the bedside.31,37–39 In addition, since DCS probes microvessels rather than major arteries, it has the potential to be more sensitive to vascular compliance and vasomotor tone than TCD. We utilize a Windkessel model40 of the arteriole microvasculature to calculate CrCP from steady-state DCS waveforms of pulsatile cerebral blood flow and concurrent pulsatile ABP waveforms during the cardiac cycle.
We hypothesized that the combination of DCS-flow and ABP waveform analysis can provide accurate estimates of CrCP in humans, and we validated the approach via comparison to TCD-flow/ABP measurements in 18 healthy adults. The DCS data also provide means to estimate a capacitive parameter of the microvasculature, which is rarely measured, the arteriole compliance, C; this compliance parameter is a potentially useful biomarker for brain diseases such as ischemic stroke. Finally, the results provide evidence that the origin of the DCS signal derives primarily from shear-induced diffusive red blood cell movement in cerebral arterioles.
Methods
DCS measurement of blood flow
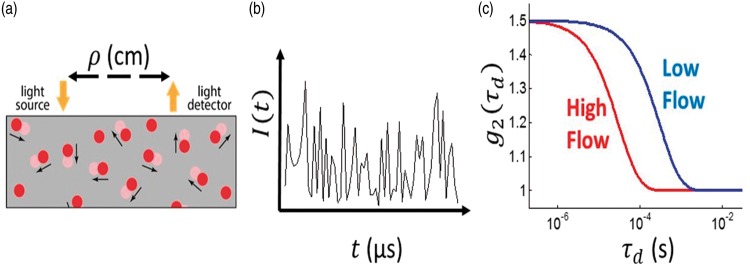
DCS estimates blood flow by quantifying rapid speckle intensity fluctuations of multiply scattered light that has traversed the tissue. These fluctuations are induced by red blood cell motion (Figure 1).41,42 Specifically, DCS measures the normalized temporal intensity autocorrelation function, , at multiple delay-times, . Here I(t) is the detected light intensity at time t, and the angular brackets, , represent time-averages. A semi-infinite tissue model is employed to derive a DCS blood flow index, F, from the decay of (Figure 1(c)).
Figure 1.
(a) Schematic of the homogeneous, semi-infinite model of the head with blood flow index of F. Blood cell position variation, e.g. represented by red disks at time, t, and light-red disks at a later time, t + τd, induce rapid temporal fluctuations in the detected speckle intensity (shown schematically in panel (b)), typically on microsecond time scales. These fluctuations are captured quantitatively by the normalized intensity autocorrelation function (g2(τd)). (c) The decay of the intensity autocorrelation function curves depends on tissue blood flow.
The DCS blood flow index, F, is directly proportional to tissue blood flow and has been compared to various independent methods.43–45 Recently, DCS measurements resolving the pulsatile heart-beat fluctuations of cerebral blood flow in arterioles have been demonstrated.21,22,34,46 Note that DCS is thought to be much more sensitive to arterioles than to large arteries because near-infrared light passing through large arteries is strongly absorbed, and hence not detected.
Vascular model of arterioles
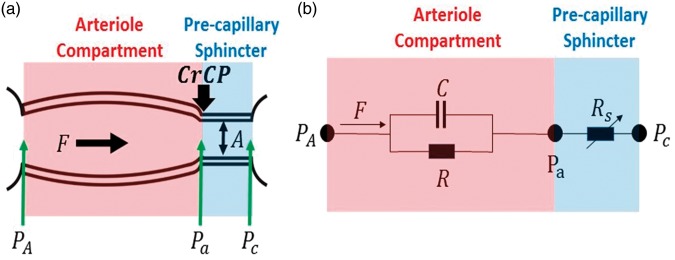
We modeled the cerebral arteriolar compartment between the large arteries and capillaries (Figure 2) as a two-compartment Windkessel model.40,47,48 The arteriole vascular compartment was defined as a tube with resistance R and compliance C. Additionally, we placed a narrow cylindrical tube (pre-capillary sphincter) with cross sectional area A and resistance Rs between the arterioles and the capillaries. The pre-capillary sphincter resistance varies as needed under normal conditions to maintain a pressure of CrCP at the distal end of the arteriole compartment.
Figure 2.
(a) Model schematic for the vasculature between arteries and capillaries. A Windkessel model is employed for the cerebral arteriole vasculature compartment (shaded pink) with resistance R and compliance C. The approach also introduces a pre-capillary sphincter, i.e. a narrow cylindrical tube between the arterioles and capillaries (shaded blue). The in-flow blood pressure at the entrance to the arteriole compartment is PA; the blood pressure at the distal ends of arteriole compartment and entrance to the pre-capillary sphincter is Pa. The external pressure (CrCP) on the pre-capillary sphincter is set by ICP and active wall tension. The sphincter adjusts to maintain Pa = CrCP under normal conditions. In normal conditions, PA > Pa = CrCP > Pc. The pressure gradient driving the arteriole blood flow, F, is PA − CrCP. (b) Electrical analogue of the vascular model consists of a parallel resistor, R, and capacitor, C; pressure (flow) is analogous to voltage (current). The variable resistor, Rs, represents the impedance of the pre-capillary sphincter and adjusts as needed to maintain the relation: (i.e. the steady-state blood flow through R and Rs is the same).
Within this model, PA is the in-flow blood pressure at the entrance to the arteriole compartment, and Pa is the blood pressure at the distal end of the arteriole compartment (i.e. at the entrance to the pre-capillary sphincter). Pc is the out-flow pressure at the entrance to the capillaries (i.e. at the exit of the pre-capillary sphincter). The isotropic pressure compressing the pre-capillary sphincter at its entrance in normal operation is CrCP; the factors responsible for this external pressure are ICP and active wall tension.9,10 During normal operation, Pa =CrCP > Pc. Note, Pa is not affected by Pc, an effect that is known as the vascular waterfall.9,12–15 Generally, PA tracks ABP and is substantially larger than CrCP, but if PA drops below CrCP, then the sphincter collapses and flow through the arteriole compartment ceases.
The model suggests that healthy microvasculature can be characterized by normative values of R, C, and CrCP. Variations in wall tension can modulate blood flow in two ways; via changes in R (by vessel dilation), and via changes in CrCP (by changing actual cerebral perfusion pressure).
Due to flow through the arteries, we expect the blood pressure, PA, at the entrance of the arteriole compartment to be lower than the systemic blood pressure, ABP. In a rat model, PA was directly measured and found to be proportional to ABP, i.e.49
| (1) |
In this animal study,49 the proportionality coefficient was Note, in utilizing a real number for γ in equation (1), we assumed that PA and ABP waveforms are synchronized.
Measurement of CrCP with DCS
Blood flow and blood pressure in arteries and arterioles is pulsatile, and for computing CrCP, we made the approximation that the arterial system is in steady-state oscillation, e.g. the heart beat is regular; the pressure and flow waveforms are repetitive periodic oscillations, as opposed to episodic transients. We can therefore represent these waveforms by a Fourier series whose frequencies are integral multiples of the frequency of repetition.50 In the arterial system, the fundamental frequency of repetition is the heart rate ().
We computed CrCP using measurements of the mean and the fundamental harmonic of the blood pressure and blood flow waveforms. The fundamental harmonic waveforms for the blood pressure and the blood flow are and respectively. Here, the amplitudes of the fundamental harmonics are denoted by and , and is the phase difference between the fundamental blood pressure and flow harmonic waveforms.
In standard linear response theory (including Ohms’ Law), the ratio is expected to be the impedance of the vascular model depicted in Figure 2. Thus, we obtained the following result for CrCP in terms of measurable parameters (see Appendix in supplementary material):
| (2) |
Here, and are the mean (time-averaged or DC) systemic blood pressure and arteriole blood flow. The factor γ is the proportionality coefficient between systemic and arteriole blood pressure, which must be less than 1 (equation (1)), and the circuit relaxation time-constant, , is called the vascular transit time for a blood volume traversing the arteriole compartment48; it is readily derived from , i.e.
| (3) |
The combination of DCS measurements of F(t) and separate/simultaneous measurements of ABP(t) thus permitted calculation of CrCP via equation (2). The values reported in the present article are based on our measurements and this calculation, which assumed .49 Finally, knowledge of τ and CrCP enabled calculation of arteriole compliance (see Appendix in supplementary material):
| (4) |
Measurement of CrCP with TCD
The TCD-based approach to measuring CrCP combines Doppler ultrasound measurements of middle cerebral artery blood velocity (Fv) and concurrent ABP waveforms. (Note: blood velocity can be converted to blood flow with knowledge of the artery diameter.) This procedure, which utilizes arterial rather than arteriole signals, was applied to our TCD data. The resultant computation of CrCP gave10,51
| (5) |
As with equation (2), the angular brackets represent temporal averages, and and are the Fourier spectral amplitudes of ABP and Fv at the heart rate frequency.
Equation (5), which has been used and validated in patients with induced ventricular fibrillation,13 is derived from a “resistive only” approximation to the model of the arterial bed that neglects the effects of arterial compliance (i.e. an electrical network model similar to Figure 2(b) but with C = 0). For TCD measurements, the error due to this “resistive only” approximation is expected to be small.13
The use of first harmonic Fourier filtering to calculate CrCP with equation (5) is theoretically equivalent to linear regression of Fv versus ABP to find the (extrapolated) blood pressure at which Fv is zero.10 This extrapolated pressure is typically taken to be the CrCP. In practice, however, Fourier filtering is preferable to the linear regression approach because it is less affected by measurement noise.13
Experimental procedures
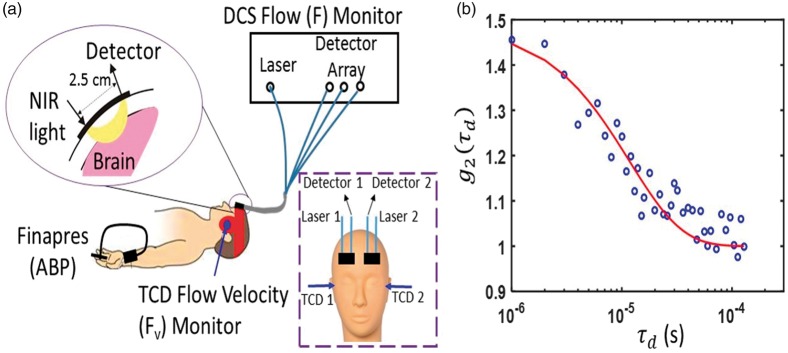
We simultaneously determined CrCP from measurements with DCS and with TCD in 20 healthy adult volunteers. Since two subjects had poor signal quality (discussed further below), we report results from 18 of the 20 subjects measured. All subjects provided written consent, and all protocols/procedures were approved by the institutional review board at the University of Pennsylvania, which adheres to the guidelines of the Common Rule and the Food and Drug Administration’s Institutional Review Board and human subject regulations. Throughout the measurement, all subjects lay supine at rest. DCS optical probes were placed bilaterally on the forehead superior to the frontal sinuses to measure blood flow downstream in the anterior middle cerebral artery distribution (Figure 3). The arteriole blood flow (F) was measured continuously at 20 Hz using a custom-built DCS instrument with a software correlator that is described in detail elsewhere.34 Specifically, arteriole blood flow was obtained from a semi-infinite fit to the DCS measurement using an assumed tissue optical absorption coefficient of 0.1 cm−1 and reduced scattering coefficient of 8 cm−1 (e.g. Figure 3(b)). Fractional blood flow changes obtained with DCS are robust to errors in these assumed tissue optical properties.52
Figure 3.
(a) Bilateral measurements of F, Fv, and noninvasive ABP were simultaneously recorded for 5 min in 20 healthy adult volunteers laying supine at rest. DCS probes were first secured with a neoprene elastic strap, and a headframe securing the TCD probes (red band) was then placed over the DCS probes; in side view, only one probe, laser, and light detector array (i.e. “Detector” in frontal view inset) are shown; in frontal view inset, the head frame and elastic strap are not shown. (b) Exemplar DCS g2(τd) measurement was averaged across three detection channels with mean photon count rate of 77 kHz and a source-detector separation of 2.5 cm, and was acquired on the left forehead of a healthy adult subject at a sampling rate of 20 Hz. The solid red line is the semi-infinite fit to the measurement. A DCS blood flow index of F = 1.4 × 10−8 cm2/s was extracted from the fit. Note, a tissue optical absorption coefficient of 0.1 cm−1 and tissue reduced scattering coefficient of 8 cm−1 were assumed for the fit.88
Concurrently, two TCD probes were secured using a Diamon Headframe for bilateral monitoring of middle cerebral artery blood velocity (Fv) with a commercial Compumetics DWL TCD system (Seigen, Germany), and continuous noninvasive arterial blood pressure (ABP) was measured on the middle finger with a finger plethysmograph system (Finometer Pro, Finapres Medical Systems, Arnhem, Netherlands).
The ABP and Fv signals were streamed continuously (in real time) to the DCS computer via output channels on the Finapres and Compumetics systems. Time synchronization of F, Fv, and ABP was achieved using software written in Labview (National Instruments, Austin, TX, USA). In all subjects, ABP, bilateral F, and bilateral Fv were measured in parallel at a sampling rate of 20 Hz for 5 min. (Note: any systemic time difference between the correlator and Finapres signals were measured and accounted for as needed.)
Pressure pulse waves propagate rapidly through the vasculature. In a rigid tube with fixed diameter, the pressure wave velocity is the speed of sound in blood, which is roughly 1500 m/s.50 In the elastic ascending aorta, the wave velocity is substantially lower, but it is still fast, i.e. between 5 and 10 m/s,53 and there is a progressive increase in wave velocity with increasing distance from the heart.53–55 We therefore assumed that the ABP waveform measured on the finger with the Finapres is in-phase with the ABP waveform immediately proximal to the cerebral arterioles (i.e. PA in Figure 2). Note, our observation of no significant phase difference between the ABP and TCD Fv (e.g. Figure 4) waveforms at the heart rate frequency also suggests that ABP immediately proximal to the MCA is in phase with ABP measured on the finger. We further expect ABP and TCD Fv waveforms to be approximately in phase, because equation (5), which makes the assumption of phase-locked ABP and Fv waveforms for calculating CrCP, has been successfully validated in humans.13 A conservative estimate of the time delay between the ABP waveform at the MCA and the PA waveform at the arterioles is provided by previous measurement of the mean time delay between the carotid artery and MCA waveforms, i.e. 7.8 ± 0.5 ms.56 The actual synchronization error between ABP and PA is likely less because we expect the pressure wave velocity to be even higher in the stiff cerebral arterial bed.13,57
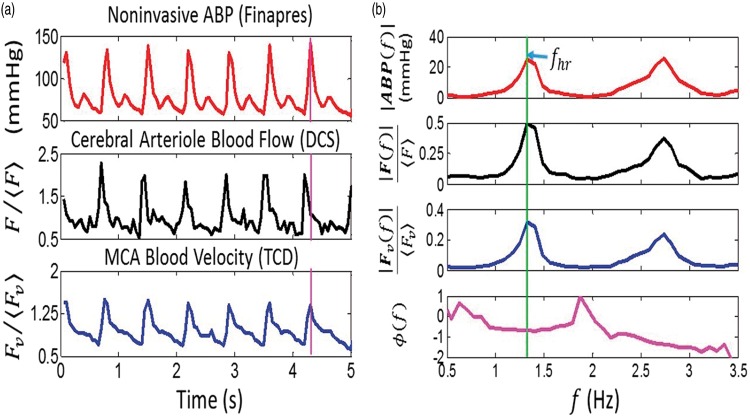
Figure 4.
Temporal measurements of noninvasive ABP, and waveforms in a representative subject (panel (a): the angular brackets, denote long-time-averaged temporal means). In panel (b), the Fourier spectral amplitudes and the phase difference between F and ABP, i.e. , are plotted against frequency, f. (See text for details on how the spectral amplitudes and phase were computed.) The vertical purple line in panel (a) shows the DCS arteriole blood flow waveform (F) is temporally shifted to the left of the ABP waveform, which corresponds to a negative phase difference at the heart rate frequency. Significant phase differences between the TCD MCA blood velocity waveform (Fv) and ABP were not observed (see text for reasons).
Data processing for CrCP calculation
In equations (2) and (5), and represent the time-averages of the measured ABP, F, and Fv time series. The frequency-dependent amplitudes and are obtained by taking the square root of their respective power spectral densities, which were computed with Welch’s method implemented in MATLAB R2014a (pwelch, Mathworks, Natick, Massachusetts).58
The phase difference between F and ABP at the heart rate frequency, i.e. in equation (3), is obtained by transfer function analysis,59,60 and is used to compute the time-constant, τ, in equation (2). Specifically, we computed the transfer function, H(f), with real and imaginary parts, that relates the frequency dependence of ABP to that of F using Welch’s method implemented in MATLAB R2014a (tfestimate). The phase difference between F and ABP at the heart rate frequency is: Here and are real and imaginary components of H at the heart rate frequency.59,60 The measurement sampling rate of 20 Hz is approximately 10 times the Nyquist rate (i.e. ), which is sufficiently high to fully resolve H(fhr), and thereby accurately estimate Note that the error in depends on the accuracy of the Fourier transform computation, which is greater with signal time (number of samples), and is much less than the 50 ms sampling interval between measurements. Given synchronization errors between the blood pressure measured on the finger and the blood pressure immediately proximal to the arterioles, a conservative estimate for the phase error is 10% (see “Experimental procedures” section). Note also that a negative phase difference corresponds to the F waveform leading the ABP waveform. Figure 4 shows exemplar ABP, F, and Fv measurements made on a healthy adult volunteer in the time-domain (Figure 4(a)) and frequency-domain (Figure 4(b)).
Since the pressure and flow waveforms can be represented as Fourier series whose frequencies are integral multiples of the heart rate frequency, one could consider analyzing the data at higher frequency harmonics (e.g. 2fhr, 3fhr). Indeed, in Figure 4(b), the amplitudes at twice the heart rate frequency are clearly visible. However, the higher harmonics are generally weaker signals and are more susceptible than the fundamental harmonic to be distorted by measurement noise.13 Therefore, higher harmonic data were not used to calculate CrCP. These noise distortions are the reason why Fourier filtering at the fundamental harmonic is preferable for CrCP calculation compared to the more traditional, linear regression approach.
It is perhaps counterintuitive for the F waveform to lead the ABP waveform driving blood flow. In fact, this observation is clearly apparent for a stationary sinusoidal ABP waveform in equilibrium. The observation is directly analogous to the well-established fact that, in steady-state, the electric current leads a stationary sinusoidal voltage signal applied to a RC circuit (Figure 2(b)).61
Data cleaning
Data analysis using equations (2) and (5) gave non-physiologic negative values for CrCP a small fraction of the time. Of the 40 CrCP measurements in the present study (i.e. 20 adults × 2 hemispheres), six were negative when computed using the DCS method (equation (2)), and three were negative when computed using the TCD method (equation (5)). We believe these negative CrCP measurements were due to poor signal quality in the DCS and TCD flow measurements.
Specifically, negative CrCP values arose whenever the measured blood flow pulsatility was smaller than the blood pressure pulsatility (i.e. when ). For DCS, this is likely caused by inadequate probe contact to the scalp. One way to assess adequate probe contact is through the fit quality of the semi-infinite correlation diffusion equation solution to the intensity autocorrelation function measurement, and the fits were substantially worse for data that yielded negative CrCP measurements compared to data that yielded positive CrCP measurements. More quantitatively, when we define the fit residual as the norm of the difference between the data and the fit, we found that the median fit-residual (±interquartile-range/2) of 0.3 ±0.08 for the negative CrCP measurements was significantly larger than the median fit-residual of 0.1 ± 0.05 for the positive CrCP measurements (p < 0.001). Thus the cases with negative CrCP were not considered in our reported comparisons of CrCP calculated with DCS and with TCD.
Statistical analysis
We carried out both linear regression and Bland–Altman analyses to assess agreement between the DCS and TCD measures of CrCP in the sample of healthy adults. To ensure independent measurements in these analyses, the average CrCP across both hemispheres was used. If CrCP assessed with either DCS or TCD was negative in one hemisphere, then the measurement in the other hemisphere wherein CrCP was positive was used in place of the average. Two of the 20 subjects were not considered because CrCP was negative for both hemispheres.
Results
For the 18 subjects investigated (11 male, 7 female), the average (mean ± SD) of arterial blood pressure, heart rate, DCS blood flow index (uncalibrated), and TCD blood velocity were 80 ± 12 mmHg, 61 ± 11 bpm (9.2 ± 3.5) × 10−9 cm2/s, and 49 ± 12 cm/s, respectively. Further, the systemic blood pressure, and the DCS and TCD blood flow pulsatility ratios were and respectively. The average phase difference and time delay between F and ABP at fhr were and 74 ± 25 ms. Note that the average detected photon count rate across the subject population of 56 ± 20 kHz for the DCS signal was sufficiently high to obtain reliable DCS blood flow pulsatility estimates.34 Finally, the average age, height, and weight across subjects were 30.6 ± 3.4 years, 173 ± 10 cm, and 75 ± 13 kg, respectively.
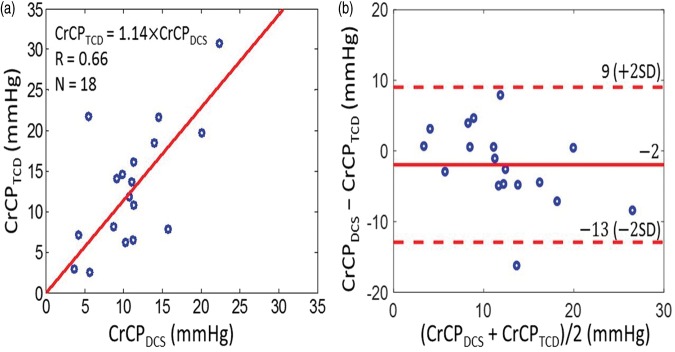
With these data, we concurrently derived CrCP in healthy adults (N = 18) with the optical DCS technique (equation (2)) and with the TCD technique (equation (5)). A linear regression analysis (Figure 5(a)) and Bland–Altman analysis (Figure 5(b)) of the data show a significant relationship between the two techniques. The slope (±95 CI) between CrCP measured by TCD and DCS is 1.14 (±0.23); the Pearson’s correlation coefficient is R = 0.66 (p = 0.003). Their mean difference (±95 CI) of −2 (±11) mmHg is not significantly different from zero (p = 0.2), and the average CrCP (mean ± SD) among these 18 subjects is CrCPTCD =13.0 ± 7.5 mmHg and CrCPDCS = 11.1 ± 5.0 mmHg, respectively.
Figure 5.
(a) CrCP measured by TCD (vertical axis) plotted against CrCP measured by DCS (horizontal axis) in 18 healthy adults. Solid red line is the linear best-fit with intercept forced to be zero (R = 0.66; slope ± 95 CI = 1.14 ± 0.23). (b) Bland–Altman plot of the difference between CrCPDCS and CrCPTCD versus the mean of these two parameters. The solid horizontal line indicates the mean difference (bias) computed across the study population (i.e. −2 mmHg), which is not significantly different from zero (p = 0.2); dashed lines indicate the 95% CI limits for the mean (i.e. −13 to 9 mmHg). The root mean square difference between DCS and TCD measurements is 4.4 mmHg.
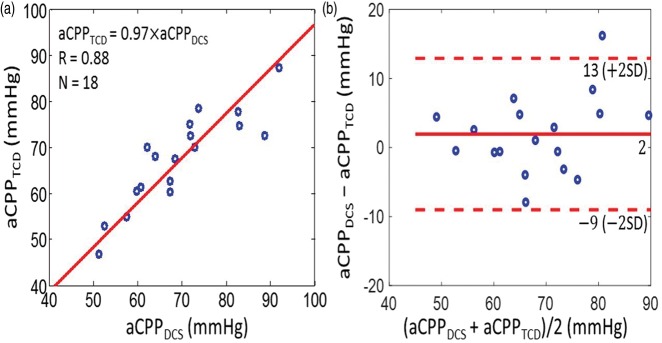
Along similar lines, we found good agreement between the CrCP-based estimates of actual cerebral perfusion pressure with TCD and DCS, i.e. and (Figure 6). The slope (±95 CI) between aCPPTCD and aCPPDCS is 0.97 (±0.04); the Pearson’s correlation coefficient is R = 0.88 (p < 0.001), and the average aCPP (mean ± SD) among the 18 subjects is aCPPTCD = 67.4 ± 10.1 mmHg and aCPPDCS =69.3 ± 11.7 mmHg for TCD and DCS, respectively.
Figure 6.
(a) CrCP-based estimates of actual cerebral perfusion pressure measured by TCD (i.e. vertical axis) and by DCS (i.e. horizontal axis) in 18 healthy adults. Solid red line is the linear best-fit with intercept forced to be zero (R = 0.88; slope ± 95 CI = 0.97 ± 0.04). (b) Bland–Altman plot of the difference between aCPPDCS and aCPPTCD versus the mean of these two parameters. The solid horizontal line indicates the mean difference (bias) computed across the study population (i.e. 2 mmHg), and the dashed lines indicate 95% CI limits for the mean (i.e. −9 to 13 mmHg).
We next considered arteriole compartment compliance effects, which are only apparent in the DCS data. The average transit time through the arteriole bed across the 18 subjects (mean ± SD) was obtained using the measured phase shift between ABP and F (equation (3)) and found to be τ = 84 ± 32 ms, giving an average arteriole compliance (equation (4)) of C = (2.2 ± 1.3) cm2/mmHg. Note, the units of C reported here reflect the units of the DCS blood flow index, F, in equation (4), i.e. cm2/s. However, these units can be calibrated.45,62
To convert the arteriole compliance measurement to traditional units, we introduce a “DCS calibration factor” of 1.3 (ml/min/100 g)/(cm2/s), which is an estimate of the proportionality coefficient between the DCS blood flow index and absolute blood flow. Similar calibration factors have been measured in previous DCS investigations, albeit of tissue types and patient types that are different from the adult subject group studied here.45,62,63 The present calibration factor was derived from 21 measurements of absolute blood flow measured with a dynamic contrast-enhanced near-infrared spectroscopy technique62,64 concurrent with DCS blood flow index measurements in a sample of 4 adults with traumatic brain injury (data not published; the standard deviation of the individual calibration factors was 47% of the mean). Utilizing this calibration factor, and noting caveats about subject similarity, we obtain an average arteriole compliance of C = (4.8 ± 2.8) ml/mmHg/100 g.
To assess the influence of the arteriole compliance on the CrCP calculation with DCS, we introduced a “resistive only” calculation of CrCP with DCS that neglects compliance and is denoted by CrCPDCS,R. This calculation employs the τ = 0 limit of equation (2). Interestingly, we see by comparison that cerebral compliance introduces a small but non-negligible effect on the full CrCP calculation with DCS (Figure 7). Indeed, while CrCPDCS and CrCPDCS,R are strongly correlated across the 18 subjects (R = 0.78), the slope between CrCPDCS,R and CrCPDCS of 1.39 ± 0.21 is different from unity, indicating that inclusion of compliance effects for the arteriole compartment is important. The data suggest that the resistive-only model overestimates CrCP. Note, we did not observe a significant phase shift between the ABP and artery flow velocity waveforms from TCD (e.g. see Figure 4(a)); thus the resistive model is probably sufficient for TCD analysis.
Figure 7.
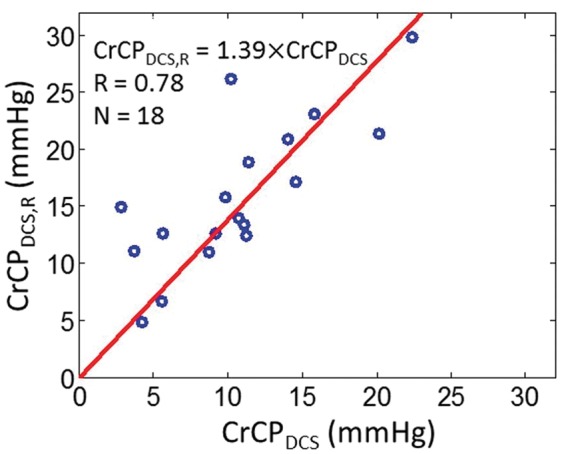
DCS estimates of CrCP computed with the resistive only vascular model (i.e. τ = 0 limit of equation (2), vertical axis) and using the full Windkessel model (i.e. equation (2), horizontal axis) in 18 healthy adults. Solid red line is the linear best-fit with intercept forced to be zero (R = 0.78; slope ± 95 CI = 1.39 ± 0.21).
Discussion
The present article introduced and demonstrated a diffuse optical technology and scheme for measurement of critical closing pressure (CrCP), actual cerebral perfusion pressure (aCPP), arteriole bed transit time (τ), and arteriole bed compliance (C) in healthy adults, which can readily be translated to patients in both inpatient and outpatient settings. The key element of the approach is to measure and compare the pulsatile ABP and flow waveforms. The CrCP and aCPP generated from the optical flow waveforms compared well with similar measurements using TCD waveforms; i.e. their mean values were the same within experimental error, and the variations measured by both techniques were significantly correlated. The work demonstrates non-invasive diffuse optical measurements of CrCP and aCPP, and suggests the optical technology as a possible alternative to TCD for use in the clinic, which also holds potential to replace invasive monitors in patients with acute brain injury. Trend monitoring and spot measurements of CrCP, for example, may be able to noninvasively identify elevated ICP episodes during acute neurocritical care. Additionally, measurements of CrCP in combination with measurements of ICP enables estimation of vasomotor tone (i.e. vascular wall tension),65 which may provide important information about autoregulation and vascular reactivity. Future work is needed to establish longitudinal reproducibility of the optical CrCP measurements in controls and in patients with brain injury and elevated ICP.
CrCP and aCPP
The DCS technique probes blood flow in the arteriole bed rather than in the major arteries. By contrast, TCD typically probes macrovascular flow in major arteries such as the MCA, which are upstream in the tissue vasculature. Thus, DCS should be more sensitive to physiological factors such as vascular compliance and vasomotor tone that contribute substantially to the responses of the downstream microvasculature. In healthy subjects, we expect that the different measurement techniques should be strongly correlated, but for patients with heterogeneous brain diseases/injuries, the DCS probe-pads can be positioned over a region of interest to directly measure localized arteriole blood flow at the disease site and thereby provide information about the cerebral vascular responses that is complementary to TCD. The DCS technique is also very well-suited for continuous, non-invasive, long-term monitoring of CrCP in all patient populations including patients on anticoagulant medications and pediatric patients.31,37–39 Lastly, the Reynolds number of flow in arterioles is substantially lower than the Reynolds number in large arteries,66 and therefore the DCS measurement is less susceptible to the consequences of turbulent flow in the vasculature, which can invalidate simple flow models.
The measured variability in CrCP and arteriole compliance across the healthy subject population was considerable (about 50% of the mean). This variability may reflect inter-subject variability in ICP and vasomotor tone. Although little data on ICP in healthy adults have been reported, some evidence of inter-subject ICP variability in healthy controls exists. In one study of 197 normal children, the average (mean ± SD) ICP measured from a lumbar puncture was 14.6 ± 5.0 mmHg and ranged from 4.4 to 35 mmHg.67 In another study, measurements of ICP in four normal adult patients ranged from −3 to 6.5 mmHg.68 It is also plausible for vasomotor tone to vary across healthy adults, since vasomotor tone changes with varying blood pressure, calmness/neural activation, hematocrit, lower limit of autoregulation, etc. Comparable variability to our measurements of CrCP and arterial compliance measured with TCD and with arterial tonometry, respectively, has been reported for healthy controls in the literature.69,70
The DCS technique has some limitations. When measurements of absolute flow are desired, some form of calibration must be utilized. In addition, CrCP analysis using DCS data requires an estimate of the ratio between systemic ABP and blood pressure at the entrance to the arteriole bed, i.e. γ in equations (1) and (2). The calculation of CrCP is directly proportional to γ (equation (2)), and therefore a 10% error in γ corresponds to a 10% error in CrCP. Arteriole compliance (equation (4)) is inversely proportional to γ. Inter-subject variations in γ likely contributed significantly to the large width of the 95% CI (in comparison to the mean) for the Bland–Altman analysis comparing CrCPDCS to CrCPTCD (Figure 5(b)). In practice, γ can be assumed from published literature measurements,49 or it can be calibrated with an “initial” measurement/estimate of CrCP on a patient by patient basis. Another source of error in the DCS measurements is signal contamination from scalp blood flow. The pulsatility (i.e. ) of scalp blood flow is lower than cerebral blood flow,71 and therefore scalp contamination would result in an underestimation of CrCP (see equation (2)). In the present study, scalp contamination was reduced by applying a moderate probe pressure against the head with the TCD head frame to maintain a low scalp flow underneath the probe.72,73
Finally, the accuracy of CrCP and compliance scales inversely with the number of samples (i.e. signal length) used in the transfer function analysis for their computation (see Data Processing for CrCP Calculation). A conservative estimate of measurement error for individual subjects was obtained by dividing the 5-min intervals of blood pressure and blood flow waveform measurements into two 2.5-min subintervals, from which two estimates of CrCP and compliance were obtained for each subject. The percent deviation between these two measurements (i.e. magnitude of the difference divided by their mean) for the CrCP and compliance are 17 ± 10% and 17 ± 7%, respectively (median ± IQR/2). Note that these two estimates wherein the signal length is half that of the original estimate are expected to be less accurate than the original estimate from the full signal.
Arteriole compliance and flow transit time
As noted above, the DCS blood flow readily exhibits a phase shift from the ABP waveform. This phase shift, which is not evident in the TCD waveforms, enables calculation of the transit time and compliance of the arteriole bed (equation (4)). These parameters require further study, but they may prove to be useful biomarkers for brain disease, and they also provide information complementary to that obtained by techniques developed for measurement of the compliance in large arteries.57,70,74–77
Comparison of DCS arteriole bed compliance to other compliance measurements reported in the literature is challenging. Here, we define compliance as the absolute mean arteriole blood volume, V, divided by the pressure difference across the arteriole bed, (see Figure 2); further, our reported numbers are normalized by tissue weight and require calibration of DCS flow to generate absolute flow (e.g. calibration by tracking an indocyanine green bolus through cerebral tissue,62,64 MRI or CT perfusion scans, or Coherent Hemodynamic Spectroscopy (CHS) NIRS78). By contrast, a different definition has been used in previously reported compliance measurements of larger arteries, i.e. the compliance is defined as the pulsatile change in blood volume of a single vessel divided by the pulsatile change in arterial blood pressure during the cardiac cycle.57,70,74–77 Thus, comparison of our measurement of the arteriole bed compliance with previously reported measurements of compliance in larger arteries (e.g. 0.05 ml/mmHg in distal arteries70) is not readily feasible without many additional approximations. Nevertheless, now that we have shown how compliance can be measured with DCS, it will be interesting to explore these comparisons in future work.
On the other hand, the arteriole bed transit time (τ) is less susceptible to differences in measurement units and calibration issues, and it may be a useful biomarker for brain disease.79 In Wistar rats, measurements of 14 mm/s and 1.2 mm were reported for cerebral pial arteriole blood velocity and length, respectively.80,81 This corresponds to a transit time of ∼86 ms, which is consistent with our arteriole transit time measurement of ∼84 ms. Previously, CHS NIRS was employed to measure a capillary transit time of 0.92 ± 0.18 s across 11 adult humans.82 This number is an upper bound on the arteriole transit time, which is expected to be considerably less than the capillary transit time where most of the oxygen exchange takes place. ASL MRI was used in 22 healthy adults to measure an arterial transit time of 0.48 ± 0.09 s,83 which is the duration for blood in the labeling region below the brain to flow into the vascular compartment imaged in the brain. This number also provides an upper bound on the arteriole transit time, since the path includes cerebral arteries and arterioles. Exploring the particular significance of arteriolar transit time measurements is an exciting avenue for future research.
In the present work, we did not observe a significant phase shift between the arterial blood pressure (ABP) and TCD flow velocity waveforms obtained from the middle cerebral artery. The larger phase shift measured with DCS is due at least in part to the fact that DCS probes local arteriole tissue (compliance) with greater sensitivity, which may in turn provide more sensitivity to vasomotor tone since variations in vasomotor tone likely give rise to variations in compliance. The origin of the observed timing difference between the DCS F and TCD Fv waveforms is an open question. Recall that DCS measures flow in the microvasculature under the probe; we modeled this microvasculature as a two-compartment Windkessel consisting of a resistor (R) and capacitor (C) in parallel (Figure 2), and thus the phase between ABP and F is given by (equation (3)). In principal, one could also model the arterial vasculature supplied by the MCA as a two-compartment Windkessel with resistance RTCD and compliance CTCD.47 Since the MCA supplies a much larger tissue volume than that measured by DCS, RTCD and CTCD can be substantially different from R and C. If the product RTCDCTCD is less than RC, then TCD Fv is more in-phase with ABP than DCS F (see equation (3)), which is consistent with our measurements. Future work with higher temporal resolution measurements may help better understand and quantify these differences, and could lead to improved modeling of the whole arterial vascular tree.
Finally, we note that the influence of compliance on the CrCP calculation was small but not negligible (Figure 7). It is therefore preferable to measure the phase shift between F and ABP and include it in the CrCP calculation, though the τ = 0 limit of equation (2) still gave a fairly good estimate of CrCP.
DCS measures arteriole blood flow
Our results provide supporting evidence for the suggestion that DCS signals are predominantly sensitive to arteriole blood flow. A priori, one would expect that the light detected by DCS interacts with blood in a mixture of arteriole, capillary, and venule compartments (recall, contributions from large arteries will be small due to their large light absorption). Consequently, the DCS blood flow index can be sub-divided to contain three contributions:
| (6) |
where Fart, Fcap, and Fven are the arteriole, capillary, and venule blood flows, respectively, and ka, kc, and kv are the weights of each compartment in the signal (). After taking time averages, and assuming further that pulsatile flow is only large in the arterioles,80,84 equation (6) simplifies to
| (7) |
Here, is the pulsatile component of blood flow in the arterioles, and is the average steady-state blood flow through the vasculature. From equation (7), it is apparent that the DCS flow amplitude at the heart-rate frequency is The observed relationship between the DCS-flow estimate of CrCP with equation (2) and the TCD estimate of CrCP (Figure 5) suggests that ka is approximately one, which in turn indicates that DCS is predominantly sensitive to flow in arterioles.
This observation and assignment of DCS signal origin are consistent with a recent investigation using Monte Carlo simulations to demonstrate that the decay of the intensity autocorrelation function measured by DCS (Figure 1(c)) is primarily due to the diffusive movement of red blood cells induced by shear flow.43 The shear-induced diffusion coefficient of red blood cells is proportional to the shear rate,85 which in turn scales linearly with blood flow.43,86 Consequently, it is reasonable to expect that among the three vascular compartments considered, the one with the highest shear rate would contribute most to the decay of the DCS intensity autocorrelation function. Shear rate is proportional to the maximum speed of red blood cells at the center of the vessel and inversely proportional to the square of the vessel radius.43,87 The combination of a steeper pressure gradient and lower compliance leads to an expectation of a much higher shear rate in arterioles compared to capillaries and venules.
Conclusion
We have introduced and demonstrated a novel optical technique for continuous, noninvasive monitoring of CrCP with near-infrared light intensity correlations. The optical measurements of CrCP were consistent with Doppler ultrasound (TCD) measurements in healthy adults. We further measured the arteriole compliance with the optical technique, a parameter which is rarely measured and which may be a useful biomarker for brain disease in the future. Our experimental measurements also support the notion that the origin of the DCS signal is primarily from shear-induced diffusive red blood cell movement in cerebral arterioles.
Supplementary Material
Acknowledgements
We gratefully acknowledge Turgut Durduran, Tiffany Ko, Rodrigo Forti, Jeff Cochran, and Sanghoon Chong for valuable discussions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health (grant numbers R01-NS082309-01A1, R01-NS060653, P41-EB015893, R01-NS072338, T32-HL007915), the American Heart Association (grant number 14POST20460161), and philanthropic support from the June and Steve Wolfson Family Foundation.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.R. Busch, W.B. Baker, A.B. Parthasarathy, and A.G. Yodh have two pending patent applications, and A.G. Yodh has two other patents relevant to this work (United States patents 8,082,015 and 6,076,010) but do not currently generate income.
Authors’ contributions
WB, AP, KG, MM, AY, and WK designed the study. AP, KG, and MM did the subject measurements. KA, DB, AP, and VK designed and built the optical instrumentation. WB and AP carried out primary data analysis. WB, with input from AP and AY, developed the theoretical modeling. WB wrote the manuscript with input from all other authors. Important editorial input, data analysis suggestions, and manuscript revisions were made by AY, RM, RB, AP, JD, JG, DL, DB, LH, and WK.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. New Engl J Med 2000; 343: 710–722. [DOI] [PubMed] [Google Scholar]
- 2.Le Roux P. Physiological monitoring of the severe traumatic brain injury patient in the intensive care unit. Curr Neurol Neurosci Rep 2013; 13: 1–16. [DOI] [PubMed] [Google Scholar]
- 3.Sandsmark DK, Kumar MA, Park S, et al. Multimodal monitoring in subarachnoid hemorrhage. Stroke 2012; 43: 1440–1445. [DOI] [PubMed] [Google Scholar]
- 4.Tzeng Y-C, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol 2014; 114: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato R, Pinsky MR. Personalizing blood pressure management in septic shock. Ann Intensive Care 2015; 5: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg 1995; 83: 949–962. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt JM, Ko S-B, Helbok R, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke 2011; 42: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkman M, Smith M. Intracranial pressure monitoring, cerebral perfusion pressure estimation, and ICP/CPP-guided therapy: a standard of care or optional extra after brain injury? Br J Anaesthesia 2014; 112: 35–46. [DOI] [PubMed] [Google Scholar]
- 9.Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics: Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg 1974; 41: 597–606. [DOI] [PubMed] [Google Scholar]
- 10.Panerai R. The critical closing pressure of the cerebral circulation. Med Eng Phys 2003; 25: 621–632. [DOI] [PubMed] [Google Scholar]
- 11.Weyland A, Buhre W, Grund S, et al. Cerebrovascular tone rather than intracranial pressure determines the effective downstream pressure of the cerebral circulation in the absence of intracranial hypertension. J Neurosurg Anesthesiol 2000; 12: 210–216. [DOI] [PubMed] [Google Scholar]
- 12.Permutt S, Riley R. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol 1963; 18: 924–932. [DOI] [PubMed] [Google Scholar]
- 13.Aaslid R, Lash SR, Bardy GH, et al. Dynamic pressure–flow velocity relationships in the human cerebral circulation. Stroke 2003; 34: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 14.Maas JJ, de Wilde RB, Aarts LP, et al. Determination of vascular waterfall phenomenon by bedside measurement of mean systemic filling pressure and critical closing pressure in the intensive care unit. Anesthesia Analgesia 2012; 114: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichol J, Girling F, Jerrard W, et al. Fundamental instability of the small blood vessels and critical closing pressures in vascular beds. Am J Physiol–Legacy Content 1951; 164: 330–344. [DOI] [PubMed] [Google Scholar]
- 16.Aaslid R, Lundar T, Lindegaard K, et al. Estimation of cerebral perfusion pressure from arterial blood pressure and transcranial Doppler recordings. In: Miller JD, Teasdale GM, Rowan JO, Galbraith SL, et al. (eds). Intracranial Pressure VI, Berlin, Heidelberg: Springer, 1986, pp. 226–229. [Google Scholar]
- 17.Czosnyka M, Matta BF, Smielewski P, et al. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg 1998; 88: 802–808. [DOI] [PubMed] [Google Scholar]
- 18.Varsos GV, Kolias AG, Smielewski P, et al. A noninvasive estimation of cerebral perfusion pressure using critical closing pressure. J Neurosurg 2015; 123: 638–648. [DOI] [PubMed] [Google Scholar]
- 19.Belfort MA, Tooke-Miller C, Varner M, et al. Evaluation of a noninvasive transcranial Doppler and blood pressure-based method for the assessment of cerebral perfusion pressure in pregnant women. Hypertens Pregnancy 2000; 19: 331–340. [DOI] [PubMed] [Google Scholar]
- 20.Rhee CJ, Fraser CD, III, Kibler K, et al. Ontogeny of cerebrovascular critical closing pressure. Pediatr Res 2015; 78: 71–75. [DOI] [PubMed] [Google Scholar]
- 21.Franceschini MA. Advances in measuring cerebral oxygen delivery and consumption in the clinic. In: Biomedical Optics 2016, OSA, Clinical and translational biophotonics, Fort Lauderdale, FL: Optical Society of America, 2016, pp. p.JTu1A. [Google Scholar]
- 22.Parthasarathy AB, Gannon KP, Baker WB, et al. Cerebral autoregulation dynamics with high-speed diffuse correlation spectroscopy. In: Biomedical Optics 2016, OSA, Fort Lauderdale, FL: Optical Society of America, 2016, pp. p.BTh4D.7. [Google Scholar]
- 23.Farzam P, Sutin J, Wu K-C, et al. Fast diffuse correlation spectroscopy for non-invasive measurement of intracranial pressure. In: Madsen SJ, Yang VXD. (eds). Clinical and translational neurophotonics, San Francisco, CA: SPIE Photonics West, 2017, pp. 10050–10028. [Google Scholar]
- 24.Yonas H, Pindzola R. Physiological determination of cerebrovascular reserves and its use in clinical management. Cerebrovasc Brain Metab Rev 1993; 6: 325–340. [PubMed] [Google Scholar]
- 25.Yonas H, Pindzola RR. Clinical application of cerebrovascular reserve assessment as a strategy for stroke prevention. Keio J Med 2000; 49: A4–A10. [PubMed] [Google Scholar]
- 26.Pindzola RR, Balzer JR, Nemoto EM, et al. Cerebrovascular reserve in patients with carotid occlusive disease assessed by stable xenon-enhanced ct cerebral blood flow and transcranial Doppler. Stroke 2001; 32: 1811–1817. [DOI] [PubMed] [Google Scholar]
- 27.Pindzola RR, Sashin D, Nemoto EM, et al. Identifying regions of compromised hemodynamics in symptomatic carotid occlusion by cerebrovascular reactivity and oxygen extraction fraction. Neurol Res 2006; 28: 149–154. [DOI] [PubMed] [Google Scholar]
- 28.Hancock SM, Mahajan RP, Athanassiou L. Noninvasive estimation of cerebral perfusion pressure and zero flow pressure in healthy volunteers: the effects of changes in end-tidal carbon dioxide. Anesthesia Analgesia 2003; 96: 847–851. [DOI] [PubMed] [Google Scholar]
- 29.Edouard A, Vanhille E, Le Moigno S, et al. Non-invasive assessment of cerebral perfusion pressure in brain injured patients with moderate intracranial hypertension. Br J Anaesthesia 2005; 94: 216–221. [DOI] [PubMed] [Google Scholar]
- 30.Cardim D, Robba C, Bohdanowicz M, et al. Non-invasive monitoring of intracranial pressure using transcranial doppler ultrasonography: Is it possible? Neurocrit Care 2016; 25: 473–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busch DR, Rusin CG, Miller-Hance W, et al. Continuous cerebral hemodynamic measurement during deep hypothermic circulatory arrest. Biomed Opt Express 2016; 7: 3461–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soehle M, Czosnyka M, Pickard JD, et al. Critical closing pressure in subarachnoid hemorrhage effect of cerebral vasospasm and limitations of a transcranial Doppler-derived estimation. Stroke 2004; 35: 1393–1398. [DOI] [PubMed] [Google Scholar]
- 33.Durduran T, Yodh AG. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. NeuroImage 2014; 85(Part 1): 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Parthasarathy AB, Baker WB, et al. Fast blood flow monitoring in deep tissues with real-time software correlators. Biomed Opt Express 2016; 7: 776–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckley EM, Parthasarathy AB, Grant EP, et al. Diffuse correlation spectroscopy for measurement of cerebral blood flow: future prospects. Neurophotonics 2014; 1: 011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durduran T, Choe R, Baker WB, et al. Diffuse optics for tissue monitoring and tomography. Rep Progr Phys 2010; 73: 076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckley EM, Lynch JM, Goff DA, et al. Early postoperative changes in cerebral oxygen metabolism following neonatal cardiac surgery: Effects of surgical duration. J Thoracic Cardiovasc Surg 2013; 145: 196–205.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferradal SL, Yuki K, Vyas R, et al. Non-invasive assessment of cerebral blood flow and oxygen metabolism in neonates during hypothermic cardiopulmonary bypass: Feasibility and clinical implications. Sci Rep 2017; 7: 44117–44126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yücel MA, Selb J, Boas DA, et al. Reducing motion artifacts for long-term clinical NIRS monitoring using collodion-fixed prism-based optical fibers. Neuroimage 2014; 85: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerhof N, Lankhaar J-W, Westerhof BE. The arterial windkessel. Med Biol Eng Comput 2009; 47: 131–141. [DOI] [PubMed] [Google Scholar]
- 41.Boas DA, Campbell LE, Yodh AG. Scattering and imaging with diffusing temporal field correlations. Phys Rev Lett 1995; 75: 1855–1858. [DOI] [PubMed] [Google Scholar]
- 42.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A 1997; 14: 192–215. [Google Scholar]
- 43.Boas DA, Sakadžić S, Selb J, et al. Establishing the diffuse correlation spectroscopy signal relationship with blood flow. Neurophotonics 2016; 3: 031412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mesquita RC, Durduran T, Yu G, et al. Direct measurement of tissue blood flow and metabolism with diffuse optics. Philos Trans Ser A: Math Phys Eng Sci 2011; 369: 4390–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain V, Buckley EM, Licht DJ, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab 2014; 34: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dietsche G, Ninck M, Ortolf C, et al. Fiber-based multispeckle detection for time-resolved diffusing-wave spectroscopy: characterization and application to blood flow detection in deep tissue. Appl Opt 2007; 46: 8506–8514. [DOI] [PubMed] [Google Scholar]
- 47.Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cerebr Blood Flow Metab 2013; 33: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandeville JB, Marota JJ, Ayata C, et al. Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cerebr Blood Flow Metab 1999; 19: 679–689. [DOI] [PubMed] [Google Scholar]
- 49.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 1989; 13: 968–972. [DOI] [PubMed] [Google Scholar]
- 50.Nichols W, O'Rourke M, Vlachopoulos C. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles, Boca Raton, Florida: CRC Press, 2011. [Google Scholar]
- 51.Michel E, Hillebrand S, von Twickel J, et al. Frequency dependence of cerebrovascular impedance in preterm neonates: A different view on critical closing pressure. J Cerebr Blood Flow Metab 1997; 17: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 52.Baker WB, Parthasarathy AB, Busch DR, et al. Modified Beer-Lambert law for blood flow. Biomed Opt Express 2014; 5: 4053–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichols W, O'Rourke M, Vlachopoulos C. McDonald's blood flow in arteries: Theoretical, experimental and clinical principles (Table 4.3), Boca Raton, Florida: CRC Press, 2011. [Google Scholar]
- 54.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25: 359–364. [DOI] [PubMed] [Google Scholar]
- 55.Nichols W, McDonald D. Wave-velocity in the proximal aorta. Med Biol Eng Comput 1972; 10: 327–335. [DOI] [PubMed] [Google Scholar]
- 56.Giller CA, Aaslid R. Estimates of pulse wave velocity and measurement of pulse transit time in the human cerebral circulation. Ultrasound Med Biol 1994; 20: 101–105. [DOI] [PubMed] [Google Scholar]
- 57.O’Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 2002; 15: 426–444. [DOI] [PubMed] [Google Scholar]
- 58.Welch PD. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 1967; 15: 70–73. [Google Scholar]
- 59.Cheng R, Shang Y, Hayes D, et al. Noninvasive optical evaluation of spontaneous low frequency oscillations in cerebral hemodynamics. Neuroimage 2012; 62: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R, Zuckerman JH, Giller CA, et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol-Heart Circ Physiol 1998; 274: H233–H241. [DOI] [PubMed] [Google Scholar]
- 61.Horowitz P, Hill W, Robinson I. The art of electronics, Cambridge: Cambridge University Press, 1980. [Google Scholar]
- 62.Diop M, Verdecchia K, Lee T-Y, et al. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed Opt Express 2011; 2: 2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z, Baker WB, Parthasarathy AB, et al. Calibration of diffuse correlation spectroscopy blood flow index with venous-occlusion diffuse optical spectroscopy in skeletal muscle. J Biomed Opt 2015; 20: 125005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elliott JT, Diop M, Morrison LB, et al. Quantifying cerebral blood flow in an adult pig ischemia model by a depth-resolved dynamic contrast-enhanced optical method. NeuroImage 2014; 94: 303–311. [DOI] [PubMed] [Google Scholar]
- 65.Varsos GV, Budohoski KP, Kolias AG, et al. Relationship of vascular wall tension and autoregulation following traumatic brain injury. Neurocrit Care 2014; 21: 266–274. [DOI] [PubMed] [Google Scholar]
- 66.Ku DN. Blood flow in arteries. Annu Rev Fluid Mech 1997; 29: 399–434. [Google Scholar]
- 67.Avery RA, Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. New Engl J Med 2010; 363: 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andresen M, Hadi A, Petersen LG, et al. Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochirurgica 2015; 157: 109–113. [DOI] [PubMed] [Google Scholar]
- 69.Panerai R, Deverson S, Mahony P, et al. Effect of CO2 on dynamic cerebral autoregulation measurement. Physiol Measure 1999; 20: 265–275. [DOI] [PubMed] [Google Scholar]
- 70.Duprez D, De Buyzere M, Rietzschel E, et al. Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur Heart J 1998; 19: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 71.Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 2011; 8: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesquita RC, Schenkel SS, Minkoff DL, et al. Influence of probe pressure on the diffuse correlation spectroscopy blood flow signal: extra-cerebral contributions. Biomed Opt Express 2013; 4: 978–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker WB, Parthasarathy AB, Ko TS, et al. Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts. Neurophotonics 2015; 2: 035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pannier BM, Avolio AP, Hoeks A, et al. Methods and devices for measuring arterial compliance in humans. Am J Hypertens 2002; 15: 743–753. [DOI] [PubMed] [Google Scholar]
- 75.Kim D-J, Kasprowicz M, Carrera E, et al. The monitoring of relative changes in compartmental compliances of brain. Physiol Measure 2009; 30: 647–659. [DOI] [PubMed] [Google Scholar]
- 76.Warnert EA, Murphy K, Hall JE, et al. Noninvasive assessment of arterial compliance of human cerebral arteries with short inversion time arterial spin labeling. J Cerebr Blood Flow Metab 2015; 35: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan L, Liu CY, Smith RX, et al. Assessing intracranial vascular compliance using dynamic arterial spin labeling. NeuroImage 2016; 124: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fantini S, Sassaroli A, Tgavalekos KT, et al. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016; 3: 031411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kasprowicz M, Czosnyka M, Soehle M, et al. Vasospasm shortens cerebral arterial time constant. Neurocrit Care 2012; 16: 213–218. [DOI] [PubMed] [Google Scholar]
- 80.Seki J, Satomura Y, Ooi Y, et al. Velocity profiles in the rat cerebral microvessels measured by optical coherence tomography. Clin Hemorheol Microcirc 2006; 34: 233–239. [PubMed] [Google Scholar]
- 81.Lapi D, Marchiafava P, Colantuoni A. Geometric characteristics of arterial network of rat pial microcirculation. J Vasc Res 2007; 45: 69–77. [DOI] [PubMed] [Google Scholar]
- 82.Kainerstorfer JM, Sassaroli A, Hallacoglu B, et al. Practical steps for applying a new dynamic model to near-infrared spectroscopy measurements of hemodynamic oscillations and transient changes: implications for cerebrovascular and functional brain studies. Acad Radiol 2014; 21: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshiura T, Hiwatashi A, Yamashita K, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. Am J Neuroradiol 2009; 30: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rashid S, McAllister JP, Yu Y, et al. Neocortical capillary flow pulsatility is not elevated in experimental communicating hydrocephalus. J Cerebr Blood Flow Metab 2012; 32: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldsmith H, Marlow J. Flow behavior of erythrocytes. II. Particle motions in concentrated suspensions of ghost cells. J Colloid Interface Sci 1979; 71: 383–407. [Google Scholar]
- 86.Carp SA, Roche-Labarbe N, Franceschini M-A, et al. Due to intravascular multiple sequential scattering, diffuse correlation spectroscopy of tissue primarily measures relative red blood cell motion within vessels. Biomed Opt Express 2011; 2: 2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu X, Pine D, Chaikin P, et al. Diffusing-wave spectroscopy in a shear flow. JOSA B 1990; 7: 15–20. [Google Scholar]
- 88.Hallacoglu B, Sassaroli A, Wysocki M, et al. Absolute measurement of cerebral optical coefficients, hemoglobin concentration and oxygen saturation in old and young adults with near-infrared spectroscopy. J Biomed Opt 2012; 17: 081406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.