Highlights
-
•
There have been few studies of the long-term outcomes of surgically resected intraductal papillary neoplasm of the bile duct (IPNB).
-
•
Mucus might be produced even after the R0 resection of IPNB, and frequent cholangitis or jaundice might be occurred.
-
•
The biliary tract of the remnant liver after curative resection should be managed carefully for a long time after surgical resection.
Keywords: IPNB, Intrahepatic papillary neoplasm of the bile duct, Refractory cholangitis, PTBD, Percutaneous transhepatic bile duct drainage, Case report
Abstract
Introduction
Few studies have reported the long-term outcomes of surgical resected intraductal papillary neoplasm of the bile duct (IPNB). Here, we describe the long-term observation and treatment of a case of widespread IPNB.
Presentation of case
A 57-year-old male was referred to our hospital due to jaundice and dilation of the intrahepatic bile duct. Computed tomography showed dilation and irregularities of the right intrahepatic and extrahepatic bile ducts together with a 3 cm nodule in the common hepatic duct. Peroral cholangioscopy revealed mucinous discharge from the ampulla of Vater, which resulted in a diagnosis of IPNB. A biopsy of the nodule and the bile duct revealed papillary adenoma in all of them. Right hepatectomy, caudate lobectomy, extrahepatic bile duct resection, and left hepaticojejunostomy were performed. The nodule was histologically diagnosed as papillary carcinoma in situ, and R0 resection was performed. However, mucus production from the papillary adenoma in the B3 and B4 was observed. We carefully managed the patient's biliary tract by inserting a biliary drainage tube into the segment 2, and he has survived for more than 7 years since the initial treatment.
Discussion
Mucus might be produced after the surgical resection of IPNB even if s surgical margin was benign. Five-year survival rate of benign IPNB was reported from 85% to 100%. That might be caused by difference of the postoperative management of the biliary tract.
Conclusions
Careful management of the biliary tract should be performed after surgical resection of IPNB.
1. Introduction
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare tumor that was only recently classified as a distinct pathological entity [1]. It can produce multifocal lesions, develop within any part of the biliary tree, and typically displays an exophytic growth pattern [2]. One-third of IPNB cases are associated with macroscopic mucin hypersecretion, and dilation of the bile duct is often observed [3]. IPNB is considered to be the biliary equivalent of intraductal papillary mucinous neoplasm of the pancreas (IPMN-P), and identical histological subclassifications have been described for these types of tumor [4]. However, the mucus produced by IPNB is more a serious problem compared with that produced by IPMN-P because the obstruction caused by the mucus within the biliary tree can induce cholangitis or obstructive jaundice.
There have been few studies of the long-term outcomes and recurrence pattern of surgically resected IPNB [3], [5], [6], [7]. IPNB includes adenoma, carcinoma-in-situ, and invasive carcinoma. The surgical margins of IPNB are usually evaluated histologically. If adenoma is detected in the surgical margins, the surgical resection is classified as R0. However, mucus production is even observed in cases of adenoma [1]. Even if the malignant region of an IPNB is completely resected, the mucus produced by the benign component can cause liver dysfunction or cholangitis. In such cases, the remnant IPNB could be considered clinically malignant, even if it is histologically benign.
Here, we report the long-term observation and treatment of a case of IPNB, in which the patient was finally diagnosed with widespread IPNB extending from the lower bile duct to the bilateral intrahepatic bile ducts. The mucus production in the remnant liver was observed even after R0 resection, and frequent cholangitis were occurred. The patient has survived for over 7 years after surgical resection due to careful management of his biliary tract.
This work has been reported in line with the SCARE criteria [8], [9].
2. Case reports
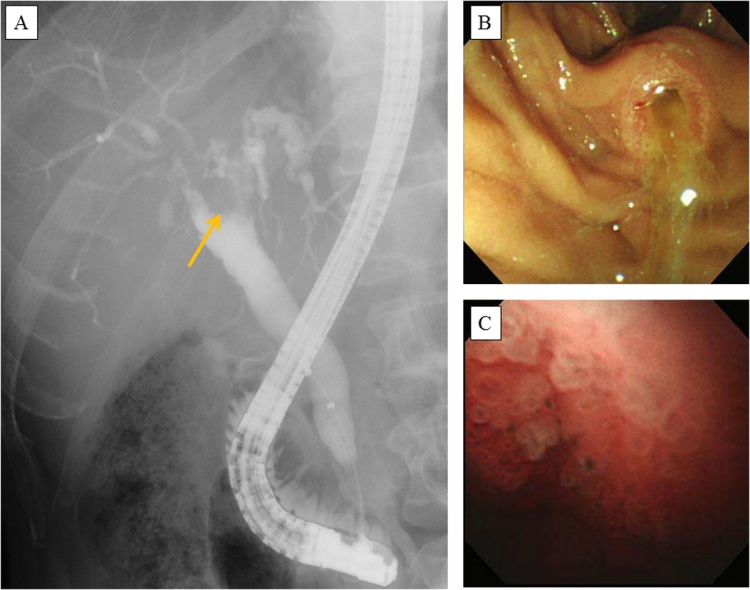
A 57-year-old male was referred to our hospital due to symptomless dilation of the intrahepatic bile duct in January 2010. Mild liver dysfunction was detected during a laboratory examination, while jaundice was not observed [total bilirubin (T-bil), 0.9 mg/dL; alkaline phosphatase (ALP), 599 U/L; aspartate aminotransferase (AST), 44 U/L]. Computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) showed dilation of the bilateral intrahepatic and extrahepatic bile ducts and a nodule measuring 1.5 cm in the common hepatic duct. Endoscopic retrograde cholangiography (ERC) revealed bile duct irregularities extending from the bilateral hepatic ducts to the peripheral bile duct. In addition, the common bile duct was dilated (to 1.5 cm in diameter) and contained a nodule measuring 1 cm in diameter (Fig. 1A). Peroral cholangioscopy (POCS) revealed mucinous discharge from the ampulla of Vater, and the bile duct was full of mucus. In addition, many round tumors (salmon roe-like lesions) were observed in the region from the middle common bile duct to the common hepatic duct (Fig. 1B/C). The nodule in the common hepatic duct was biopsied, and a pathological examination revealed intraductal papillary adenoma. The lower bile duct and the left hepatic duct were also biopsied, which led to the lesions within them being diagnosed as intraductal papillary adenoma. A cytological examination of the mucus did not reveal any evidence of malignancy. Based on these findings, the patient was diagnosed with IPNB, which was mainly located in the region from the middle common bile duct to the intrahepatic bile duct of the right and left hepatic lobes.
Fig. 1.
A The findings of endoscopic retrograde cholangiography performed at the initial diagnosis are shown. Bile duct irregularities were observed in the region from the bilateral hepatic ducts to the peripheral bile duct, and a nodule measuring 1.5 cm in diameter was seen in the common hepatic duct (arrow). B: An endoscopic examination showed mucinous discharge from the ampulla of Vater. C: Peroral cholangioscopy showed papillary tumors (salmon roe-like lesions) located in the common hepatic duct.
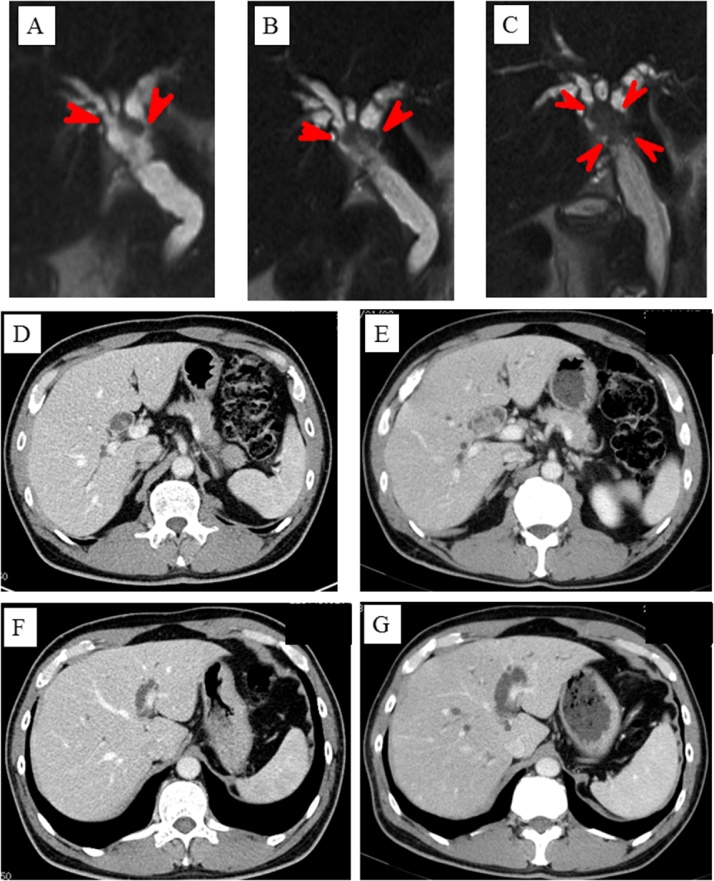
A watchful waiting strategy was employed because total resection of the irregular bile duct was impossible, and none of the histological examinations had detected findings that were suggestive of malignancy. However, 6 months later he was admitted to our hospital with jaundice and liver dysfunction [T-bil, 6.1 mg/dL; ALP, 1456 U/L; AST, 827 U/L]. MRCP and CT revealed that the nodule in the common hepatic duct had increased in size from 1.5 cm to 3 cm and that the hilar bile duct was obstructed (Fig. 2A/B/C/D/E). On the other hand, CT demonstrated that the irregularities extending from the left hepatic duct to the umbilical portion had disappeared (Fig. 2F/G). At this time, we determined that the IPNB, including the nodule and the irregular bile duct, could be resected via right hepatectomy and extrahepatic bile duct resection. An endoscopic nasobiliary drainage tube was inserted into the left hepatic duct, and the patient’s jaundice was ameliorated. At the same time, the nodule was biopsied again, and a pathological examination revealed intraductal papillary adenoma. It was estimated that the remnant liver would be 27.7% of its original size after right hepatectomy combined with resection of the caudate lobe, and percutaneous transhepatic portal vein embolization was performed. Three weeks later, the estimated size of the remnant liver had increased to 36.6%. Right hepatectomy combined with resection of the caudate lobe and extra-hepatic bile duct followed by reconstruction via left hepaticojejunostomy combined with regional lymph node resection was conducted in April 2010.
Fig. 2.
A/B/C Magnetic resonance cholangiopancreatography showed that the nodule in the common hepatic duct had grown from 1.5 cm to 3 cm. A: the initial diagnosis, B: 3 months later, C: 6 months later; D/E: Computed tomography (CT) showed that the nodule in the common hepatic duct had grown. D: the initial diagnosis, E: 6 months later; F/G: CT revealed that the irregularities in the region from the left hepatic duct to the umbilical portion had disappeared. F: the initial diagnosis, G: 6 months later.
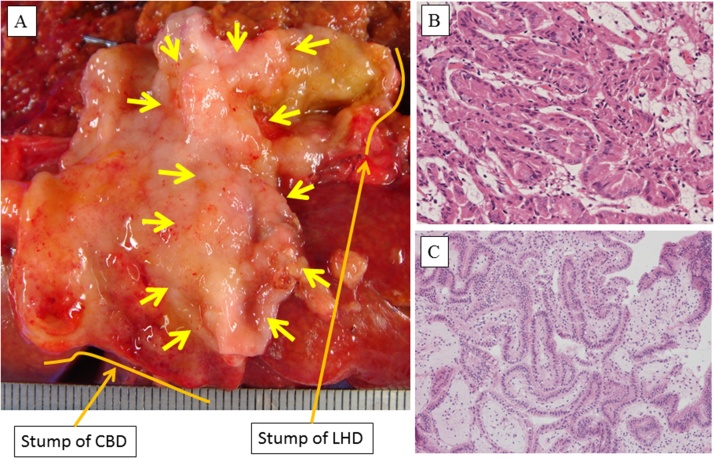
An intraoperative histological examination of frozen sections demonstrated that the stumps of the left hepatic duct and lower common bile duct were not malignant, but intraductal papillary adenoma was detected. The duration of the operation was 400 min, and the estimated amount of intraoperative blood loss was 1400 g. During a macroscopic examination, a papillary tumor measuring 3.0 × 1.5 cm was observed in the region extending from the right hepatic duct to the middle common bile duct (Fig. 3A). A histological examination showed that the tumor was mainly composed of intraductal papillary adenoma; however, some part of it was composed of intraductal papillary adenocarcinoma in situ (Fig. 3B/C). An immunohistochemical examination of the tumor revealed positivity for mucin 6 (MUC-6) and negativity for mesothelin, cyclin D1, caudal type homeobox transcription factor 2 (CDX-2), and HIK. Consequently, the tumor was diagnosed as a gastric type IPNB containing carcinoma in situ. No lymph node metastasis (0/8) was detected, and the lesions in the stump of the lower bile duct and the left hepatic duct were diagnosed as intraductal papillary adenoma. Postoperative bile leakage (International Study Group of Liver Surgery grade B) occurred and was cured by drain management. The patient was discharged at 41 days after surgery.
Fig. 3.
A The macroscopic appearance of the resected extrahepatic bile duct is shown. A papillary tumor measuring 3.0 × 1.5 cm was observed in the region from the right hepatic duct to the middle common bile duct (arrow). CBD: common bile duct, LHD: left hepatic duct; B: A histological image of the papillary tumor is shown. Intraductal papillary adenocarcinoma in situ was observed in part of the tumor. C: A histological image of the stump of the left hepatic duct is shown. Intraductal papillary adenoma in situ was detected.
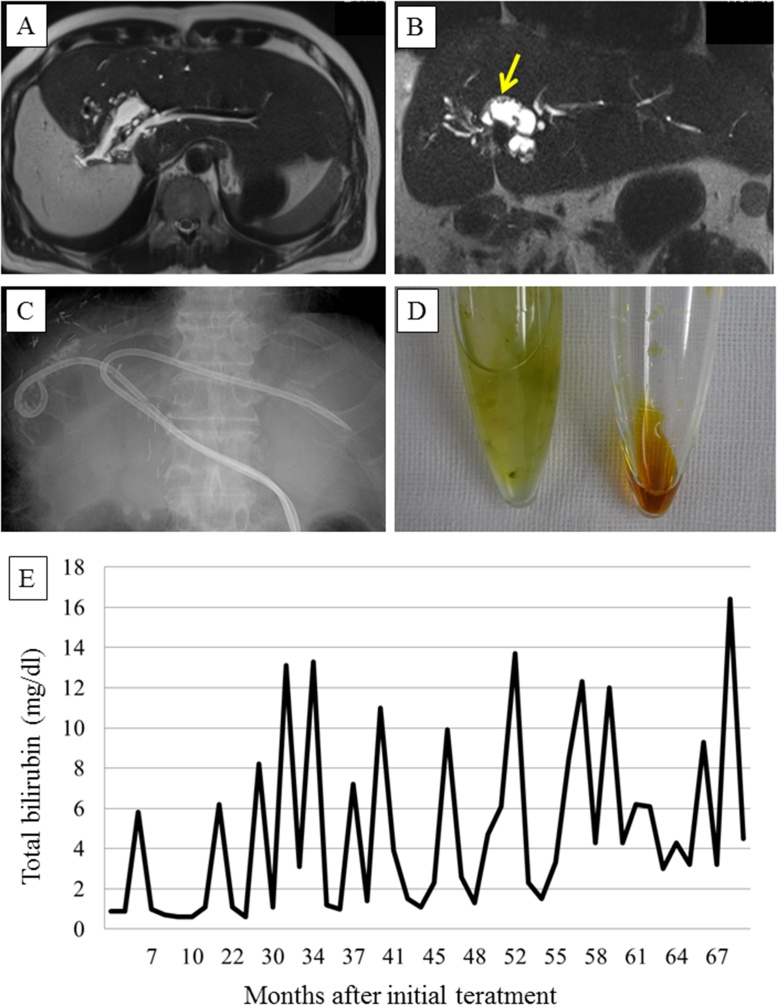
No adjuvant chemotherapy was administered, but 3-monthly abdominal CT or magnetic resonance imaging (MRI) scans and blood examinations were performed. The patient’s T-bil, ALP, and AST levels normalized after the liver resection, while the intrahepatic bile duct of the remnant liver was slightly dilated (to 1 cm) at its junction with the left hepatic duct. At 14 postoperative months, the patient was admitted to our hospital due to cholangitis combined with jaundice and liver dysfunction [C-reactive protein (CRP), 7.7 mg/dL; T-bil, 6.2 mg/dL; ALP, 1486 U/L; AST, 140 U/L]. CT revealed dilation of the B2 and B3 ducts (to 0.5 cm). However, no tumors or stones were present in the hepaticojejunostomy or the intrahepatic bile duct. Antibiotic treatment improved the patient’s jaundice and liver dysfunction. At 23 postoperative months, cholangitis combined with jaundice and liver dysfunction recurred, but they were successfully treated with antibiotics. At 39 postoperative months, cholangitis combined with severe jaundice and liver dysfunction was detected [CRP, 12.6 mg/dL; T-bil, 13.1 mg/dL; ALP, 3236 U/L; AST, 327 U/L]. MRI revealed dilation of the B3 and B4 ducts (to 1 cm), and small papillary tumors were detected in the intrahepatic bile duct (Fig. 4A/B). We considered that the IPNB had recurred and that the cholangitis and liver dysfunction had been caused by mucus. Antibiotic treatment was administered, and the cholangitis disappeared. However, 1 month later the cholangitis recurred again. We decided to perform percutaneous transhepatic bile duct drainage (PTBD) to drain the mucus in the intrahepatic bile duct. A PTBD tube was inserted into the region from the B3 duct to the reconstructed jejunal limb through the hepaticojejunostomy. The discharge from the PTBD tube contained crystallized mucus, but not bile, and could not be drained through a thin tube. The size of the PTBD tube was increased from 7.2 Fr to 12 Fr, and irrigation of the bile duct via the PTBD tube was performed every day concurrently with antibiotic treatment. A cytological examination of the mucus did not reveal any signs of malignancy. The discharge from the PTBD tube gradually changed from crystallized mucus to pure bile, and the patient’s jaundice and liver dysfunction were ameliorated. However, cholangitis recurred frequently (at 44, 45, and 47 postoperative months). At 48 postoperative months, a PTBD tube was inserted from the B3 to B2 duct (Fig. 4C). Thereafter, the frequency of cholangitis decreased (it was seen at 51, 57, 63, 65, and 74 postoperative months). The discharge collected from the PTBD tube inserted into the region extending from the B3 duct to the reconstructed jejunum limb contained crystallized mucin, but not bile, while the PTBD discharge collected from the region extending from the B3 duct to the B2 duct was composed of pure bile (amount: 200 ml daily) (Fig. 4D). MRI revealed dilation of the B3 and B4 ducts together with small papillary tumors and dilation of the bile duct in the pancreas combined with papillary tumors. The peripheral side of the B2 duct was not dilated, and no papillary tumors were observed in this region. Thus, the PTBD discharge collected from the region extending from the B3 duct to the B2 duct was important for biliary drainage. The patient has survived for over 7 years since the initial treatment in spite of the frequent occurrence of cholangitis and jaundice, which was treated via careful management of his biliary tract (Fig. 4E).
Fig. 4.
A/B Magnetic resonance imaging revealed dilation of the B3 and B4 ducts (to 1 cm) and small papillary tumors in the intrahepatic bile duct (arrow). The dilation of the B2 duct was mild. C: Percutaneous transhepatic bile duct (PTBD) tubes were inserted from the B3 duct to the reconstructed jejunal limb through the hepaticojejunostomy and from the B3 duct to the B2 duct. D: The discharge from the PTBD tube inserted from the B3 duct to the reconstructed jejunal limb contained crystallized mucin, but not bile (left side), while the discharge from the PTBD tube inserted from the B3 to the B2 duct contained pure bile (right side). E: The observed changes in the serum total bilirubin (T-bil) level are shown. Cholangitis and jaundice occurred frequently.
3. Discussion
The long-term outcomes of surgically resected IPNB have recently been reported [3], [5], [6], [7], [10], and its 5-year overall survival rate ranged from 47.0–82.0%. R1 resection, invasive carcinoma, extraductal invasion, lymphovascular invasion, lymph node metastasis, positivity for MUC1, and positivity for carcinoembryonic antigen (CEA) have been reported to be poor prognostic factors after the surgical resection of IPNB. However, few studies have examined the pattern of recurrence or optimal treatment for recurrence in such cases [11], [12]. IPNB that include invasive carcinoma carry a similar risk of recurrence to bile duct cancer. R1 resection was found to be correlated with a poor prognosis after the surgical resection of IPNB that included a malignant component. However, the prognosis of benign IPNB after surgical resection differed from study to study. Some studies have reported that the 5-year survival rate of benign IPNB was 100% [3], [7], while another study reported that it was 85% [11]. As shown in the present case, mucus production can occur after surgical resection, even if no malignant IPNB tissue remains. The biliary management strategy might affect the prognosis of benign IPNB. Even in malignant cases of IPNB, the careful management of the biliary tract after surgical resection is important.
It is difficult to accurately determine the extent of IPNB based on imaging because mucus in the biliary tract can cause bile duct irregularities to appear on MRCP, ERC, or CT. In the present case, the right and left hepatic ducts both exhibited irregularities at the initial diagnosis. However, the irregularity of the left hepatic duct had disappeared 6 months later. Decisions regarding the optimal surgical procedure for treating tumors in the hilar bile duct should be taken with caution. POCS and biopsies of the biliary epithelium might be useful for assessing the extent of IPNB.
In the current case, 7 years have passed since the initial treatment. After right hepatectomy, caudate lobectomy, and extrahepatic bile duct resection, MRI revealed dilation of the B3, B4, and intrapancreatic bile ducts together with small papillary tumors. Namely, the patient’s IPNB ranged from the intrapancreatic bile duct to the bilateral intrahepatic bile ducts. The peripheral side of the B2 duct was not dilated, and no papillary tumors were observed in this region. The discharge from the PTBD tube inserted from the B3 duct to the reconstructed jejunum limb only contained crystallized mucus. The PTBD tube inserted into the B2 duct was the only drainage route for bile of this patient.
In conclusion, we experienced a case of widespread IPNB extending from the intrapancreatic bile duct to the bilateral intrahepatic bile ducts, in which the patient survived for over 7 years after the initial treatment due to careful management of the biliary tract. Mucus might be produced even after the R0 resection of IPNB, and frequent cholangitis or jaundice might be occurred. The biliary tract of the remnant liver after curative resection should be managed carefully for a long time after surgical resection.
The SCARE statement
This case report has been reported in line with the SCARE criteria.
Conflicts of interest
DH, TN, SY, TY, KI, TY, TA, KN, MN, KN, SO, HK, and MS have no conflicts of interest.
Funding
Not applicable.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contribution
DH wrote this manuscript. TN, SY, TY, and TY performed the operation. KI, TA, KN, MN, KN, and SO managed the patient. HK and MS advised the best treatment of choice. All authors read and approved the final manuscript.
Guarantor
Not applicable.
References
- 1.Zen Y., Fujii T., Itatsu K., Nakamura K., Minato H., Kasashima S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology (Baltimore, Md) 2006;44(November (5)):1333–1343. doi: 10.1002/hep.21387. (PubMed PMID: 17058219. Epub 2006/10/24.eng.) [DOI] [PubMed] [Google Scholar]
- 2.Paik K.Y., Heo J.S., Choi S.H., Choi D.W. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J. Surg. Oncol. 2008;97(May (6)):508–512. doi: 10.1002/jso.20994. (PubMed PMID: 18314868. Epub 2008/03/05.eng) [DOI] [PubMed] [Google Scholar]
- 3.Kubota K., Nakanuma Y., Kondo F., Hachiya H., Miyazaki M., Nagino M. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J. Hepato-Biliary-Pancreatic Sci. 2014;21(March (3)):176–185. doi: 10.1002/jhbp.23. (PubMed PMID: 23908126. Epub 2013/08/03.eng) [DOI] [PubMed] [Google Scholar]
- 4.Gordon-Weeks A.N., Jones K., Harriss E., Smith A., Silva M. Systematic review and meta-analysis of Current Experience in Treating IPNB: clinical and pathological correlates. Ann. Surg. 2016;263(April (4)):656–663. doi: 10.1097/SLA.0000000000001426. (PubMed PMID: 26501712. Epub 2015/10/27.eng) [DOI] [PubMed] [Google Scholar]
- 5.Kim W.J., Hwang S., Lee Y.J., Kim K.H., Park K.M., Ahn C.S. Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct. J. Gastrointest. Surg. 2016;20(July(7)):1368–1375. doi: 10.1007/s11605-016-3103-5. (PubMed PMID: 26873016. Epub 2016/02/14.eng) [DOI] [PubMed] [Google Scholar]
- 6.Jung G., Park K.M., Lee S.S., Yu E., Hong S.M., Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J. Hepatol. 2012;57(October (4)):787–793. doi: 10.1016/j.jhep.2012.05.008. (PubMed PMID: 22634127. Epub 2012/05/29.eng) [DOI] [PubMed] [Google Scholar]
- 7.Rocha F.G., Lee H., Katabi N., DeMatteo R.P., Fong Y., D’Angelica M.I. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology (Baltimore, Md) 2012;56(October (4)):1352–1360. doi: 10.1002/hep.25786. (PubMed PMID: 22504729. Epub 2012/04/17.eng) [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P. A protocol for the development of reporting criteria for surgical case reports: the SCARE statement. Int. J. Surg. (London, England) 2016;27(March):187–189. doi: 10.1016/j.ijsu.2016.01.094. (PubMed PMID: 26828281. Epub 2016/02/02.eng) [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. (Lond. Engl.) 2016;34(October):180–186. doi: 10.1016/j.ijsu.2016.08.014. (PubMed PMID: 27613565. Epub 2016/10/21.eng) [DOI] [PubMed] [Google Scholar]
- 10.Luvira V., Pugkhem A., Bhudhisawasdi V., Pairojkul C., Sathitkarnmanee E., Luvira V. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J. Gastroenterol. Hepatol. 2016;29(June) doi: 10.1111/jgh.13481. (PubMed PMID: 27356284. Epub 2016/06/30.eng) [DOI] [PubMed] [Google Scholar]
- 11.Kim K.M., Lee J.K., Shin J.U., Lee K.H., Lee K.T., Sung J.Y. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am. J. Gastroenterol. 2012;107(January (1)):118–125. doi: 10.1038/ajg.2011.316. (PubMed PMID: 21946282. Epub 2011/09/29.eng) [DOI] [PubMed] [Google Scholar]
- 12.Narita M., Endo B., Mizumoto Y., Matsusue R., Hata H., Yamaguchi T. Multicentric recurrence of intraductal papillary neoplasms of bile duct in the remnant intrahepatic bile duct after curative resection. Int. J. Surg. Case Rep. 2015;12:123–127. doi: 10.1016/j.ijscr.2015.05.033. (PubMed PMID: 26070186. Pubmed Central PMCID: PMC4486396. Epub 2015/06/13.eng) [DOI] [PMC free article] [PubMed] [Google Scholar]