Abstract
Background
Previous studies suggest that a higher ratio of primary to secondary metabolites of di-2-ethylhexyl phthalate (DEHP), reflective of a slower DEHP conversion rate, is associated with a greater physiologic effect. We examined associations of several individual characteristics and lifestyle factors with the ratio of mono-2-ethylhexyl phthalate to mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHP:MEHHP) and %MEHP (the ratio of MEHP to the sum of the secondary metabolites).
Methods
We used the data from the National Health and Nutrition Examination Survey, 2001–2012. The study included adults with BMI<30 and no diabetes. Pregnant women were excluded. We examined associations of age, race, gender, Body Mass Index, smoking, alcohol and caffeine consumption, medication use, cancer history, and menopausal status and postmenopausal hormone use (in women) with MEHP:MEHHP and %MEHP using multivariable linear regression. The values for %MEHP were log-transformed in the analysis.
Results
In multivariable analysis, non-Caucasian individuals had higher %MEHP (non-Hispanic Blacks: β=0.114, 95% Confidence interval [CI]: 0.050, 0.177; Hispanic: β=0.089, 95% CI: 0.024, 0.154; other race: β=0.126, 95% CI: 0.033, 0.219). Age was inversely associated with MEHP:MEHHP (β= −0.001, 95% CI: −0.002, −0.001) and %MEHP (β= −0.006, 95% CI: −0.008, −0.004). Overweight individuals had lower MEHP:MEHHP and lower %MEHP (β= −0.035, 95% CI: 0.062, −0.008 and β= −0.104, 95% CI: −0.162, −0.046, respectively). Alcohol consumption was inversely associated with %MEHP among men (p-trend=0.03).
Conclusions
Individual and lifestyle characteristics are associated with differences in DEHP metabolism. Understanding underlying biological mechanisms could help to identify individuals at a greater risk of adverse effects from DEHP exposure.
Keywords: phthalate exposure, lifestyle, risk factors, endocrine disruptors
1. Introduction
Phthalates are industrial chemicals extensively used in a variety of consumer products and certain medical products such as blood bags and pharmaceutical coatings (1). Because of their wide-spread use and variety of biological effects, phthalates were included on the list of endocrine-disrupting compounds with high exposure concern and highest priority for research (1, 2). Di-2-ethylhexyl phthalate (DEHP) is the most common phthalate that the general population is exposed to ubiquitously, with ingestion being the main non-occupational exposure route (1, 3–5). DEHP is rapidly metabolized within hours (half-life 6–12 hours) (6–8). Secondary DEHP metabolites are detected in 100% of the samples from the general U.S. population with wide variation in their concentration (3, 9).
DEHP is rapidly hydrolyzed to its corresponding monoester (mono-(2-ethyl-hexyl) phthalate, MEHP) by phase I enzymes, lipases and esterases in the intestinal epithelium (4, 10–12). Upon absorption, the monoester undergo further hydroxylation and oxidation (Figure 1) (4). A greater proportion of the dose of DEHP is accounted for by the more downstream metabolites, including mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) (13). A smaller portion of absorbed monoester undergoes phase II biotransformation into glucuronide-conjugated monoesters that are excreted in urine (12). The portion of DEHP and MEHP that was not absorbed in the intestines is excreted in the feces (1).
Figure 1. Di-2-ethylhexyl phthalate (DEHP) metabolism.
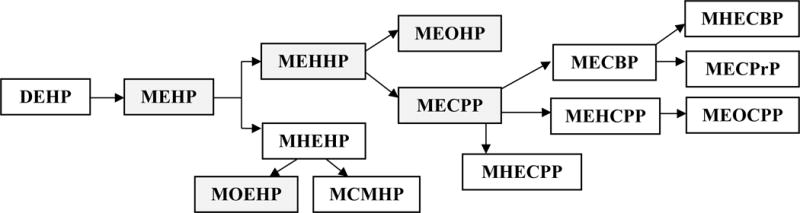
Note: Shaded boxes represent analytes measured as part of NHANES
Abbreviations: MBP - monobutyl phthalate; MBzP - Mono-benzyl phthalate; MEHP - Mono-2-ethylhexyl phthalate; MEP - Mono-ethyl phthalate; MEHHP - Mono-(2-ethyl-5- hydroxyhexyl) phthalate; MEOHP - Mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - Mono- (2-ethyl-5-carboxypentyl) phthalate; MOEHP - Mono-2-(1-oxoethyl)hexyl-d4 phthalate; MCMHP - mono(2-carboxymethylhexyl) phthalate; MHEHP – mono-2-(hydrohyethyl)hexyl phthalate, MHECPP – mono-e-(1-hydroxyethyl)-5-carboxypentyl phthalate, MEHCPP – mono-(2-ethyl-4-hydroxy-5-carboxypentyl) phthalate, MEOCPP - mono-(2-ethyl-4-oxo-5- carboxypentyl) phthalate; MECBP – mono-(2-ehtyl-4-carboxybutyl) phthalate, MHECBP – mono-2-(1-hydroxyethyl)-4-carboxybutyl phthalate, MECPrP - Mono(2-ethyl-3- carboxypropyl) phthalate
Previous studies suggested an association of exposure to DEHP with the risk of premature thelarche (14), higher risk of endometriosis (15, 16), low sperm quality (17, 18), reduced testosterone levels (19–21), obesity, diabetes, and possibly breast cancer (22–28). The primary metabolite, MEHP, is a well-known ligand for the peroxisome proliferator-activated receptor (PPAR) family (29), is a mitochondrial toxicant and disruptor of lipid and glucose metabolism (29–31), and is the most potent DEHP metabolite in its toxicity (32, 33). Even though some studies suggested differences in susceptibility to the toxic effects of peroxisome proliferators across species with lower potential in humans, the basis for species differences in peroxisome proliferation and carcinogenesis by phthalate esters has not been fully described (33).
It has been previously shown that a higher ratio of MEHP to MEHHP or MEHP to MEOHP in humans is associated with a greater physiologic effect and potentially greater endocrine disrupting capacity (4, 29). It was also suggested that the ratio of MEHP to the secondary metabolites could represent a phenotypic marker of the rate of DEHP metabolism to less toxic metabolites (4). However, it remains unknown what individual characteristics and lifestyle factors are associated with differences in metabolic conversion of DEHP. This knowledge could potentially help to identify population subgroups that might be more susceptible to adverse effects of phthalates. Some of these factors could be related to underlying genetic differences resulting in different conversion rates. Other factors may potentially influence the DEHP metabolism rate by other mechanisms such as inhibition or activation of metabolic enzymes.
To address these knowledge gaps, we investigated, for the first time, the association of selected individual characteristics (age, gender, race/ethnicity, BMI, cancer history, and, menopausal status [in women]) and lifestyle factors (smoking, alcohol use, caffeine consumption, prescription medication use, postmenopausal hormone use [in women]) with the ratios of the major DEHP metabolites in a representative sample of the U.S. population using the data from the National Health and Nutrition Examination Survey (NHANES), 2001–2012. As the ratio of MEHP to the sum of the secondary DEHP metabolite concentrations (MEHHP, MEOHP, and MECPP), referred to as %MEHP, and the ratio of MEHP to MEHHP (MEHP:MEHHP) are reflective of the conversion rate for the most potent DEHP metabolite, MEHP, we primarily presented the results of the analyses for these two ratios. In a secondary analysis, we also investigated the associations of selected characteristics and lifestyle factors with ratios of secondary DEHP metabolites: MEHHP to MECPP (MEHHP:MECPP), MEOHP to MECPP (MEOHP:MECPP), and MEHHP to MEOHP (MEHHP:MEOHP).
2. Materials and Methods
2.1 Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing survey conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC), to assess the health of the U.S. population. The survey utilizes a multistage, stratified, clustered design that selects a representative sample of the civilian, non-institutionalized U.S. population with oversampling of specific subgroups defined by age, race/ethnicity and income in various NHANES years (34). The survey data are collected through household interviews and standardized examinations at mobile examination centers throughout the United States as described in detail previously (34). Blood, serum and urine samples were collected as part of the NHANES examination. Environmental chemicals were measured in randomly selected subsamples of participants within specific age groups. All NHANES protocols were approved by the NCHS’ Research Ethics Review Board, and all participants signed a consent form before their participation.
This analysis used Continuous NHANES data from 2001–2012 and was restricted to individuals who were 18 and older at the time of the survey, had a body mass index (BMI)<30, had no history of diabetes or insulin use, and had urinary phthalates measurements. Additionally, pregnant women were also excluded. In total, 6,653 adults met these criteria (Figure 2).
Figure 2. Participant selection flowchart.
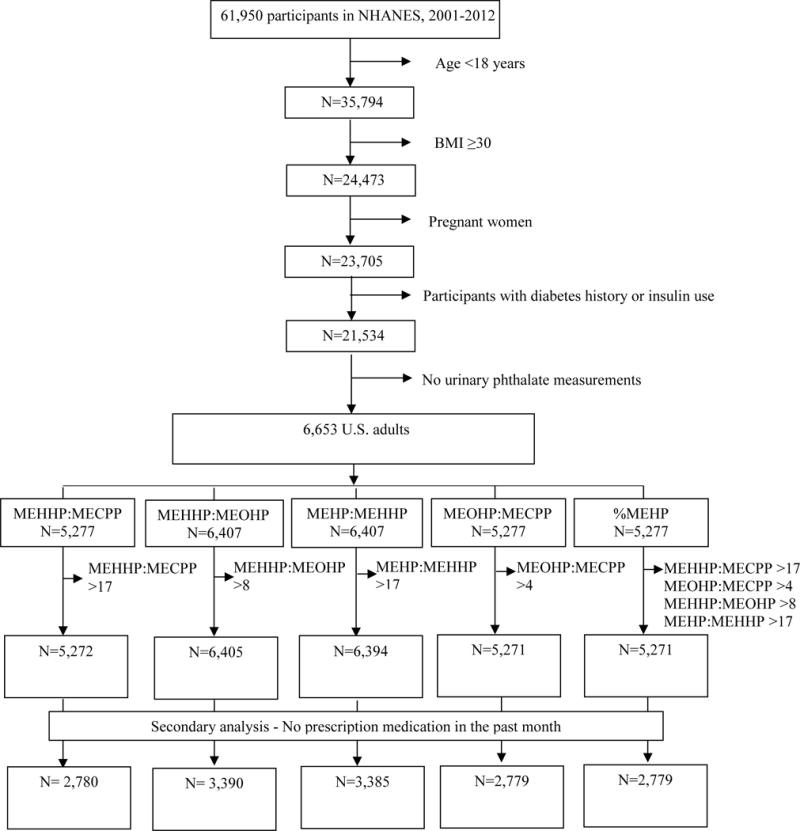
Among four DEHP metabolites that were measured between 2001 and 2012 (MEHP, MEHHP, MEOHP, and MECPP), MECPP measurements were available for NHANES 2003–2012 only, thus reducing the sample size of the analyses utilizing this metabolite (Figure 2). Of the eligible adults, 6,407 participants had values for MEHP:MEHHP and MEHHP:MEOHP, while 5,277 participants had values for MEHP:MECPP, MEOHP:MECPP, and %MEHP. As further explained under the Urinary Phthalates Section, a few participants were further excluded as the result of unreasonably large ratio values in instances where the second metabolite was below the limit of detection (LOD). The final sample ranged between 5,271 and 6,405 participants, depending on the ratio (Figure 2).
2.2 Individual characteristics and lifestyle factors
Information on the following variables was extracted from the NHANES questionnaires: age, gender, race/ethnicity, menopausal status and postmenopausal hormone use (women only), history of cancer, alcohol and caffeine consumption, smoking, and prescription medication use in the past month. Participants’ height and weight were measured as a part of the physical examination. BMI was calculated as weight in kilograms divided by height in meters squared and was categorized as <18.5 kg/m2 (underweight), 18.5–<25 kg/m2 (normal weight), 25–<30 kg/m2 (overweight) (35). Alcohol consumption was available from the alcohol use questionnaire (consumption over the past 12 months). Caffeine consumption (in mg) was estimated from the data collected in a dietary interview that recorded all food and beverages consumed during the 24-hour period preceding the interview.
2.3 Urinary phthalates
Urine samples were collected according to the standard protocol from participants aged 6 and older at the time of the medical examination. Urine specimens were processed, stored at −20°C, and then shipped to the Division of Laboratory Sciences, National Center for Environmental Health at CDC for analysis (36–38). As the current analysis focused on ratios of phthalate metabolites rather than concentrations of individual metabolites, no creatinine adjustment was needed. Urinary phthalates were quantified with high performance liquid chromatography–tandem mass spectrometry as previously described (39–41). We examined the associations of individual characteristics and lifestyle factors with the following ratios of DEHP metabolites: MEHP to the sum of the secondary DEHP metabolite concentrations (MEHHP, MEOHP, and MECPP) referred to as %MEHP, MEHP:MEHHP, MEHHP:MECPP, MEHHP:MEOHP, and MEOHP:MECPP. As %MEHP and MEHP:MEHHP are reflective of the conversion rate of MEHP, we primarily present the results of the analyses for these two ratios, followed by the findings for other ratios as supplementary material.
The values below LOD were substituted with LOD for the given analyte (specific to the NHANES survey year) divided by the square root of 2 (Supplementary table 1) (42). Upon initial examination of the distribution of the phthalate metabolite ratios, we excluded individuals with ratio values >17 for MEHHP:MECPP and MEHP:MEHHP, >8 for MEHHP:MEOHP, and >4 for MEOHP:MECPP, because these individuals had a value <LOD for the second metabolite in the ratio which resulted in an unreasonably large ratio value. However, we kept in the analysis observations where both the first and the second analyte were represented by a value <LOD or the first analyte was represented by a very small value. In these instances, the value for the ratio did not meet the exclusion criterion. All observations that were excluded from analyses of individual ratios based on the aforementioned ratio cut-offs were also excluded from the analysis for %MEHP. After excluding individuals with the extreme values (Figure 2), the distributions of all ratios were approximately normal, except for %MEHP. We thus log-transformed %MEHP in the analysis.
2.4 Statistical analysis
We accounted for the complex survey structure (multistage probability sample design) throughout the analyses following the NHANES analytic guidelines (43). The weighted numbers based on the sample represent the estimated number of the study population in the United States. Our analyses included 5,271 participants for %MEHP, 6,394 for MEHP:MEHHP, 5,272 for MEHHP:MECPP, 6,405 for MEHHP:MEOHP, and 5,271 for MEOHP:MECPP (Figure 2).
We presented distributions of socio-demographic characteristics and lifestyle factors in the study sample by quartiles of %MEHP. Differences across the quartiles were tested using Chi-Square test for categorical variables and F-test for continuous variables.
The individual characteristics and lifestyle factors of interest were modeled as follows: age (continuous), race (Caucasian [reference], Non-Hispanic Black, Mexican-American or other Hispanic, and Other race), BMI (normal [reference], underweight, overweight), history of cancer (any vs. none), alcohol consumption (none [reference], below limit of 14 drinks/week for men or 7 drinks/week for women, above the limit of 14 drinks/week for men or 7 drinks/week for women) (44), smoking status (never smoker [reference], past smoker, current smoker), prescription medication use in the past month (none [reference], any), caffeine use (quartiles based on the distribution in the study sample: <10 [reference]; 10-<91; 91-<208; ≥208 mg). In women, we additionally examined the associations of menopausal status and postmenopausal hormone use (premenopausal [reference], postmenopausal with no history of hormone use, postmenopausal with past hormone use, postmenopausal with current hormone use, postmenopausal with past but unknown current hormone use) with the phthalate ratios.
We used multivariable linear regression to analyze the association of the selected individual characteristics with the phthalate metabolite ratios. First, we explored the associations of each individual characteristic or lifestyle factor with each of the ratios, adjusting for age, gender, race, and BMI (Model 1). We additionally examined the effects of poverty and education on the risk estimates, but as they did not affect the estimates and were not statistically significant, they were not retained in the final models. We then examined the associations in a fully adjusted model that simultaneously included all of the individual characteristics and lifestyle factors (Model 2). Missing values for each of the variables were represented by an “unknown” category. An additional Model 2 was run on women to examine the associations of menopausal status and postmenopausal hormone use with phthalate metabolite ratios. Furthermore we used respective medians within each category to assess the overall trend for BMI, alcohol and caffeine consumption. As alcohol consumption was categorized based on gender-specific cutoffs, the test for trend for alcohol consumption was performed separately in each of the gender stratum.
Finally, a secondary analysis was limited to participants without prescription medication use (including hormones) in the past month; both Model 1 and Model 2 were examined in this subset. Analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). For all analyses, the statistical significance was assessed at the 0.05 level. All tests were two-sided.
3. Results
Characteristics of the study population by quartiles of %MEHP are presented in Table 1. As compared to the individuals in the lowest %MEHP quartile, those in the highest %MEHP quartile were younger (p<0.0001), more likely to be non-Caucasian (p<0.0001) and less likely to be overweight (p=0.04). Individuals in the highest %MEHP quartile were more likely to be current smokers (30.0% vs. 21.2%, p<0.0001). A greater proportion of individuals in the lowest quartile had a history of cancer (12.0% vs. 6.5%, p<0.001) and a history of prescription medication use in the past month (59.6% vs. 45.5%, p<0.0001). Among women, those in the lowest %MEHP quartile were less likely to be premenopausal and were more likely to be current postmenopausal hormone users (p<0.001).
Table 1.
Characteristics of the Study Population, by the Log-transformed %MEHP Quartile (% or Mean [SD]), NHANES 2001–2012
| Characteristic | 1st quartile Sample N=1,317 | 2nd quartile Sample N=1,318 | 3rd quartile Sample N=1,318 | 4th quartile Sample N= 1,318 |
|---|---|---|---|---|
| Age, years* | 48.5 (0.7) | 44.9 (0.6) | 42.2 (0.5) | 40.6 (0.6) |
| Race* | ||||
| Caucasian | 76.8% | 74.1% | 68.7% | 64.4% |
| Other Race | 5.9% | 6.5% | 7.7% | 9.9% |
| Mexican-American or other Hispanic | 9.3% | 12.4% | 13.2% | 14.2% |
| Non-Hispanic Black | 8.0% | 7.0% | 10.4% | 11.5% |
| Gender | ||||
| Male | 47.5% | 49.8% | 48.1% | 49.2% |
| Female | 52.5% | 50.2% | 51.9% | 50.8% |
| BMI† | ||||
| Normal | 44.7% | 47.9% | 51.2% | 52.1% |
| Underweight | 2.4% | 3.2% | 3.2% | 3.1% |
| Overweight | 52.9% | 48.9% | 45.5% | 44.8% |
| Smoking* | ||||
| Never smoker | 52.6% | 51.6% | 52.9% | 53.7% |
| Past smoker | 26.7% | 20.6% | 19.9% | 16.3% |
| Current smoker | 21.2% | 27.8% | 27.2% | 30.0% |
| Alcohol use | ||||
| None | 14.7% | 16.0% | 14.5% | 13.3% |
| Below limit | 69.2% | 69.4% | 72.9% | 74.6% |
| Above limit | 16.0% | 14.6% | 12.6% | 12.1% |
| Cancer history* | ||||
| No | 88.0% | 90.9% | 93.6% | 93.5% |
| Yes | 12.0% | 9.1% | 6.4% | 6.5% |
| Caffeine, g/day* a | ||||
| 1st Quartile | 19.1% | 14.9% | 18.3% | 23.5% |
| 2nd Quartile | 21.0% | 24.0% | 23.8% | 22.1% |
| 3rd Quartile | 26.4% | 29.1% | 25.4% | 25.2% |
| 4th Quartile | 33.5% | 32.0% | 32.5% | 29.3% |
| Prescription medication* | ||||
| Did not use in past month | 40.4% | 49.6% | 53.5% | 54.5% |
| Used in past month | 59.6% | 50.4% | 46.5% | 45.5% |
| Menopausal status/postmenopausal hormone use (women only)*b | ||||
| Premenopausal | 56.3% | 60.3% | 67.5% | 73.1% |
| Postmenopausal/Never used | 22.7% | 18.7% | 17.5% | 14.1% |
| Postmenopausal/Past, not current | 12.4% | 13.7% | 8.6% | 8.8% |
| Postmenopausal, Past/unknown current | 3.0% | 3.5% | 2.2% | 0.3% |
| Postmenopausal/Current | 5.5% | 3.8% | 4.2% | 3.7% |
differences across quartiles significant at 0.001 level
differences across quartiles significant at 0.05 level
1st − <10; 2nd: 10−<91 ; 3rd − 91−<208; and 4th −>208 mg
N of women for each % quartile: 1st quartile: Sample N = 603, Weighted N =11,899,351; 2nd quartile: Sample N = 616, Weighted N = 12,182,219; 3rd quartile: Sample N = 633, Weighted N =12,507,047; 4th quartile: Sample N = 586 Weighted N =11,381,258
In multivariable analysis (Model 2), compared to Caucasian, other racial/ethnic groups had higher %MEHP (non-Hispanic Blacks: β=0.114, 95% confidence interval [CI]: 0.050, 0.177; Hispanic: β=0.089, 95% CI: 0.024, 0.154; other race: β=0.126, 95% CI: 0.033, 0.219) (Table 2). Age was inversely associated with MEHP:MEHHP (β= −0.001, 95% CI: −0.002, −0.001) and %MEHP (β= −0.006, 95% CI: −0.008, −0.004). Overweight individuals had lower MEHP:MEHHP and lower %MEHP (β= −0.035, 95% CI: −0.062, −0.008, p-trend=0.01 and β= −0.104, 95% CI: −0.162, −0.046, p-trend<0.001, respectively). Current smokers had higher %MEHP (β = 0.069, 95% CI: 0.011, 0.126) as compared to non-smokers. Even though alcohol consumption was not associated with either of the ratios in the overall analysis, the test for trend in the analysis for %MEHP was significant among men (p-trend=0.04). Cancer history, caffeine consumption, prescription medication use and in women, menopausal status and postmenopausal hormone use, were not associated with either %MEHP or MEHP:MEHHP.
Table 2.
Associations of Individual Characteristics and Lifestyle Factors with MEHP:MEHHP and %MEHP (Regression Estimates and 95% Confidence Intervals), All Participants, NHANES 2001–2012.
| MEHP:MEHHP | log(%MEHP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | Model 1a | Model 2b | Model 1a | Model 2b | ||||||
|
|
||||||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |||
| Age, years | −0.001 | −0.001, −0.001 | −0.001 | −0.002, −0.001 | −0.006 | −0.007, −0.004 | −0.006 | −0.008, −0.004 | ||
| Race | ||||||||||
| Caucasian | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Other Race | 0.033 | −0.002, 0.069 | 0.020 | −0.017, 0.057 | 0.158 | 0.065, 0.251 | 0.126 | 0.033, 0.219 | ||
| Mexican-American or other | 0.022 | −0.015, 0.059 | 0.014 | −0.027, 0.055 | 0.111 | 0.052, 0.170 | 0.089 | 0.024, 0.154 | ||
| Hispanic | ||||||||||
| Non-Hispanic Black | 0.010 | −0.025, 0.044 | −0.005 | −0.045, 0.035 | 0.136 | 0.082, 0.189 | 0.114 | 0.050, 0.177 | ||
| Gender | ||||||||||
| Male | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Female | 0.024 | −0.001, 0.049 | 0.016 | −0.007, 0.040 | 0.006 | −0.042, 0.054 | 0.015 | −0.032, 0.062 | ||
| BMI | ||||||||||
| Normal | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Underweight | −0.013 | −0.078, 0.053 | −0.010 | −0.075, 0.056 | −0.007 | −0.148, 0.133 | −0.010 | −0.160, 0.141 | ||
| Overweight | −0.034 | −0.060, −0.053 | −0.035 | −0.062, −0.008 | −0.101 | −0.158, −0.044 | −0.104 | −0.162, −0.046 | ||
| Smoking | ||||||||||
| Never smoker | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Past smoker | −0.027 | −0.047, −0.007 | −0.022 | −0.045, 0.002 | −0.054 | −0.117, 0.010 | −0.049 | −0.114, 0.015 | ||
| Current smoker | −0.008 | −0.033, 0.017 | 0.001 | −0.029, 0.031 | 0.058 | −0.001, 0.116 | 0.069 | 0.011, 0.126 | ||
| Alcohol use | ||||||||||
| None | 0.000 | Reference | 0.000 | Reference | Reference | Reference | ||||
| Below limit | −0.035 | −0.103, 0.034 | −0.029 | −0.097, 0.039 | −0.016 | −0.102, 0.070 | −0.008 | −0.094, 0.077 | ||
| Above limit | −0.060 | −0.127, 0.007 | −0.053 | −0.116, 0.010 | −0.073 | −0.183, 0.037 | −0.081 | −0.190, 0.027 | ||
| Cancer history | ||||||||||
| No | Reference | Reference | Reference | Reference | ||||||
| Yes | 0.009 | −0.036, 0.054 | 0.010 | −0.036, 0.056 | −0.049 | −0.171, 0.073 | −0.036 | −0.155, 0.083 | ||
| Caffeine, mgc | ||||||||||
| 1st quartile | Reference | Reference | Reference | Reference | ||||||
| 2nd quartile | −0.024 | −0.075, 0.026 | −0.026 | −0.077, 0.025 | −0.031 | −0.108, 0.046 | −0.044 | −0.123, 0.036 | ||
| 3rd quartile | −0.031 | −0.082, 0.020 | −0.030 | −0.080, 0.020 | −0.023 | −0.105, 0.060 | −0.039 | −0.122, 0.045 | ||
| 4th quartile | −0.037 | −0.078, 0.004 | −0.036 | −0.076, 0.004 | 0.007 | −0.084, 0.098 | −0.018 | −0112, 0.077 | ||
| Prescription medication in past month | ||||||||||
| None | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Any | 0.011 | −0.020, 0.042 | 0.013 | −0.017, 0.042 | −0.055 | −0.115, 0.005 | −0.049 | −0.108, 0.011 | ||
| Menopausal status/hormone use (women only) | ||||||||||
| Premenopausal | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | ||
| Postmenopausal/Never used | −0.010 | −0.063, 0.043 | −0.004 | −0.062, 0.055 | −0.127 | −0.291, 0.037 | −0.097 | −0.259, 0.064 | ||
| Postmenopausal/Past, not current | −0.033 | −0.086, 0.020 | −0.024 | −0.086, 0.039 | −0.106 | −0.297, 0.084 | −0.052 | −0.239, 0.134 | ||
| Postmenopausal/Past, unknown current | −0.036 | −0.110, 0.038 | −0.035 | −0.120, 0.050 | −0.227 | −0.448, −0.006 | −0.169 | −0.392, 0.054 | ||
| Postmenopausal/Current | 0.022 | −0.049, 0.092 | 0.033 | −0.047, 0.113 | −0.080 | −0.334, 0.175 | −0.038 | −0.294, 0.219 | ||
Abbreviations: CI, Confidence Interval
Adjusted for age, BMI, and race
Adjusted for age, BMI, race, and all other factors in the analyses
1st quartile <10; 2nd quartile 10−<91 ; 3rd quartile 91−<208; and 4th quartile >208 mg
In the secondary analysis restricted to individuals with no prescription medication use in the past month, associations of race, age and BMI, and alcohol consumption with %MEHP were similar (Table 3). Associations of smoking with %MEHP were marginally significant, though the magnitude of the estimates was greater than in the overall study sample (β= 0.114, 95% CI: 0.033, 0.195). Cancer history was inversely associated with MEHP:MEHHP (β= −0.051, 95% CI: −0.084, −0.018). Other characteristics and lifestyle factors were not associated with either of the two ratios.
Table 3.
Associations of Individual Characteristics and Lifestyle Factors with MEHP:MEHHP and %MEHP (Regression Estimates and 95% Confidence Intervals), Participants without Prescription Medication Use in the Past Month, NHANES 2001–2012.
| MEHP:MEHHP | log(%MEHP) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | Model 1a | Model 2b | Model 1a | Model 2b | ||||
|
|
||||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Age, years | ||||||||
| Estimates | −0.001 | −0.002, 0.001 | −0.001 | −0.002, 0.001 | −0.004 | −0.006, −0.002 | −0.006 | −0.008, −0.003 |
| Race | ||||||||
| Caucasian | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Other Race | 0.028 | −0.018, 0.073 | 0.017 | −0.032, 0.066 | 0.172 | 0.046, 0.299 | 0.170 | 0.037, 0.302 |
| Mexican-American or other | 0.010 | −0.021, 0.040 | 0.003 | −0.033, 0.038 | 0.137 | 0.060, 0.214 | 0.135 | 0.049, 0.220 |
| Hispanic Non-Hispanic Black | 0.004 | −0.038, 0.046 | −0.008 | −0.055, 0.038 | 0.134 | 0.059, 0.209 | 0.128 | 0.038, 0.218 |
| Gender | ||||||||
| Male | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Female | 0.015 | −0.14, 0.045 | 0.014 | −0.015, 0.043 | −0.001 | −0.078, 0.077 | 0.009 | −0.070, 0.088 |
| BMI | ||||||||
| Normal | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Underweight | −0.005 | −0.095, 0.085 | 0.006 | −0.087, 0.098 | −0.051 | −0.214, 0.112 | −0.015 | −0.203, 0.172 |
| Overweight | −0.039 | −0.068, −0.009 | −0.039 | −0.069, −0.009 | −0.103 | −0.164, −0.041 | −0.103 | −0.164,−0.042 |
| Smoking | ||||||||
| Never smoker | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Past smoker | −0.016 | −0.042, 0.011 | −0.011 | −0.036, 0.015 | 0.009 | −0.097, 0.116 | 0.007 | −0.108, 0.122 |
| Current smoker | 0.022 | −0.004, 0.048 | 0.028 | −0.001, 0.057 | 0.105 | 0.023, 0.188 | 0.114 | 0.033, 0.195 |
| Alcohol use | ||||||||
| None | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Below limit | −0.004 | −0.032, 0.023 | −0.004 | −0.033, 0.025 | −0.063 | −0.174, 0.049 | −0.048 | −0.157, 0.062 |
| Above limit | −0.022 | −0.060, 0.016 | −0.027 | −0.065, 0.011 | −0.112 | −0.243, 0.020 | −0.125 | −0.256, 0.005 |
| Cancer history | ||||||||
| No | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Yes | −0.052 | −0.085, −0.019 | −0.051 | −0.084, −0.018 | −0.008 | −0.228, 0.212 | −0.001 | −0.215, 0.214 |
| Caffeine, mgc | ||||||||
| 1st quartile | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| 2nd quartile | −0.009 | −0.059, 0.040 | −0.012 | −0.062, 0.039 | −0.029 | −0.135, 0.077 | −0.048 | −0.156, 0.060 |
| 3rd quartile | −0.029 | −0.067, 0.010 | −0.030 | −0.071, 0.010 | −0.058 | −0.176, 0.061 | −0.079 | −0.199, 0.041 |
| 4th quartile | −0.019 | −0.062, 0.024 | −0.023 | −0.067, 0.022 | 0.016 | −0.127, 0.159 | −0.020 | −0.163, 0.124 |
| Menopausal status/hormone use (women only) | ||||||||
| Premenopausal | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference | 0.000 | Reference |
| Postmenopausal/Never used | 0.037 | −0.046, 0.121 | 0.039 | −0.049, 0.128 | −0.185 | −0.419, 0.049 | −0.171 | −0.402, 0.060 |
| Postmenopausal/Past, not current | −0.018 | −0.087, 0.051 | −0.003 | −0.081, 0.076 | −0.068 | −0.321, 0.184 | −0.040 | −0.305, 0.224 |
| Postmenopausal/Past, unknown current | −0.006 | −0.102, 0.090 | −0.006 | −0.094, 0.081 | −0.187 | −0.457, 0.083 | −0.012 | −0.292, 0.268 |
| Postmenopausal/Current | NA | NA | NA | NA | ||||
Abbreviations: CI, Confidence Interval; MEHP, mono-2-ethylhexyl phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; %MEHP, ratio of MEHP to the sum of the secondary metabolites; NA, Not Applicable
Adjusted for age, BMI, and race
Adjusted for age, BMI, race, and all other factors in the analyses
1st quartile <10; 2nd quartile 10−<91 ; 3rd quartile 91−<208; and 4th quartile >208 mg
In secondary analyses for the other phthalate metabolite ratios, race, gender, alcohol consumption and caffeine consumption, and medication use in the past month, were associated with the ratios of secondary phthalate metabolites (Supplementary Table 2, 3 and 4).
4. Discussion
In this large representative sample of the U.S. population, we found significant associations of several individual characteristics and lifestyle factors with ratios of urinary phthalate metabolites. These findings indicate that the presence of certain lifestyle factors may put individuals at an increased risk from DEHP exposure because of less favorable metabolic patterns. This shift in balance towards more potent MEHP could subsequently result in a greater biological effect.
Previous studies suggest a great inter-individual variation in the metabolism of DEHP in humans, which can have implications for the DEHP exposure risk assessment. DEHP is metabolized to MEHP by lipase or esterase and further to the secondary metabolites by the cytochrome P450 system, alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH), and UDP-glucuronyl transferase (45–47). Prior studies indicate that activity of these enzymes could be influenced by gender, medication, smoking, caffeine, and BMI (48). For example, higher BMI, smoking, and caffeine consumption induce activity of CYP3A4 explaining as much as 20% variation in inter-individual differences in enzymatic activity (48). Importantly, CYP3A4 is responsible for metabolism of more than 50% of xenobiotics, including DEHP (48). Smoking and long-term alcohol consumption also induce activity of CYP1A2, CYP2A6, CYP2E1 and reduce activity of CYP2A6 which together with CYP3A4 and CYP2C9, CYP2C19 metabolize up to 90% of xenobiotics, though the role of these enzymes in phthalate metabolism is unclear (49–51).
We observed lower ratios of MEHP:MEHHP and %MEHP in individuals with greater BMI, which could potentially be explained by the aforementioned stimulating effect of BMI on CYP450 system genes (48). A previous study in women found a positive association of BMI with MEHP:MEHHP ratio (27). However, the study population and methods in the current study are different from the previous analysis. First, we included both men and women with a BMI<30 in this study, while the previous study focused on women without restrictions on BMI. Second, BMI was an outcome of interest in the previous study and as required in such instances, all analyses were adjusted for total calorie and fat intake. Finally, these studies also differed in terms of statistical modeling and included covariates.
ADH is reversely inhibited by high levels of acetaldehyde and ALDH activity is reduced by smoking (52). A few polymorphisms in these genes have been linked to susceptibility to alcohol dependence due to the varying enzymatic activity leading to different rates of acetaldehyde accumulation (53). Individuals with higher alcohol consumption might potentially represent individuals with some of these genetic polymorphisms reflective of slower acetaldehyde accumulation, higher tolerance to ethanol, and increased risk of alcohol dependence. Increased activity of ADH in these individuals could potentially explain changes in the rate of MEHP metabolism as reflected in lower %MEHP though it is unclear why this association was limited to men.
We observed a higher %MEHP in current smokers as compared to non-smokers. This finding cannot be explained by the inducing effects of smoking on CYP450 genes, but could potentially result from reduced ALDH activity. Interestingly, when the analysis was restricted to individuals without prescription medication use, thus removing the potential stimulating effect of medication on CYP450 genes, the risk estimates for smoking became larger. However, future studies are warranted to explain the underlying biological mechanisms behind increased %MEHP levels in smokers.
Our findings suggest that non-Caucasian individuals might be at a greater risk of adverse health effects from exposure to DEHP. Previous studies on health outcome disparities have demonstrated that individuals from Hispanic, non-Hispanic Black and other mixed racial groups have an increased risk of various health outcomes as compared to their Caucasian counterparts and that socioeconomic and clinical factors can only partially explain these disparities (54–57). Though the underlying biological causes of these disparities are not well understood, the differences in metabolism of environmental exposures, including phthalates, could potentially in part explain some of the observed patterns.
For some of the lifestyle factors and individual characteristics, we observed differences for the ratios of the secondary metabolites but not for the primary to secondary DEHP metabolite ratios. Even though we did not see differences by gender for MEHP:MEHHP and %MEHP, women appeared to have lower ratios of MEHHP:MECPP and MEHHP:MEOHP as compared to men suggesting that this metabolic step might be occurring at a greater rate in females. Alcohol use and caffeine consumption both were associated with an increase in secondary metabolite ratios, as was use of prescription medication. However, interpretation of these findings in the context of the changed risk of adverse effects from DEHP exposure is hindered by the lack of knowledge on relative toxicity of these secondary metabolites in humans. Finally, while we found an association of BMI with both %MEHP and MEHP:MEHHP, we observed no differences in the ratios of the secondary metabolites.
Some exposures in our analysis differed with respect to the reference period for their assessment: past 24-h for caffeine consumption, past 12 months for alcohol consumption, past month for prescription medication, and life-long history for smoking (never, past, current), Exposure misclassification may increase while using some of the reference periods for specific exposures. For example, some previous studies suggest that 24-h recall for caffeine consumption may underestimate the usual caffeine consumption levels (58). For alcohol consumption, longer reference periods are recommended to capture varying patterns by season, holidays, and irregular drinking (59). The accuracy of the recall of current medication use may vary by the type of medication and repetitiveness of its use (60, 61). Concurrent use of instruments assessing recent and usual exposures could help to assess accuracy of reporting, reduce misclassification, and identify most relevant windows of exposure to these factors.
This is the first study to examine the association of several individual characteristics and lifestyle factors with metabolism of phthalates as reflected by the ratios of primary and secondary metabolites. A few previous papers discussed associations of certain lifestyle factors and individual characteristics with levels of individual DEHP metabolites (62–65). The differences in the ratios of phthalate metabolites are reflective of inter-individual differences in oxidative metabolism of DEHP, the most common phthalate (66). Previous studies suggest that a higher ratio of MEHP to MEHHP may have a potentially greater physiologic effect as compared to individual metabolites (4, 13). This analysis used the data from a large representative sample of the U.S. population. Nonetheless, our study has a few limitations. The cross-sectional nature of the data does not allow us to determine the temporal relationship. In addition, phthalates were measured in a single urine sample. Even though phthalates are rapidly metabolized within hours (half-life 6–12 hours), previous studies suggest that a single urine sample can accurately classify phthalate exposure over the previous 3 months (67, 68). A few studies demonstrated that a single sample could reliably represent a long-term exposure levels for phthalates in women, children, and men, some for up to three years (69). To assess the temporal variability in phthalate concentrations, some studies examined correlation between levels within the same individuals, though only a few studies examined this correlation over a period of time longer than 6 months. The strength of the correlation varied by metabolite and study population (general population, women, men, children, and pregnant women). The strongest correlation over the period of time >6 months was observed among women for MEOHP and sum of DEHP metabolites (0.46 and 0.42, respectively). Among men, the interclass correlation over period of approximately 3 months ranged between 0.51 and 0.54 for MEHP. The worst reproducibility was noted in pregnant women and children (69). Both children and pregnant women were excluded from our current analysis. While overall results from these studies suggest that a single sample may adequately represent individual’s exposure over several months or a few years, use of multiple urine samples in future studies would help to reduce exposure misclassification, especially if certain subpopulations such as children and pregnant women are included.
5. Conclusions
The findings of our study suggest an association of several individual characteristics and lifestyle factors, including age, race, BMI, smoking, and alcohol use with the metabolic conversion rate of MEHP. The exact mechanisms driving the differences in these metabolic patterns in relation to each of these factors remain unclear. Future prospective studies are needed to examine the temporal relationship between lifestyle factors and DEHP metabolism and to elucidate the causal links, if any.
Supplementary Material
Acknowledgments
Funding support was provided by the National Cancer Institute at the National Institutes of Health (Grant U54 CA155496); the Agency for Healthcare Research and Quality (Grant K01 HS022330); and the Foundation for Barnes-Jewish Hospital.
Financial support: This work was supported by the National Cancer Institute at the National Institutes of Health (U54 CA155496), the Agency for Healthcare Research and Quality (K01 HS022330) and the Foundation for Barnes-Jewish Hospital.
Footnotes
Research on human subjects: All NHANES protocols were approved by the NCHS’ Research Ethics Review Board, and all participants signed a consent form before their participation.
References
- 1.U.S. Department of Health and Human Services. Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile for Di(2-ethylhexyl)phthalate. 2002 [PubMed] [Google Scholar]
- 2.Groshart C, Okkerman P. Towards the establishment of a priority list of substances for further evaluation of their role in endocrine disruption. European Commission DG ENV; 2000. [Google Scholar]
- 3.CDC. Center for Control Disease and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. 2009 [Google Scholar]
- 4.Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Molecular Nutrition & Food Research. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- 5.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure–an update and latest results. Int J Androl. 2006;29:155–65. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181–5. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113:1530–5. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer. Di(2-ethylhexyl)phthalate; Sup 7, 77, 101. Lyon, France: 2013. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 9.Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–91. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- 10.Albro PW, Corbett JT, Schroeder JL, Jordan S, Matthews HB. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982;45:19–25. doi: 10.1289/ehp.824519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albro PW, Lavenhar SR. Metabolism of di(2-ethylhexyl)phthalate. Drug Metab Rev. 1989;21:13–34. doi: 10.3109/03602538909029953. [DOI] [PubMed] [Google Scholar]
- 12.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch HM, Rossbach B, Drexler H, Angerer J. Internal exposure of the general population to DEHP and other phthalates–determination of secondary and primary phthalate monoester metabolites in urine. Environ Res. 2003;93:177–85. doi: 10.1016/s0013-9351(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 14.Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108:895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, Mazzeo P, Petraglia F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–5. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- 16.Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006;113:515–20. doi: 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 17.Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. EPIDEMIOLOGY. 2003;14:269–77. [PubMed] [Google Scholar]
- 18.Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. EPIDEMIOLOGY. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 19.Joensen UN, Frederiksen H, Jensen MB, Lauritsen MP, Olesen IA, Lassen TH, Andersson AM, Jorgensen N. Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Environ Health Perspect. 2012;120:1397–403. doi: 10.1289/ehp.1205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–84. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Tellez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117:1587–92. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364:2097–113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, Rich-Edwards J. Urinary Phthalate Metabolite Concentrations and Diabetes among Women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect. 2012;120:1307–13. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson K, Hernandez-Ramirez RU, Burguete-Garcia A, Cebrian ME, Calafat AM, Needham LL, Claudio L, Lopez-Carrillo L. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res. 2011;111:792–6. doi: 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Carrillo L, Hernandez-Ramirez RU, Calafat AM, Torres-Sanchez L, Galvan-Portillo M, Needham LL, Ruiz-Ramos R, Cebrian ME. Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010;118:539–44. doi: 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaghjyan L, Sites S, Ruan Y, Chang SH. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int J Obes (Lond) 2015 doi: 10.1038/ijo.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meeker J, Ferguson K. Phthalates: Human Exposure and Related Health Effects. In: Schecter A, editor. Dioxins and Health. 3rd. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 29.Campioli E, Batarseh A, Li J, Papadopoulos V. The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872) PLoS One. 2011;6:e28750. doi: 10.1371/journal.pone.0028750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dees JH, Gazouli M, Papadopoulos V. Effect of mono-ethylhexyl phthalate on MA-10 Leydig tumor cells. Reproductive Toxicology. 2001;15:171–187. doi: 10.1016/s0890-6238(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Arguelles DB, Papadopoulos V. Mechanisms Mediating Environmental Chemical-Induced Endocrine Disruption in the Adrenal Gland. Frontiers in Endocrinology. 2015;6:29. doi: 10.3389/fendo.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber WW, Grasl-Kraupp B, Schulte-Hermann R. Hepatocarcinogenic potential of di(2-ethylhexyl)phthalate in rodents and its implications on human risk. Crit Rev Toxicol. 1996;26:365–481. doi: 10.3109/10408449609048302. [DOI] [PubMed] [Google Scholar]
- 33.Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard S, Swanson C, Cattley RC, Corton JC. Role of PPARα in mediating the effects of phthalates and metabolites in the liver. Toxicology. 2005;207:149–163. doi: 10.1016/j.tox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.CDC. CDC (Centers for Disease Control and Prevention) National Health and Nutrition Examination Survey. 2011b Available: http://www.cdc.gov/nchs/nhanes.htm [accessed 22 May 2014] 2011b.
- 35.NHLBI. National Heart Lung, and Blood Institute and North American Association for the Study of Obesity. Practical Guide on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; Oct, 2000. NIH publication No. 00-4084 2000. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Naional Center for Health Statistics. The National Health and Nutrition Examination Survey: 2003–2004. Laboratory Procedures Manual. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/lab.pdf.
- 37.Centers for Disease Control and Prevention. Naional Center for Health Statistics. The National Health and Nutrition Examination Survey: 1999–2000. Laboratory Procedures Manual. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/lab.pdf.
- 38.Centers for Disease Control and Prevention. Naional Center for Health Statistics. The National Health and Nutrition Examination Survey: 2001–2002. Laboratory Procedures Manual. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/specimen_collection_year_3.pdf.
- 39.Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789:393–404. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 40.Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72:4127–34. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- 41.Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr, Herbert AR, Samandar E, Needham LL, Calafat AM. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:161–7. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 42.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- 43.Johnson C, Paulose-Ram R, Ogden C, Carroll M, Kruszon-Moran D, Dohrmann S, Curtin L. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;2(161) [PubMed] [Google Scholar]
- 44.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans, 2005. 6th. Washington, DC: U.S. Government Printing Office; Jan, 2005. [Google Scholar]
- 45.Ito Y, Nakajima T. PPARalpha- and DEHP-Induced Cancers. PPAR Res. 2008;2008:759716. doi: 10.1155/2008/759716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakajima T, Hopf N, Schulte P. Di(2-ethylhexyl) phthalate (DEHP) IARC Monographs. 2000;77 [Google Scholar]
- 47.Occupational Safety & Health Administration. Di-(2-Ethylhexyl)phthalate. 2005 Available from: https://www.osha.gov/dts/chemicalsampling/data/CH_235155.html.
- 48.Gralow JR. Bone density in breast cancer: when to intervene? J Clin Oncol. 2007;25:3194–7. doi: 10.1200/JCO.2007.12.3430. [DOI] [PubMed] [Google Scholar]
- 49.Farvid M, Eliassen A, Cho E, Liao X, Chen W, Willett W. Dietary Fiber Intake in Young Adults and Breast Cancer Risk. Pediatrics. 2016;137:e20151226. doi: 10.1542/peds.2015-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hukkanen J, Jacob P, 3rd, Peng M, Dempsey D, Benowitz NL. Effect of nicotine on cytochrome P450 1A2 activity. Br J Clin Pharmacol. 2011;72:836–8. doi: 10.1111/j.1365-2125.2011.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Weide J, Steijns LS, van Weelden MJ. The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics. 2003;13:169–72. doi: 10.1097/00008571-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Helander A, Curvall M. Comparison of Blood Aldehyde Dehydrogenase Activities in Moist Snuff Users, Cigarette Smokers and Nontobacco Users. Alcoholism: Clinical and Experimental Research. 1991;15:1–6. doi: 10.1111/j.1530-0277.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 53.Quertemont E. Genetic polymorphism in ethanol metabolism: acetaldehyde contribution to alcohol abuse and alcoholism. Mol Psychiatry. 2004;9:570–81. doi: 10.1038/sj.mp.4001497. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Rodriguez-Monguio R. Racial disparities in the risk of developing obesity-related diseases: a cross-sectional study. Ethn Dis. 2012;22:308–16. [PubMed] [Google Scholar]
- 55.Diehl AK, Stern MP. Special health problems of Mexican-Americans: obesity, gallbladder disease, diabetes mellitus, and cardiovascular disease. Adv Intern Med. 1989;34:73–96. [PubMed] [Google Scholar]
- 56.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey 1988–1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, Albertson PC, Hamilton AS, Hunt WC, Potosky AL. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93:388–95. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 58.Schliep KC, Schisterman EF, Mumford SL, Perkins NJ, Ye A, Pollack AZ, Zhang C, Porucznik CA, VanDerslice JA, Stanford JB, Wactawski-Wende J. Validation of Different Instruments for Caffeine Measurement Among Premenopausal Women in the BioCycle Study. American Journal of Epidemiology. 2013;177:690–699. doi: 10.1093/aje/kws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawson D. National Institute on Alcohol Abuse and Alcoholism. Methodological Issues in Measuring Alcohol Use. 2003 [PMC free article] [PubMed] [Google Scholar]
- 60.West SL, Savitz DA, Koch G, Sheff KL, Strom BL, Guess HA, Hartzema AG. Demographics, health behaviors, and past drug use as predictors of recall accuracy for previous prescription medication use. Journal of Clinical Epidemiology. 1997;50:975–980. doi: 10.1016/s0895-4356(97)00026-7. [DOI] [PubMed] [Google Scholar]
- 61.Lacasse A, Ware M, Bourgault P, Lanctot H, Dorais M, Boulanger A, Cloutier C, Shir Y, Choiniere M. Accuracy of Self-reported Prescribed Analgesic Medication Use: Linkage Between the Quebec Pain Registry and the Quebec Administrative Prescription Claims Databases. Clinical Journal of Pain. 2016;32:95–102. doi: 10.1097/AJP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 62.Starling AP, Engel LS, Calafat AM, Koutros S, Satagopan JM, Yang G, Matthews CE, Cai Q, Buckley JP, Ji BT, Cai H, Chow WH, Zheng W, Gao YT, Rothman N, Xiang YB, Shu XO. Predictors and long-term reproducibility of urinary phthalate metabolites in middle-aged men and women living in urban Shanghai. Environ Int. 2015;84:94–106. doi: 10.1016/j.envint.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou J-W, Lin C-L, Tsai Y-A, Chang C-H, Liao K-W, Yu C-J, Yang W, Lee M-J, Huang P-C, Sun C-W, Wang Y-H, Lin F-R, Wu W-C, Lee M-C, Pan W-H, Chen B-H, Wu M-T, Chen C-C, Wang S-L, Lee C-C, Hsiung CA, Chen ML. The effects of phthalate and nonylphenol exposure on body size and secondary sexual characteristics during puberty. International Journal of Hygiene and Environmental Health. 2015;218:603–615. doi: 10.1016/j.ijheh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. International Journal of Hygiene and Environmental Health. 2015;218:220–231. doi: 10.1016/j.ijheh.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Buckley JP, Engel SM, Braun JM, Whyatt RM, Daniels JL, Mendez MA, Richardson DB, Xu Y, Calafat AM, Wolff MS, Lanphear BP, Herring AH, Rundle AG. Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. EPIDEMIOLOGY. 2016;27:449–458. doi: 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–51. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–8. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environment International. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


