Abstract
We typically explain other people’s behaviors by attributing them to the beliefs and motives of an unobservable mind. Although such attributional inferences are critical for understanding the social world, it is unclear whether they rely on processes distinct from those used to understand the nonsocial world. We used functional magnetic resonance imaging to identify brain regions associated with making attributions about social and nonsocial situations. Attributions in both domains activated a common set of brain regions, and individual differences in the domain-specific recruitment of one of these regions in the dorsomedial prefrontal cortex correlated with attributional accuracy in each respective domain. Overall, however, this brain region showed greater activated for attributions about social situations, and this selective response to the social domain was greatest in those participants reporting the highest levels of social expertise. We conclude that folk explanations of behavior are an expert use of a domain-general cognitive ability.
1. Introduction
Psychologists and laypeople both desire explanations for human behavior. Whereas the psychologist’s efforts to explain behavior demand years of formal training, the layperson’s efforts seem to demand no formal training at all. In fact, most three-year-old children already have the ability to produce culturally acceptable explanations of other people’s behavior (Bartsch & Wellman, 1989). This fundamental aspect of human social cognition has been the object of theories and research in disciplines ranging from social psychology (Heider, 1958; Fiske & Taylor, 1984), to developmental psychology (Gopnik & Schulz, 2004), and the philosophy of mind (Davidson, 1963; Dennett, 1989). These disciplines largely converge on the view that folk explanations of human behavior are the product of a cognitive ability that is distinct from the one used to explain non-human events of a natural origin. Here, we used functional magnetic resonance imaging (fMRI) in conjunction with a novel behavioral task to examine the idea that folk explanations of behavior require specialized cognition.
Lay explanations of human behavior usually rest on attributing a person’s observable behavior to an inferred mental state, such as their motive or belief about the behavior (Davidson, 1963; Dennett, 1989; Malle, 2004). These causal attributions draw on what has been variously termed a folk psychology or theory-of-mind, that is, a culturally-shared conceptual framework that specifies the meaning of mental terms, both as they causally relate to each other (e.g., anger causes aggression) and to observable behaviors (e.g., embarrassment causes blushing) (Bartsch & Wellman, 1989; Malle, 2004; Apperly, 2012). Thus, folk psychology provides a powerful conceptual framework for making attributions about the social world (Heider, 1958). For attributions about changes observed in the physical world, however, folk psychological concepts are much less powerful. Such changes are best understood using a different set of concepts that describe (either lay or scientific) laws of nature (Dennett, 1989).
Thus, attributions about social and nonsocial situations clearly demand the use of different domains of causal knowledge. However, it remains unclear whether they also require distinct cognitive processes operating on that knowledge. In prior work, we have shown that attributions about human behavior selectively activate an anatomically well-defined left hemisphere brain network that prominently includes regions of the medial and orbitofrontal prefrontal cortex, precuneus, temporoparietal junction, and anterior superior temporal sulcus (Spunt, Falk, & Lieberman, 2010; Spunt, Satpute, & Lieberman, 2011; Spunt & Lieberman, 2012a; Spunt & Lieberman, 2012b; Spunt & Adolphs, 2014). By exclusively focusing on attributions about human behavior, these studies could not examine an important unanswered question: Does this network implement an attributional process that is domain-general, or one that is specifically tied to the social domain?
Extant work in social neuroscience suggests that the functions of this network might be specific to the social domain. It has long been known that psychiatric illness and brain lesions can cause dramatic changes in social cognition while sparing ostensibly domain-general executive functions (Leslie & Thaiss, 1992; Samson, Apperly, Chiavarino, & Humphreys, 2004). Similarly, numerous neuroimaging studies suggest that brain regions supporting social judgments dissociate from those that support similar nonsocial judgments (Mitchell, 2009; Van Overwalle, 2011; Kennedy & Adolphs, 2012). However, since these studies have typically relied on directly comparing responses to social versus nonsocial stimuli (e.g., Mitchell, Heatherton, & Macrae, 2002; Martin & Weisberg, 2003; Mitchell, Macrae, & Banaji, 2005; Mason et al., 2010), their findings could be, in part, driven by domain-specific expertise, attention, and motivation – factors that might lead the social domain to place relatively stronger demands on cognitive processes that are intrinsically domain-general (Tarr & Gauthier, 2000; Grelotti, Gauthier, & Schultz, 2002). To address this, we used fMRI in conjunction with a novel behavioral task that allowed attributional processing to be isolated within each domain separately.
2. Materials and Methods
2.1. Participants
Twenty-one adults from the Los Angeles metropolitan area participated in the study. Two individuals were excluded from the study after their participation, one due to the incidental discovery of a brain abnormality, and the other due to a recruitment screening error. This left a total of 19 participants for the analysis (13 males, 6 females; mean age = 28.32, age range = 21–46). Sample size determination was based on the first author’s previous fMRI studies of social attribution in healthy adults, which used experimental manipulations that are conceptually similar to the one used in the present study and consistently found robust effects (Mean N = 19, SD = 5.67, RANGE = 10–29) (Spunt et al., 2010; Spunt et al., 2011; Spunt & Lieberman, 2012a; Spunt & Lieberman, 2012b; Spunt & Lieberman, 2013; Spunt & Adolphs, 2014). Participants in the present study were screened to ensure that they were right-handed, neurologically and psychiatrically healthy, had normal or corrected-to-normal vision, spoke English fluently, had IQ in the normal range (as assessed using the Wechsler Abbreviated Scales of Intelligence), and were not pregnant or taking any psychotropic medications at the time of the study. All participants provided written informed consent according to a protocol approved by the Institutional Review Board of the California Institute of Technology, and received financial compensation in exchange for participating.
2.2. Social/Nonsocial Why/How Task
The task used here is a modified version of the Yes/No Why/How Task which we recently validated for investigating social attributional processing both behaviorally and neurally (Spunt & Adolphs, 2014). The task has participants answer attributional (Why) and factual (How) questions about the emotional facial expressions and intentional hand actions depicted in photographs (Figure 1). This basic protocol permits an isolation of the attributional process of interest by subtracting brain activity associated with factual (“How”) questions from brain activity associated with attributional (“Why”) questions. Since the photographs being evaluated are the same for both question types, only the level of attributional processing differs. In this way, the Why/How manipulation has been used to isolate the brain regions specific to attributional processing in the Social domain.
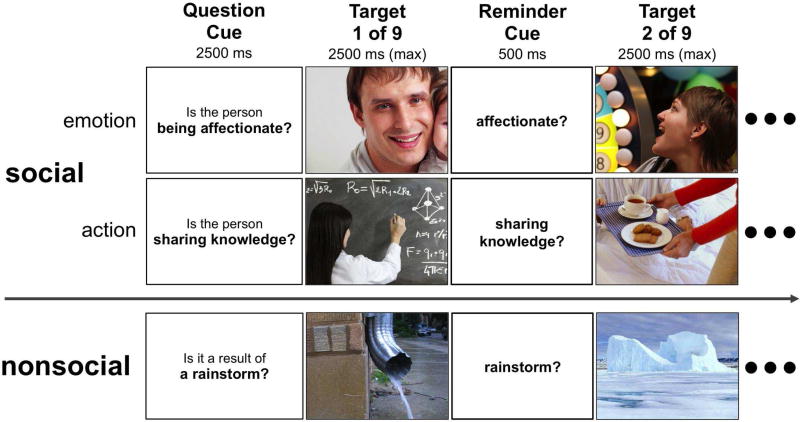
Figure 1.
Design of the Social/Nonsocial Why/How Task. Each block is formed by pairing a yes/no question that is either attributional (Why) or factual (How) with 9 target photographs. In an independent sample of respondents, 5 photographs in every block elicited a consensus response of ‘yes’, while the remaining 4 elicited a consensus response of ‘no’. Participants have 2500 ms to respond to each photograph. Once the participant responds, the task advances. Given this, block durations were contingent on response times. However, total task duration was not, as block onsets were fixed. Finally, between each photograph is a brief reminder of the question for that block. The diagram below displays the first two trials of an attribution block for each of the three stimulus categories. The same photographs were used to create the factual question blocks. See Table 1 for a list of all questions.
For the present study, we followed three criteria in constructing an orthogonal Why/How manipulation in the Nonsocial domain:
Stimulus Criterion. The stimulus being evaluated does not show humans, humanlike entities, or outcomes that are typically the result of intentional human behavior.
Task Content Criterion. The attributions participants are evaluating do not contain the mental and social concepts typically featured in attributions about human behavior.
Task Structure Criterion. The task period during which brain activity is measured is structured so as to prevent the occurrence of task-irrelevant social inferences. This criterion is important given the well-documented human tendency to spontaneously anthropomorphize non-social objects and events (Epley, Waytz, & Cacioppo, 2007). In fact, the possibility of task-irrelevant social cognition has been used to explain previous findings showing that some nonsocial reasoning tasks also activate regions involved in social reasoning (Van Overwalle, 2011).
The resulting task featured a 2 (Question: Why vs. How) x 3 (Stimulus: Non-Social Scenes vs. Emotional Expressions vs. Intentional Actions) factorial design (Figure 1). Henceforth, we use the label “Social” to collectively refer to the two categories of human behavior (emotional expressions, and intentional actions). Each of the three stimulus categories featured 54 naturalistic photographs acquired from a variety of online stock photography sources (see Figure 1 for examples, and Figure S1–Figure S3 for the full stimulus sets). The Nonsocial stimuli depicted events commonly attributed to changes in nature, for instance, extreme weather and seasonal changes. We used Amazon. com’s web service Mechanical Turk to collect normative ratings of photograph valence from approximately 30 native English speaking U.S. citizens. This was achieved by asking participants “How POSITIVE (pleasant) vs. NEGATIVE (unpleasant) is each photo?”, and then presenting each photo with a 7-Likert scale (1 = Extremely Positive, 4 = Neutral, 7 = Extremely Negative). An independent samples t-test showed that photograph valence did not differ across the Social (M = 3.647, SD = 0.577) and Nonsocial (M = 3.731, SD = 0.742) stimulus domains, t(160) = 0.307, p = 0.759.
Table 1 displays the 36 questions featured in the study, broken down by Question and Stimulus Category.
Table 1.
The questions used to achieve the why/how manipulation in the three stimulus categories. For Nonsocial blocks, why-questions began with either the string “Is it going to result in” or “It is the result of”, while all how-questions begin with the string “Is the photo showing”. For both categores of Social block, both why-questions and how-questions began with the string “Is the person”.
| Stimulus | Question | |
|---|---|---|
|
| ||
| Attributional (Why) | Factual (How) | |
|
|
|
|
| Nonsocial | Spring season? | clouds? |
| Scenes | a drought? | colorful flowers? |
| a forest fire? | dry ground? | |
| a hurricane? | moving water? | |
| a rainstorm? | palm trees? | |
| result in rain? | smoke? | |
| Emotional | being affectionate? | gazing up? |
| Expressions | celebrating something? | looking at the camera? |
| expressing gratitude? | looking to the side? | |
| expressing self-doubt? | opening their mouth? | |
| in an argument? | showing their teeth? | |
| proud of themselves? | smiling? | |
| Intentional | competing against others? | carrying something? |
| Actions | doing their job? | lifting something up? |
| expressing themselves? | putting something on? | |
| helping someone? | reaching for something? | |
| protecting themselves? | using a writing utensil? | |
| sharing knowledge? | using both hands? | |
For the Social situations, attributional questions regarded the mental state of the focal person in the photograph (e.g., Is the person expressing gratitude?), while factual questions regarded an observable motor behavior (e.g., Is the person reaching for something?). For Nonsocial situations, attributional questions regarded a natural causal process or occurence (e.g., Is it the result of Spring season?), while factual questions regarded an observable object or event (e.g., Is the photo showing colorful flowers?). Each question is paired with 5 photographs designed to elicit the response ‘yes’, and 4 photographs designed to elicit the response ‘no’. These pairings were selected based on the responses of an independent sample of Mechanical Turk respondents. Each pairing was evaluated by approximately 30 native English speaking U.S. citizens. We retained only those pairings that elicited a consensus response. An independent-samples t-test showed that consensus for Nonsocial pairs (M = 92.704%, SD = 6.532) did not differ from consensus for Social pairs (M = 92.593%, SD = 6.650), t(160) = 0.101, p = 0.920. In our subsequent analysis of participant performance, consensus data was used to code responses as correct or incorrect.
During MRI scanning, items were presented to participants in blocks of 9 corresponding to each of the 36 questions (Figure 1). The order and onsets of question-blocks was optimized to maximize the efficiency of separately estimating the Why > How contrast for each of the three stimulus categories. This was achieved by generating the design matrices for one million pseudorandomly generated designs, and for each summing the efficiencies of Why > How contrast estimation for the three categories. The most efficient design was retained and used for all participants.
Prior to entering the scanner, participants were told they would be performing a “Photograph Judgment Test” in which they would answer yes/no questions about various kinds of photographs. They were then shown two example trials and were invited to ask the experimenter questions if they did not fully understand the task. Finally, they were told that they would have a limited amount of time to respond to each photograph. Immediately prior to performing the task in the scanner, participants performed a brief practice version of the test featuring stimuli not used in the experimental task.
2.3. Stimulus Presentation and Response Recording
Stimulus presentation and response recording used the Psychophysics Toolbox (version 3.0.9; Brainard, 1997) operating in MATLAB (version 2012a; MathWorks Inc., Natick, MA, USA). An LCD projector was used to show stimuli on a screen at the rear of the scanner bore that was visible to participants through a mirror positioned on the head coil. Participants were given a button box and made their responses using their right hand index and middle fingers.
2.4. Personality Measurement
For the purposes of exploratory individual difference analyses, participants also completed several self-report questionnaires measuring aspects of an individual’s motivation and ability to understand other people’s behavior. We explored the moderating effect of these measures to add additional constraint on interpreting the nature of effects observed in our primary analyses.
The short form of the Empathy Quotient (EQ; Wakabayashi et al., 2006; Baron-cohen & Wheelwright, 2004; α = .871) measures the drive to understand and respond appropriately to the internal states of others (e.g., “I am good at predicting how someone will feel”). The Social Curiosity Scale (SCS; Renner, 2006; α = .885) measures a general interest in acquiring novel information about others (e.g., “Other people’s life stories interest me”). Items 10 and 11 on the SCS were deemed inappropriate and were omitted from the study1. Finally, participants completed the Attributional Complexity Questionnaire (ACQ; Fletcher, Danilovics, Fernandez, Peterson, & Reeder, 1986; α = .929), which measures the tendency to produce relatively complex and sophisticated explanations of human behavior (e.g., “I really enjoy analyzing the reasons or causes for people’s behavior”). Participants also completed the Gossip Functions Questionnaire (Foster, 2004). However, because responses to this questionnaire demonstrated poor reliability (average scale α = .359), they were not retained for further analysis.
2.5. Image Acquisition
All imaging data was acquired at the Caltech Brain Imaging Center acquired using a Siemens Trio 3.0 Tesla MRI scanner outfitted with a 32 channel phased-array headcoil. For the Social/Nonsocial Why/How Task, we acquired 394 T2*-weighted echoplanar image volumes (EPIs; slice thickness=3 mm, 46 slices, TR=2500 ms, TE=30 ms, flip angle=85°, matrix=64 x 64, FOV=192 mm). Participants’ in-scan head motion was minimal (max translation = 2.08 mm, max rotation = 1.86°). Following this scan, we acquired an additional 386 EPI volumes for each participant while they performed two additional tasks as part of a separate study. Finally, we acquired a high-resolution anatomical T1-weighted image (1 mm isotropic) and field maps for use in image preprocessing (described below).
2.6. Image Preprocessing
Images were analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) operating in MATLAB. Prior to statistical analysis, each participants’ images were subjected to the following preprocessing steps: (1) the first two EPI volumes were discarded to account for T1-equilibration effects; (2) the remaining EPI volumes were corrected for slice-timing differences; (3) within each run, EPI volumes were realigned to the first EPI volume of the run; (4) the participants’ T1 structural volume was co-registered to the mean EPI volume; (5) the group-wise DARTEL registration method included in SPM8 (Ashburner, 2007) was used to normalize the T1 structural volume to a common group-specific space, with subsequent affine registration to Montreal Neurological Institute (MNI) space; and finally, (6) all EPI volumes were normalized to MNI space using the deformation flow fields generated in the previous step, which simultaneously re-sampled volumes (3 mm isotropic) and applied spatial smoothing (Gaussian kernel of 8 mm isotropic, full width at half maximum).
2.7. Within-Subject Contrast Estimation
We used a General Linear Model to estimate the effects of performing the Social/Nonsocial Why/How Task on the EPI timeseries for each participant. Each model included six covariates of interest corresponding to the six cells created by crossing factors corresponding to the Question (Why vs. How) and Stimulus (Nonsocial vs. Emotion vs. Action). These regressors were defined using a variable epoch model (Grinband, Wager, Lindquist, Ferrera, & Hirsch, 2008), with the epochs for each block spanning the onset of the first trial and the offset of the last trial. Each model also included several covariates of no interest. Two parametric covariates of no interest were included that modeled variability in the amplitude of the BOLD response that could be explained by differences in response accuracy and RTs across task blocks (regardless of condition). These covariates were deemed necessary to account for differences in performance that were observed across conditions (Table 2).
Table 2.
Results of group-level whole-brain conjunction analyses (N = 19; minimum statistic image cluster-corrected at a famly-wise error rate of .05). PFC = Prefrontal Cortex; STS = Superior Temporal Sulcus; OFC = Orbitofrontal Cortex; x, y, and z = Montreal Neurological Institute (MNI) coordinates in the left-right, anterior-posterior, and inferior-superior dimensions, respectively.
| Effect Name | MNI Coordinates
|
|||||
|---|---|---|---|---|---|---|
| ; Region Name | L/R | Extent | t-value | x | y | z |
| Response to Why > How for All Stimulus Categories | ||||||
| Anterior STS | L | 124 | 5.837 | −57 | 0 | −21 |
| Dorsomedial PFC | L | 69 | 5.376 | −9 | 45 | 51 |
| Lateral OFC | L | 92 | 5.148 | −48 | 33 | −12 |
| L | - | 4.153 | −45 | 24 | 15 | |
| Temporoparietal Junction | L | 59 | 5.043 | −51 | −69 | 33 |
| L | - | 4.539 | −42 | −51 | 21 | |
| Stronger Response to Why > How for Social Stimuli | ||||||
| Dorsomedial PFC | L | 303 | 6.245 | −9 | 57 | 18 |
| L/R | - | 5.942 | 0 | 51 | 36 | |
| L | - | 5.346 | −9 | 29 | 58 | |
| R | - | 4.649 | 12 | 57 | 12 | |
The remaining covariates of no interest included the 6 motion parameters estimated from image realignment as well as a predictor for every timepoint where in-brain global signal change (GSC) exceeded 2.5 SDs of the mean GSC or where estimated motion exceed 0.5 mm of translation or 0.5 degrees of rotation. The hemodynamic response was modeled using the canonical (double-gamma) response function, and the predicted and actual signals were high-pass filtered at 1/128 Hz. Finally, all models were estimated using the SPM8 RobustWLS toolbox, which implements the robust weighted least-squares estimation algorithm (Diedrichsen & Shadmehr, 2005).
2.8. Group-Level Analyses
Our primary analyses were conducted at the group-level. Given extensive prior work identifying those brain regions most reliably associated with social attribution (Spunt & Adolphs, 2014; Spunt & Lieberman, 2012b; Spunt & Lieberman, 2012a; Spunt et al., 2011; Spunt et al., 2010), we first tested our group-level hypotheses on a set of independently-defined regions of interest (ROI). We focused on six left hemisphere ROIs that were defined based on the Why > How contrasts reported in Study 1 (N = 29) and Study 3 (N = 21) of Spunt and Adolphs (2014). These contrast images are publicly available for download on the first author’s website (http://bobspunt.com/whyhow-localizer). ROI sections are depicted in Figure 2a, and further details can be found in Table S3. For each ROI, we extracted percent signal change (PSC) in the six conditions of the experimental design. To test for domain-generality, we used paired-samples t-tests to identify those regions that independently demonstrated an association with the Why > How contrast for all three stimulus categories. To test for domain-specificity, we used one-sample t-tests to identify those regions that showed a positive effect in two interaction contrasts: ([Emotion-Why > Emotion-How] > [Nonsocial-Why > Nonsocial-How]) and ([Action-Why > Action-How] > [Nonsocial-Why > Nonsocial-How]). For each test, we report p-values corrected for multiple comparisons across ROIs using the false-discovery rate (FDR) procedure described in Benjamini and Yekutieli (Benjamini & Yekutieli, 2001). Confidence intervals (CIs) for these effects were estimated using the bias corrected and accelerated percentile method (10,000 random samples with replacement; implemented using the BOOTCI function in MATLAB).
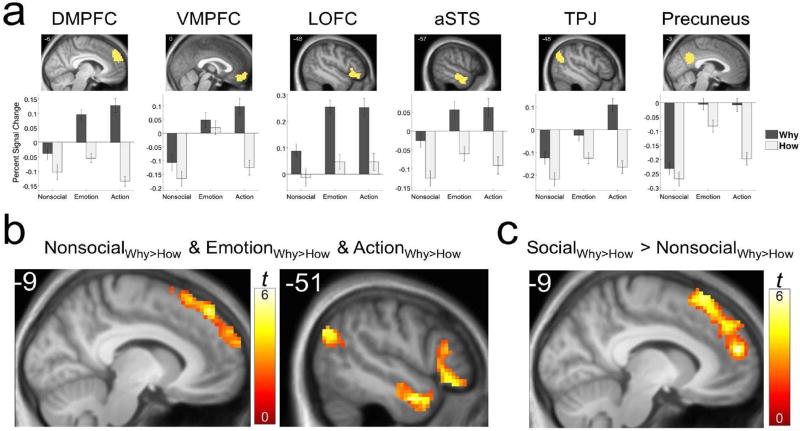
Figure 2.
Region of interest (ROI) and whole-brain analyses. (a) Percent signal change (PSC) from fixation baseline in the independently defined regions of interest (ROIs; see Table S3 for details). Test results for all ROIs are provided in Tables S4-S5. (b) Whole-brain analysis showing regions independently associated with evaluating attributions versus facts for all three stimulus categories. (c) Whole-brain analysis showing the region of the dorsomedial prefrontal cortex (DMPFC) that displayed an elevated response to attributions about both emotions and actions when compared to attributions about nonsocial situations. For both whole-brain analyses, regions were identified from a group-level (N = 19) search using a cluster-forming threshold of p < .001 and a cluster-level family-wise error rate of 0.05. Activity is overlaid on the group mean anatomical at a threshold of p < .005 to show extent. VMPFC = Ventromedial Prefrontal Cortex; LOFC = Lateral Orbitofrontal Cortex; aSTS = Anterior Superior Temporal Sulcus; TPJ = Temporoparietal Junction.
Region-of-interest (ROI) analyses were followed by whole-brain analyses. For this, contrast images were computed for the Why > How contrast in each of the three Stimulus Categories (Nonsocial, Emotion, Action). To determine the extent to which the Why > How contrast isolates regional activation that is domain-general, we subjected the contrast images for each Stimulus Cateogry to one-sample t-tests and used the resulting t-statistic images to compute the minimum t-statistic image required for valid conjunction inference (Nichols, Brett, Andersson, Wager, & Poline, 2005). Next, two interaction contrast images were computed, one indexing the relative strength of the Why > How effect for Emotions compared to Nonsocial Scenes ([Emotion-Why > Emotion-How] > [Nonsocial-Why > Nonsocial-How]), and one indexing the relative strength of the Why > How effect for Actions compared to Nonsocial Scenes ([Action-Why > Action-How] > [Nonsocial-Why > Nonsocial-How]). To determine the extent to which the Why > How contrast isolates a response that is selective for the Social domain, we subjected the contrast images for each interaction to one-sample t-tests and used the resulting t-statistic images to compute the minimum t-statistic image. This conjunction isolates regions that show a stronger response to the Why > How contrast for both Emotions and Actions relative to Nonsocial Scenes. Whole-brain analyses were conducted by applying a cluster-forming (voxel-level) threshold of p < .001 followed by cluster-level correction for multiple comparisons at a family-wise error (FWE) of .05. For visual presentation, thresholded t-statistic maps were overlaid on the average of the participants’ T1-weighted anatomical images.
2.9. Individual Difference Analyses
For each participant, MATLAB was used to score and assess the reliability of responses to the personality measures, and to compute measures of mean percent accuracy and response time (RT) for the Social/Nonsocial Why/How Task. Prior to computing accuracy, we omitted trials with no response, which were rare (across participants, M = 0.97%, SD = 1.94%, MAX = 8.33%). To address negative skewness, accuracy scores were subjected to a Box-Cox transformation (Box & Cox, 1964). Mean RT was computed on the distribution of correct trials only, and after deleting values that were greater than 3 SDs from the remaining distribution mean. Group-level descriptive statistics are reported in the Supplementary Materials (Table S1).
To examine the brain-behavior relationships reported in Table 3, we computed each participant’s mean percent signal change (PSC) within the DMPFC ROI in each domain’s Why > How contrast. Then, we used the MATLAB Statistics Toolbox to perform a series of robust multiple regressions analyses that simultaneously modeled the influence of DMPFC responses to the two domains on five outcomes of interest: attributional accuracy in the social and nonsocial domains2, and the three personality measures described above. Robust regressions were carried out using iteratively reweighted least squares estimated as implemented in the MATLAB Statistics Toolbox. Finally, zero-order Pearson correlations among all examined variables are reported in Table S6.
Table 3.
Robust multiple regression analyses of domain-specific activation in the dorsomedial prefrontal cortex (DMPFC) during attributional processing. Each row displays the results of one of five separate regression models, all of which contained the same two predictors: DMPFC activation to the Why > How contrast in the nonsocial domain, and DMPFC activation to the Why > How contrast in the social domain. The DMPFC region was independently defined using the region of interest depicted in Figure 2 and described in Table S3. Zero-order correlations among all examined variables are provided in Table S6.
| DMPFC Response to Why > How
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonsocial Domain
|
Social Domain
|
|||||||||
| Outcome Name | Beta | t | p | 95% CI | Beta | t | p | 95% CI | ||
| Accuracy – Nonsocial Attribution | 0.55 | 2.65 | 0.017 | 0.11 | 0.99 | 0.24 | 1.17 | 0.260 | −0.20 | 0.69 |
| Accuracy – Social Attribution | 0.14 | 0.75 | 0.466 | −0.26 | 0.54 | 0.67 | 3.56 | 0.003 | 0.27 | 1.07 |
| Empathy Quotient | −0.37 | −1.86 | 0.081 | −0.78 | 0.05 | 0.59 | 3.02 | 0.008 | 0.18 | 1.01 |
| Social Curiosity | −0.14 | −0.73 | 0.474 | −0.56 | 0.27 | 0.69 | 3.52 | 0.003 | 0.28 | 1.11 |
| Attributional Complexity | −0.30 | −1.44 | 0.170 | −0.74 | 0.14 | 0.62 | 3.01 | 0.008 | 0.18 | 1.06 |
3. Results
3.1. Domain-General Effects
As displayed in Figure 2a and listed in Table S4, four of the six ROIs examined demonstrated evidence of a domain-general association with the Why > How contrast. These were the dorsomedial prefrontal cortex (DMPFC), the lateral orbitofrontal cortex (LOFC), the anterior superior temporal sulcus (aSTS), and the temporoparietal junction (TPJ). As displayed in Figure 2b and listed in Table 2, each of these regions was also observed in whole-brain analyses based on the conjunction of the the Why > How contrast for the three stimulus categories. While these findings still leave open the possibility of representational differences within the observed regions in the way that social and nonsocial attributions correspond to brain activation patterns (as could be revealed through pattern-information analysis, e.g., Kriegeskorte & Kievit, 2013), they argue strongly for a broadly similar set of psychological processes engaged in both cases.
3.2. Effects Specific to the Social Domain
The results presented above thus suggest that many of the brain regions associated with the Why > How contrast in the Social domain are also associated with that contrast in the Nonsocial. In our next analysis, we sought to identify those regions demonstrating evidence of a response to attributional processing that is specific to the social domain. To do so, we capitalized on the fact that we operationally defined the social domain in two ways: photographs of emotional facial expressions, and photographs of intentional hand actions. For a region to be characterized as functionally sensitive to the social domain, it should demonstrate this sensitivity in response to both emotions and actions. To identify such regions, we examined the conjunction of two interaction contrasts: [(WhyEmotion > HowEmotion) > (WhyNonsocial > HowNonsocial)], and [(WhyAction > HowAction) > (WhyNonsocial > HowNonsocial)]. Although every ROI except for the anterior STS displayed an elevated response to at least one of the two social stimulus categories (Table S5), only the left DMPFC showed such a response both in ROI and whole-brain analyses (Figure 2c; Table 2). That is, largely the same area of DMPFC that demonstrated a domain-general association with making attributions, nevertheless demonstrated a stronger association with making attributions about the social world.
3.3. Dispositional Moderators of DMPFC Responses
In order to provide further constraint on interpreting the nature of the elevated DMPFC response to social attributions, we capitalized on individual variability in both performance on the social and nonsocial attribution tasks, as well as in self-reported personality traits relevant to social cognition. We used these to rule out a potent alternative interpretation of the domain-general effects observed in this region. That is, the presence of the DMPFC in the nonsocial Why > How contrast may be caused by task-irrelevant social attributional processing that may occur spontaneously during performance of the nonsocial attributional task. This interpretation has been used before to explain observations of DMPFC activation in nonsocial reasoning tasks (Van Overwalle, 2011), and is made especially viable given the strong human tendency to anthropomorphize nonsocial objects and events (Epley, Waytz, & Cacioppo, 2007). Moreover, this could also account for the fact that the DMPFC response to nonsocial attributions was globally weaker when compared to its response to social attributions.
To evaluate this possibility, we tested two hypotheses. First, if DMPFC function is irrelevant to the nonsocial attribution task participants are performing, individual differences in the level of DMPFC activation to nonsocial attributions should be either uncorrelated or negatively correlated with levels of performance on the nonsocial attribution task. Such an effect would be consistent with the well-established association of the DMPFC with mind wandering, stimulus-independent thought, and the default-mode network of the brain more broadly (Mason et al., 2007). If instead the DMPFC is critical to performance of the nonsocial attribution task, its levels of activation in that domain should be positively associated with making more accurate attributions in that domain. Moreover, this association should be evident even when accounting for the DMPFC response to attributions in the social domain. To evaluate these alternatives, we used multiple regression to simultaneously model the influence of domain-specific DMPFC activation and attributional accuracy in both domains (results shown in Table 3, with zero-order correlations provided in Table S6). The results strongly support the proposition that the DMPFC association with attributional processing is domain-general: DMPFC activation in the nonsocial (but not social) domain was uniquely associated with attributional accuracy in the nonsocial domain, while the DMPFC activation in the social (but not nonsocial) domain was uniquely associated with attributional accuracy in the social domain. This evidence argues against the possibility that the domain-general effects observed in the DMPFC are simply a byproduct of incidental processing shared across the domains.
If the DMPFC response to nonsocial attribution were to reflect spontaneous social cognition, a second prediction would be that this task-irrelevant response should be strongest in those individuals most inclined to show spontaneous social cognition. Indeed, Wagner and colleagues (2011) recently showed that individual differences in task-irrelevant DMPFC responses to social scenes positively correlate with scores on the Empathy Quotient (EQ). Similarly, we found evidence that DMPFC responses to social attributions in our task were uniquely positively associated not only with scores on the EQ, but also with self-reported measures of social curiosity and attributional complexity (Table 3). In contrast, the DMPFC response to nonsocial attribution demonstrated no unique association with these measures. Hence, though the DMPFC response to nonsocial attribution was generally weaker than its response to social attribution, these secondary analyses strongly argue that it nonetheless comes into play during both kinds of attributions, and moreover does so as a function of the relative expertise and performance abilities of each participant with respect to that domain of attribution.
4. Discussion
We observed that most of the brain regions activated by social attributions were also activated by nonsocial attributions. Moreover, individual differences in the domain-specific recruitment of one of these regions – the DMPFC – correlated with attributional accuracy in each domain. DMPFC also showed an elevated response to attributions about social situations, and this socially-selective response was strongest in those participants reporting the highest levels of social expertise. Based on these findings, we suggest that attributions about the social world reflect a specialized use of a domain-general cognitive abiity.
The regions associated with attributional processing in present study are anatomically similar to those observed in extant neuroimaging studies of social reasoning (Schurz, Radua, Aichhorn, Richlan, & Perner, 2014; Amodio & Frith, 2006). Given proposals that these regions may implement processes that are dedicated to social cognition (Mitchell, 2009; Van Overwalle, 2011; Kennedy & Adolphs, 2012), it may be surprising that these regions demonstrated an association with attributions about nonsocial situations. We believe this apparent discrepancy with prior research is explained by several distinctive features of the present study’s methods.
First, we used a factorial design to independently manipulate attributional processing (Why vs. How questions) and stimulus domain (Social vs. Nonsocial scenes). This allowed us to conduct independent tests for attribution-specific brain activity within each domain. Previous studies either could not or did not conduct such tests, and instead relied on directly comparing brain activity elicited by judgments of social and nonsocial stimuli (e.g., Mitchell, Heatherton, & Macrae, 2002; Martin & Weisberg, 2003; Mitchell, Macrae, & Banaji, 2005; Mason et al., 2010). As the present study findings suggest, brain activity observed in such direct comparisons could be caused by social and nonsocial stimuli placing different demands on the same cognitive process.
Second, normative data allowed us to assess the accuracy of participants’ attributional judgments. This data, in turn, allowed us to demonstrate that, in addition to showing a domain-general association with attributional processing, DMPFC activity also shows a domain-general association with attributional accuracy. This degree of experimental control over both stimulus and response is largely absent from previous neuroimaging studies comparing social and nonsocial reasoning (Baetens, Ma, Steen, & Van Overwalle, 2014; Mitchell et al., 2005). Given that people spontaneously generate social interpretations of nonsocial stimuli (Epley et al., 2007), it is difficult to interpret brain activity associated with nonsocial reasoning in tasks where participant responses are unconstrained and/or unmeasured.
Finally, we acknowledged that the social world is heterogeneous and therefore operationally defined it with two distinct classes of human behavior: emotional expressions and intentional actions. The importance of this is demonstrated by Table S5, which shows that several regions show a socially-selective response to attributions about either emotions or actions, but not to both. This strengthens the conclusion that the DMPFC is selective for the abstract domain shared by emotions and actions, namely, the social domain.
What domain-general process could implement social attributions? One answer emerges from neuroimaging studies of semantic memory use (Binder & Desai, 2011). In a meta-analysis of 120 fMRI studies, Binder and colleagues (2009) identified a network that includes the set of regions that showed a domain-general association with attribution in the present study. Other reviews and meta-analyses have identified similar anatomical correspondences across mental-state reasoning tasks and task groups with strong semantic memory demands, for instance, narrative comprehension, prospection, and autobiographical memory (Schacter et al., 2012; Mar, 2011; Spreng, Mar, & Kim, 2009). Such data fits with a view of folk attribution as heavily dependent on the efficient retrieval, selection, and integration of semantic memory, that is, world-knowledge that is not reliably tied to observable objects and events (Fletcher et al., 1986). Hence, social and nonsocial attribution appear as isomorphic uses of semantic memory, in that both demand comprehending the contents of a visual scene (e.g., smiling face; blooming flower) using relatively abstract causal schemata (e.g., friendliness; Spring season).
The plausibility of this domain-general view depends on its ability to account for the socially-selective response of the DMPFC to attributional processing. Our individual difference analyses provide valuable constraint on interpreting this effect. First, although nonsocial attributions elicited modest DMPFC response across participants, individual differences in the magnitude of this response nevertheless uniquely correlated with attributional accuracy in the nonsocial domain. Second, individual differences in responses to social attribution was uniquely associated not only with attributional accuracy in the social domain, but also with self-reported levels of empathy, social curiosity, and attributional complexity. In other words, those individuals who demonstrated the greatest ability in a given domain also tended to show the strongest DMPFC responses to that domain.
To accommodate these findings, a semantic memory account need only assume the following: Most people are substantially interested in and knowledgeable about the causal factors at play in their social worlds. Such depth of domain-specific knowledge would be predicted to place strong demands on the executive aspects of semantic memory use that includes the retrieval, selection, and integration of conceptual knowledge. Regions of the DMPFC have been linked with these functions in both nonsocial and social semantic memory tasks (Satpute, Badre, & Ochsner, 2014; Binder & Desai, 2011; Binder et al., 2009; Goel, Gold, Kapur, & Houle, 1997; Jenkins & Mitchell, 2010; Mitchell, 2009). Moreover, neuroimaging studies of social reasoning indicate the DMPFC plays a central executive role in the use of social knowledge (Spunt & Lieberman, 2013; Meyer et al., 2012). Hence, in the same way that the face-selective response of the fusiform face area may be explained by acquired perceptual expertise (Tarr & Gauthier, 2000), the socially-selective response of the DMPFC during attributional processing may be best explained by conceptual expertise acquired as a natural consequence of living in a world that is thoroughly and inescapably social (Fiske & Taylor, 1984; Fletcher et al., 1986; Grelotti et al., 2002; Roy, Shohamy, & Wager, 2012; Barrett & Satpute, 2013). Future studies will be needed to evaluate additional predictions of a conceptual expertise account of attributional processing. For instance, DMPFC selectivity for the social domain should be atypical in psychiatric conditions that severely impede the acquisition of social expertise (Grelotti et al., 2002; Kennedy & Adolphs, 2012). In fact, work in progress in our lab is investigating this in a group of high-functioning adults with autism. A second prediction regards acquired expertise in (nonsocial) knowledge domains, such as for instance medicine. Here, DMPFC selectivity for attributions within that domain should parametrically track individual differences in domain-specific experience (e.g., year in medical school) and performance (e.g., diagnostic accuracy).
The present study suggests that social attributions rely on a process, possibly centralized in the DMPFC, that can be used to make attributions about nonsocial situations. By showing only this, our findings remain consistent with proposals that the basic cognitive mechanisms supporting social reasoning evolved specifically in response to the increasing complexity of primate social structures (Cosmides & Tooby, 1992; Dunbar, 1998; Herrmann, Call, Hernandez-Lloreda, Hare, & Tomasello, 2007), and later were re-purposed for use in other domains. In fact, the complexity and heterogeneity of the social world may be the very reason why this ability can be flexibly re-purposed for use in other domains.
Indeed, Herrmann and colleagues (2007) reached a similar conclusion in a study comparing the social and nonsocial cognitive skills of 2.5-year-old human children to those of chimpanzees. While the children generally outperformed the chimpanzees in the social but not the nonsocial domains, there was one nonsocial skill area where the children exceled: causal understanding. Based on this finding, they speculate that “…what is distinctively human is not social-cultural cognition as a specialized domain, as we have hypothesized. Rather, what may be distinctive is the ability to understand unobserved causal forces in general, including (as a special case) the mental states of others as causes of behavior (p. 22).” While the findings presented here support such a “special case” perspective on social causal attribution, they suggest that of all the possible uses humans have for this ability, its use in the service of understanding the social world is the most important.
Acknowledgments
We acknowledge Mike Tyszka and the Caltech Brain Imaging Center for help with neuroimaging; the Della Martin Foundation and Caltech Conte Center for Social Decision-Making for funding support; and Elliot Berkman, Meghan Meyer, Ajay Satpute, and Damian Stanley for helpful feedback.
Footnotes
Both items can be interpreted as references to a stereotypical stalking behavior: “I like to stand at the window and watch what my neighbors are doing” (10) and “I like to look into other people’s windows” (11). We note that item exclusion did not substantively change the results.
Given that DMPFC activity was theorized to be functionally relevant for answering only Why questions, attributional accuracy was defined by the percentage of correct responses to Why questions. We chose not to examine the Why > How accuracy difference because it assumes that accuracy and DMPFC activity during How questions will have a negative association that is as strong as the predicted positive association between accuracy and DMPFC activity during Why questions.
References
- Amodio D, Frith C. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apperly I. Mindreaders: The Cognitive Basis of “Theory of Mind”. 1. New York: Psychology Press; 2012. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baetens K, Ma N, Steen J, Van Overwalle F. Involvement of the mentalizing network in social and non-social high construal. Soc Cogn Affect Neurosci. 2014;9(6):817–824. doi: 10.1093/scan/nst048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: An investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34(2):163–175. doi: 10.1023/B:JADD.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Barrett L, Satpute A. Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Current Opinion in Neurobiology. 2013;23(3):361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics. 2001:1165–1188. [Google Scholar]
- Binder J, Desai R. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Desai R, Graves W, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society, Series B. 1964;26(2):211–252. [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. doi: 10.1163/156856897×00357. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J. Cognitive adaptations for social exchange. The adapted mind. 1992:163–228. [Google Scholar]
- Davidson D. Actions, reasons, and causes. J of Philosophy. 1963;60(23):685–700. [Google Scholar]
- Dennett DC. The intentional stance. Cambridge: The MIT Press; 1989. [Google Scholar]
- Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. Neuroimage. 2005;27(3):624–634. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;9:178–190. [Google Scholar]
- Epley N, Waytz A, Cacioppo J. On seeing human: A three-factor theory of anthropomorphism. Psychol Rev. 2007;114(4):864–886. doi: 10.1037/0033-295X.114.4.864. [DOI] [PubMed] [Google Scholar]
- Fletcher GJO, Danilovics P, Fernandez G, Peterson D, Reeder GD. Attributional complexity: An individual differences measure. Journal of Pers Soc Psychol. 1986;51(4):875–884. doi: 10.1037/0022-3514.51.4.875. [DOI] [Google Scholar]
- Foster EK. Research on gossip: Taxonomy, methods, and future directions. Rev of General Psychol. 2004;8(2):78–99. doi: 10.1037/1089-2680.8.2.78. [DOI] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. Neuroreport. 1997;8(5):1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Gopnik A, Schulz L. Mechanisms of theory formation in young children. Trends Cogn Sci. 2004;8(8):371–377. doi: 10.1016/j.tics.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Grelotti D, Gauthier I, Schultz R. Social interest and the development of cortical face specialization: What autism teaches us about face processing. Dev Psychobiol. 2002;40(3):213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Grinband J, Wager T, Lindquist M, Ferrera V, Hirsch J. Detection of time-varying signals in event-related fMRI designs. Neuroimage. 2008;43(3):509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F. The psychology of interpersonal relations. New York: Wiley; 1958. [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317(5843):1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Heyes CM, Frith CD. The cultural evolution of mind reading. Science. 2014;344(6190):1243091. doi: 10.1126/science.1243091. [DOI] [PubMed] [Google Scholar]
- Jack A, Dawson A, Begany K, Leckie R, Barry K, Ciccia A, Snyder A. fMRI reveals reciprocal inhibition between social and physical cognitive domains. Neuroimage. 2012;66C:385–401. doi: 10.1016/j.neuroimage.2012.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A, Mitchell J. Mentalizing under uncertainty: Dissociated neural responses to ambiguous and unambiguous mental state inferences. Cereb Cortex. 2010;20(2):404–410. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16(11):559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Kievit R. Representational geometry: Integrating cognition, computation, and the brain. Trends Cogn Sci. 2013;17(8):401–412. doi: 10.1016/j.tics.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AM, Thaiss L. Domain specificity in conceptual development: Neuropsychological evidence from autism. Cognition. 1992;43(3):225–251. doi: 10.1016/0010-0277(92)90013-8. [DOI] [PubMed] [Google Scholar]
- Lillard A. Ethnopsychologies: cultural variations in theories of mind. Psychological bulletin. 1998;123(1):3. doi: 10.1037/0033-2909.123.1.3. [DOI] [PubMed] [Google Scholar]
- Malle BF. How the mind explains behavior: Folk explanations, meaning, and social interaction. Cambridge: MIT Press; 2004. [Google Scholar]
- Mar R. The neural bases of social cognition and story comprehension. Annual Review of Psychology. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol. 2003;20(3–6):575–587. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M, Norton M, Van Horn J, Wegner D, Grafton S, Macrae C. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Magee JC, Kuwabara K, Nind L. Specialization in relational reasoning: The efficiency, accuracy, and neural substrates of social versus nonsocial inferences. Soc Psychol Pers Sci. 2010;1(4):318–326. doi: 10.1177/1948550610366166. [DOI] [Google Scholar]
- Meyer M, Spunt R, Berkman E, Taylor S, Lieberman M. Evidence for social working memory from a parametric functional MRI study. Proc Natl Acad Sci USA. 2012;109(6):1883–1888. doi: 10.1073/pnas.1121077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99(23):15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Neil Macrae C, Banaji M. Forming impressions of people versus inanimate objects: Social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mitchell J. Social psychology as a natural kind. Trends Cogn Sci. 2009;13(6):246–251. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1(04):515. doi: 10.1017/S0140525×00076512. [DOI] [Google Scholar]
- Renner B. Curiosity about people: The development of a social curiosity measure in adults. J Pers Assess. 2006;87(3):305–316. doi: 10.1207/s15327752jpa8703_11. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager T. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly I, Chiavarino C, Humphreys G. Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience. 2004;7(5):499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Badre D, Ochsner KN. Distinct regions of prefrontal cortex are associated with the controlled retrieval and selection of social information. Cereb Cortex. 2014;24(5):1269–1277. doi: 10.1093/cercor/bhs408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D, Addis D, Hassabis D, Martin V, Spreng R, Szpunar K. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42C:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Spreng R, Mar R, Kim A. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R. Validating the why/how contrast for functional MRI studies of theory of mind. Neuroimage. 2014;99:301–311. doi: 10.1016/j.neuroimage.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R, Falk E, Lieberman M. Dissociable neural systems support retrieval of how and why action knowledge. Psychol Sci. 2010;21(11):1593–1598. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- Spunt R, Lieberman M. An integrative model of the neural systems supporting the comprehension of observed emotional behavior. Neuroimage. 2012a;59(3):3050–3059. doi: 10.1016/j.neuroimage.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Spunt R, Lieberman M. Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience. 2012b;32(10):3575–3583. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R, Lieberman M. The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychol Sci. 2013;24(1):80–86. doi: 10.1177/0956797612450884. [DOI] [PubMed] [Google Scholar]
- Spunt R, Satpute A, Lieberman M. Identifying the what, why, and how of an observed action: An fMRI study of mentalizing and mechanizing during action observation. J Cogn Neurosci. 2011;23(1):63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Tarr M, Gauthier I. Ffa: A flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3(8):764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Taylor SE. Social Cognition. 1. Reading, Massachusetts; Random House: 1984. [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54(2):1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wagner D, Kelley W, Heatherton T. Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cereb Cortex. 2011;21(12):2788–2796. doi: 10.1093/cercor/bhr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A, Baron-Cohen S, Wheelwright S, Goldenfeld N, Delaney J, Fine D, Weil L. Development of short forms of the empathy quotient (eq-short) and the systemizing quotient (sq-short) Pers Indiv Differ. 2006;41(5):929–940. doi: 10.1016/j.paid.2006.03.017. [DOI] [Google Scholar]
- Bartsch K, Wellman H. Young children’s attribution of action to beliefs and desires. Child development. 1989:946–964. [PubMed] [Google Scholar]