Abstract
Toxic shock syndrome (TSS) is a rare but life-threatening multisystem disease known to develop in the early postoperative period after various surgery. We report a rare case in which a patient who underwent Caesarean section developed TSS caused by methicillin-resistant Staphylococcus aureus (MRSA) on the 39th postoperative day. She was treated with debridement because of the possible diagnosis of necrotizing soft tissue infections. Culture test from the resected specimen was positive for MRSA. She was diagnosed with TSS caused by suture abscess and was treated with intensive care including antimicrobials. After a good postoperative course, she was discharged on the 30th postoperative day. TSS occurring 4 weeks after operation is extremely rare, but late-onset of suture abscess is known to occur. We should becognizant of development with TSS beyond early postoperative period.
Keywords: Toxic shock syndrome, Methicillin-resistant staphylococcus aureus, Surgical site infection, Caesarean section, Late onset
Introduction
Toxic shock syndrome (TSS) is caused by staphylococcal and streptococcal super antigens that produce a rapid hyper-inflammatory response typified by a cytokine storm [1], [2], [3]. TSS can progress rapidly to multiple organ failure and death [4], [5]. This syndrome was first reported by Todd et al. in 1978, as a rare complication of staphylococcus aureus infection [4]. Methicillin-resistant Staphylococcus aureus (MRSA) Infections in the puerperium period often lead to TSS and become severe conditions. TSS is known to develop in the early postoperative period after various surgery [6], [7]. In this case report we present a patient who developed TSS which was provoked by suture abscess with MRSA with a late onset after surgery.
Case
A 33-year-old female delivered a baby by Caesarean section at 29 weeks gestation with premature rupture, amniotic fluid turbidity and chorioamnionitis. Her past medical history was negative. The patient had placenta abscesses, but the vaginal discharge sample was negative for culture except for normal vaginal flora. The postoperative course was uneventful and the patient discharged on the 6th postoperative day.
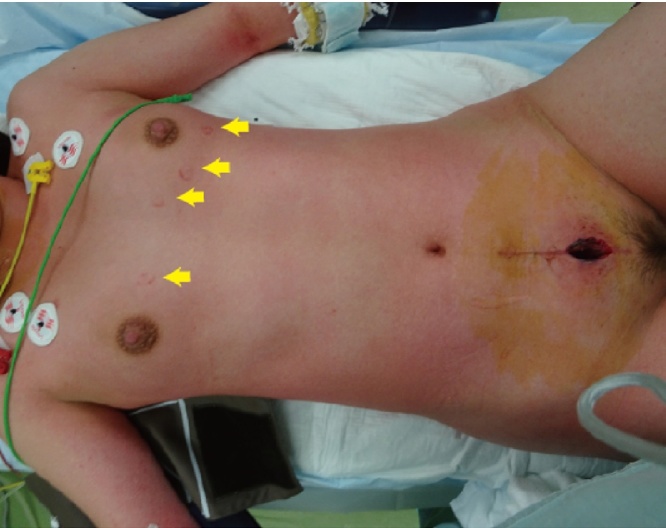
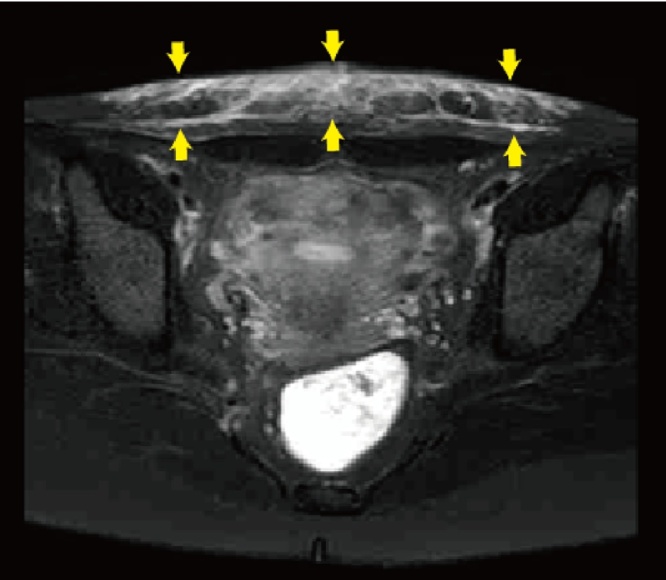
On the 37th postoperative day, the patient had a fever more than 38 °C and felt rigidity and pain in the abdominal surgical scar. Her pain gradually increased, and the fever rose up to 39.0 °C in two days and she visited our hospital. On physical examination, her blood pressure was 84/45 mmHg with a pulse rate of 143 beats, respiratory rate of 24 per minute, and body temperature of 41.2 °C. Her conjunctivae were hyperemic. She had significant tenderness, swelling, redness and induration around the surgical scar in the abdomen, a rash on the trunk of her body and arms, and subcutaneous bleeding in the area from where the ECG pads had been removed (Fig. 1). Concurrently, she had purulent vaginal discharge. Laboratory analysis showed WBC was 16,700/mm3 and hemoglobin and platelets were within normal range, AST, ALT and LDH were high at 221, 136 and 408 U/L respectively, BUN and creatinine were also high at 26.5 and 2.12 mg/dL respectively, and a C-reactive protein level was high at 17.57 mg/dL. Abdominal MRI examination revealed high signal-intensity lesion around the surgical scar in T2 weighted images with fat-suppression (Fig. 2).
Fig. 1.
The patient had a rash on the trunk of her body and arms, and also subcutaneous bleeding in the area from where the ECG pads had been removed (arrows).
Fig. 2.
Abdominal MRI examination revealed high signal-intensity lesion (arrows) around the surgical scar in T2 weighted images with fat-suppression.
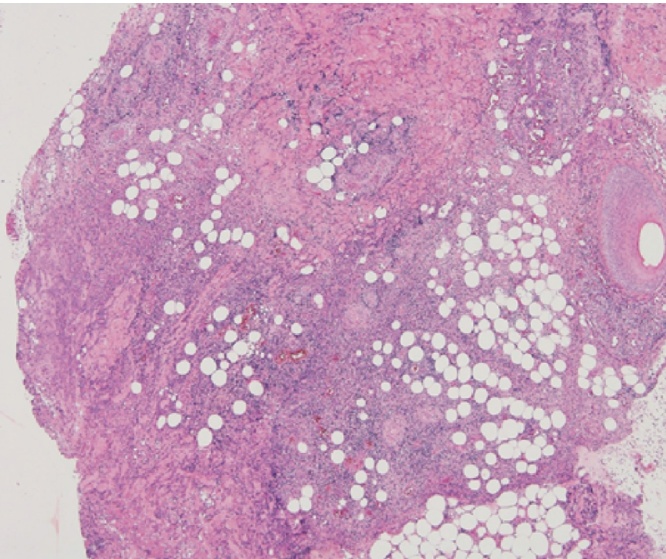
She was diagnosed with sepsis caused by surgical site infection. Debridement was performed for the induration area that involved local necrosis of the skin and adipose tissue. The rectus abdominis fascia under the area was not necrotic. We removed the suture threads of Vicryl plus® which were used for the rectus abdominis fascia suture. The pathological findings of the resected specimens revealed granulation tissue formation with multinuclear foreign-body giant cells and inflammatory cells around the suture threads (Fig. 3). The abscess formation was observed most strongly in the midline incision. Culture tests of the specimens demonstrated pure growth of MRSA, sensitive to vancomycin. Blood culture was negative. The vaginal discharge sample was negative for culture except normal vaginal flora. From these findings, we diagnosed the patient with TSS caused by MRSA with a stitch abscess of the rectus abdominis fascia closure. We changed the antibacterial treatment to vancomycin on the 3rd postoperative day. In addition, negative pressure wound therapy had been performed for the skin defect. Simple closure for skin defect was performed on the 22nd postoperative day. She had a good postoperative course and she was discharged on the 30th postoperative day.
Fig. 3.
Histological analysis of the resected specimen showed that inflammatory cells infiltration under the deep dermis were observed. It showed panniculitis was been there.
Discussion
We present a case of TSS caused by MRSA on the 39th day post Cesarean section. As well as an association with tampon use, TSS is known to develop in the early postoperative period after various surgeries [6], [7]. Compared with menstrual TSS, postoperative TSS is the life-threatening syndrome, the mortality of postoperative TSS has been reported between 5 and 22% [5], [8], [9], [10].
Bartlett et al. [6] reported patients of postoperative TSS, among which, focal signs of surgical site infection were minimal or absent in 85% cases. This may be explained by the fact that most strains causing TSS produce very small quantities of alpha-hemolysin, but large amounts of toxins such as TSST-1 [11], [12], [13]. In vivo, these strains can cause local infections that are remarkably apurulent, but potentially fatal, owing to their superantigen expression [6], [8], [9], [13], [14].
In a retrospective review, the mean time to onset of these symptoms was generally within 2 to 4 days after surgery [7], [8]. Among 40 cases of TSS reported in Japan, it has reported that the median interval of development with TSS was 3 days after surgery [15]. The presented patient developed delayed TSS on 39th day post Cesarean section. We suspected that the origin of the MRSA infection was uterus because of the existence of vaginal discharge, but it was low probability since a vaginal discharge sample was negative for culture. From pathological findings that the patient developed infection by suture abscess was the most reasonable explanation. Once it was infected, braided suture contains of bacteria and polymorphonuclear cells, even after 70 days implantation [16]. We suggest that MRSA infected the suture during or after surgery, and consequently TSS developed in late-onset. In the present case, the braided suture threads of Vicryl plus® were used for abdominal fascial closure. It has been well established that monofilament and absorbable threads are good for prevention of development of suture abscess [17].
The case definition of TSS is described in the diagnostic criteria of Centers for Disease Control and Prevention (https://wwwn.cdc.gov/nndss/conditions/toxic-shock-syndrome-other-than-streptococcal/case-definition/2011/). Both confirmed and probable cases of TSS were included. The presented patient did not meet all the criteria but she fulfilled the conditions for probable TSS. The basic treatments of TSS are identification and decontamination of the site of toxin production, aggressive fluid resuscitation, administration of antistaphylococcal the antibacterial treatment, as well as general supportive care [18]. When a patient develops fever, rash, and a shock like state during the postoperative course, postoperative TSS should be considered even in case on month after previous surgery.
In conclusion, we encountered a patient with TSS caused by MRSA in late onset, on postoperative day 39. Most of TSS develop short interval after surgery, but few patients develop TSS late onset especially used braid sutures, thus it is crucial for all medical doctors to be aware of the possibility of occult focal infections in patients with symptoms of TSS, regardless of the long interval after surgery.
Ethics approval and consent to participate
All procedures performed in the treatment pf this patient were in accordance with the ethical standards of our institution and with the ethical guidelines of the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Funding
Not applicable.
Authors’ contributions
HK, TK participated in the patient’s treatment, data analysis and writing of the article. SO, KN, RM, KN, IH and TS participated in the patient’s care and data collection.IM and TS performed the pathological examination. HK, TK and TS participated in revising the manuscript critically. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Contributor Information
Hiroyasu Komuro, Email: hiroyasu_101819@yahoo.co.jp.
Takaharu Kato, Email: tkato@jichi.ac.jp.
Shinichiro Okada, Email: okada@jichi.ac.jp.
Kensuke Nakatani, Email: kensuken@jadecom.jp.
Risa Matsumoto, Email: risamatsumoto1217@gmail.com.
Kazuhiro Nishida, Email: pandadebanda4@gmail.com.
Hiroyuki Iida, Email: hiroakkhsn@yahoo.co.jp.
Maki Iida, Email: maki14f@ybb.ne.jp.
Shiro Tsujimoto, Email: tujimts@jadecom.jp.
Toshiyuki Suganuma, Email: toshiyukis@jadecom.jp.
References
- 1.Bergdoll M.S., Crass B.A., Reiser R.F., Robbins R.N., Davis J.P. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–1021. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y.W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin superantigens with human T cells. Proc Natl Acad Sci U S A. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchiyama T., Yan X.J., Imanishi K., Yagi J. Bacterial superantigens-mechanism of T cell activation by the superantigens and their role in the pathogenesis of infectious diseases. Microbiol Immunol. 1994;38:245–256. doi: 10.1111/j.1348-0421.1994.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 4.Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978;2:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 5.Descloux E., Perpoint T., Ferry T., Lina G., Bes M., Vandenesch F. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis. 2008;27:37–43. doi: 10.1007/s10096-007-0405-2. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett P., Reingold A.L., Graham D.R., Dan B.B., Selinger D.S., Tank G.W. Toxic shock syndrome associated with surgical wound infections. JAMA. 1982;247:1448–1450. [PubMed] [Google Scholar]
- 7.Graham D.R., O'Brien M., Hayes J.M., Raab M.G. Postoperative toxic shock syndrome. Clin Infect Dis. 1995;20:895–899. doi: 10.1093/clinids/20.4.895. [DOI] [PubMed] [Google Scholar]
- 8.Reingold A.L., Hargrett N.T., Dan B.B., Shands K.N., Strickland B.Y., Broome C.V. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann Intern Med. 1982;96:871–874. doi: 10.7326/0003-4819-96-6-871. [DOI] [PubMed] [Google Scholar]
- 9.Kain K.C., Schulzer M., Chow A.W. Clinical spectrum of nonmenstrual toxic shock syndrome (TSS): comparison with menstrual TSS by multivariate discriminant analyses. Clin Infect Dis. 1993;16:100–106. doi: 10.1093/clinids/16.1.100. [DOI] [PubMed] [Google Scholar]
- 10.Hajjeh R.A., Reingold A., Weil A., Shutt K., Schuchat A., Perkins B.A. Toxic shock syndrome in the United States: surveillance update, 1979 1996. Emerg Infect Dis. 1999;5:807–810. doi: 10.3201/eid0506.990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlievert P.M., Osterholm M.T., Kelly J.A., Nishimura R.D. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 12.Clyne M., De Azavedo J., Carlson E., Arbuthnott J. Production of gamma-hemolysin and lack of production of alpha-hemolysin by Staphylococcus aureus strains associated with toxic shock syndrome. J Clin Microbiol. 1988;26:535–539. doi: 10.1128/jcm.26.3.535-539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vojtov N., Ross H.F., Novick R.P. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc Natl Acad Sci U S A. 2002;99:10102–10107. doi: 10.1073/pnas.152152499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresler M.J. Toxic shock syndrome due to occult postoperative wound infection. West J Med. 1983;139:710–713. [PMC free article] [PubMed] [Google Scholar]
- 15.Taki Y., Ohata S., Sato S., Watanabe M., Arai K., Takagi M. A cae report of toxic shock syndrome due to MRSA developed 25 days after rectal amputation for rectal cancer. Nihon Rinsho Geka Gakkai Zasshi. 2016;77:393–398. Japanease. [Google Scholar]
- 16.Bucknall T.E. Abdominal wound closure: choice of suture. J R Soc Med. 1981;74:580–585. doi: 10.1177/014107688107400805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai H., Mizutani S., Kato N., Nonaka T., Sugiura J., Hatano Y. Effectivenes of wound infectio control in open heart surgery for neonates and infants less than three months old. Jpn J Cardiovasc Surg. 2009;38:7–10. Japanease. [Google Scholar]
- 18.Centers for Disease Control and Prevention Toxic shock syndrome (other than Streptococcal) (TSS) 2011. Case definition. Available from: http://wwwn.cdc.gov/nndss/script/casedef.aspx?CondYrID=869&DatePub=1/1/2011 [Accessed 12 July 2017] [Google Scholar]