Abstract
We compared the effects of bedroom-intensity light from a standard fluorescent and a blue- (i.e., short-wavelength) depleted LED source on melatonin suppression, alertness, and sleep. Sixteen healthy participants (8 females) completed a 4-day inpatient study. Participants were exposed to blue-depleted circadian-sensitive (C-LED) light and a standard fluorescent light (FL, 4100K) of equal illuminance (50 lux) for 8 h prior to a fixed bedtime on two separate days in a within-subject, randomized, cross-over design. Each light exposure day was preceded by a dim light (<3 lux) control at the same time 24 hours earlier. Compared to the FL condition, control-adjusted melatonin suppression was significantly reduced. Although subjective sleepiness was not different between the two light conditions, auditory reaction times were significantly slower under C-LED conditions compared to FL 30 minutes prior to bedtime. EEG-based correlates of alertness corroborated the reduced alertness under C-LED conditions as shown by significantly increased EEG spectral power in the delta-theta (0.5–8.0 Hz) bands under C-LED as compared to FL exposure. There was no significant difference in total sleep time (TST), sleep efficiency (SE%), and slow-wave activity (SWA) between the two conditions. Unlike melatonin suppression and alertness, a significant order effect was observed on all three sleep variables, however. Individuals who received C-LED first and then FL had increased TST, SE% and SWA averaged across both nights compared to individuals who received FL first and then C-LED. These data show that the spectral characteristics of light can be fine-tuned to attenuate non-visual responses to light in humans.
Keywords: Light, spectrum, melatonin, sleep, alertness, circadian
1. INTRODUCTION
Light is a direct stimulant which increases brain activation and alertness (21) and impedes the ability to fall asleep and reduces sleep quality (6). Evening light suppresses the pineal hormone melatonin, the biochemical signal of darkness (31, 39). Ordinary room light (i.e., 100 lux or less) can induce these effects (5, 12, 39). These ‘non-visual’ effects of light are mediated primarily by the non-rod, non-cone melanopsin-containing photoreceptors located in intrinsically photosensitive retinal ganglion cells (ipRGCs) and are maximally sensitive to short-wavelength blue light (λmax 480 nm) (1, 4, 16, 17, 33). We and others have shown that filtering out short-wavelength light from high intensity (>1000 lux) broad-spectrum fluorescent white light can prevent melatonin suppression (14, 23, 24, 28, 29, 34), but whether this approach works under bedroom-light intensity [<100 lux (2, 30)] is not known. The relative contribution of ipRGCs is less under dim lighting (13); therefore, targeted reduction of short-wavelength light may be less effective under dim light conditions.
The effects of high CCT (blue-enriched lighting) and low CCT (blue-depleted) fluorescent lighting at bedroom-light intensity (40 lux) on melatonin suppression and alertness has been compared in one prior study (7). Since the light sources were fluorescent, they contained several peaks in the short-wavelength region (380–500 nm), even when blue-depleted (7), however. Using an LED source would enable more specific removal of these peaks in the short-wavelength region of the visible spectrum, which may further enhance the attenuation in melatonin suppression.
The effects of a custom-designed blue-depleted LED source on melatonin suppression, alertness and sleep has been compared in a comprehensive study with various different light sources varying in spectral composition and intensity (27), but light intensity for the blue-depleted condition (~240 lux) was higher than typical bedroom or pre-bedtime intensities (2, 30). Therefore, in the current study, we compared the effects of bedroom-intensity (50 lux) light from a standard fluorescent and a blue-depleted LED source on melatonin suppression, alertness, and sleep. We hypothesized that an 8-h evening light exposure with short wavelengths selectively reduced (C-LED; a novel proprietary circadian photobiology-informed Light Emitting Diode) would cause less pre-sleep melatonin suppression than a commercially available fluorescent (FL) source. Moreover, we explored the effects of C-LED exposure on subjective and objective sleepiness prior to bedtime and nocturnal sleep.
2. MATERIALS AND METHODS
2.1. Participants
Sixteen healthy participants [8 females; mean age (± SD): 24.2 ± 3.0 years)] were studied in the Intensive Physiological Monitoring (IPM) Unit in the Center for Clinical Investigation at Brigham and Women’s Hospital. The study was approved by the Partners Human Research Committee, and participants provided written informed consent. Clinical Trial Registration Number: NCT01586039. All had comprehensive but unremarkable physical, psychological and ophthalmologic exams, including a negative Ishihara color blindness test. For at least 3 weeks prior to admission, participants maintained a self-selected, constant 8-h sleep/rest/dark schedule confirmed via a time- and date-stamped voicemail at bedtime and wake time and with actigraphy for at least 1 week prior to admission. Participants were asked to refrain from prescription and nonprescription medications, supplements, recreational drugs, caffeine, alcohol, and nicotine. Compliance was verified by urine and blood toxicology during screening and urine toxicology upon admission.
2.2. Protocol Design
Participants were studied in an environment free of time cues for 6 days, the first 4 of which are described herein (Figure 1A). The last two days of the protocol constituted an additional unrelated study. All data collection relevant to the current study was completed before the start of the second study. After admission ~4 h after waketime on Day 1, dim light (<3 lux) exposure began 8 h after wake time and an initial dim light melatonin onset (DLMO) assessment performed. An 8-h sleep episode was scheduled based on the average 7 days prior to admission. Participants were randomized shortly after admission to receive either FL or blue-depleted C-LED light of equal illuminance for 8 h prior to bedtime on Day 2. On Day 3, participants completed another pre-bedtime dim light exposure identical to Day 1, followed by exposure to the alternative light on Day 4.
Figure 1. Study protocol, spectral characteristics of experimental lighting and representative melatonin profiles.
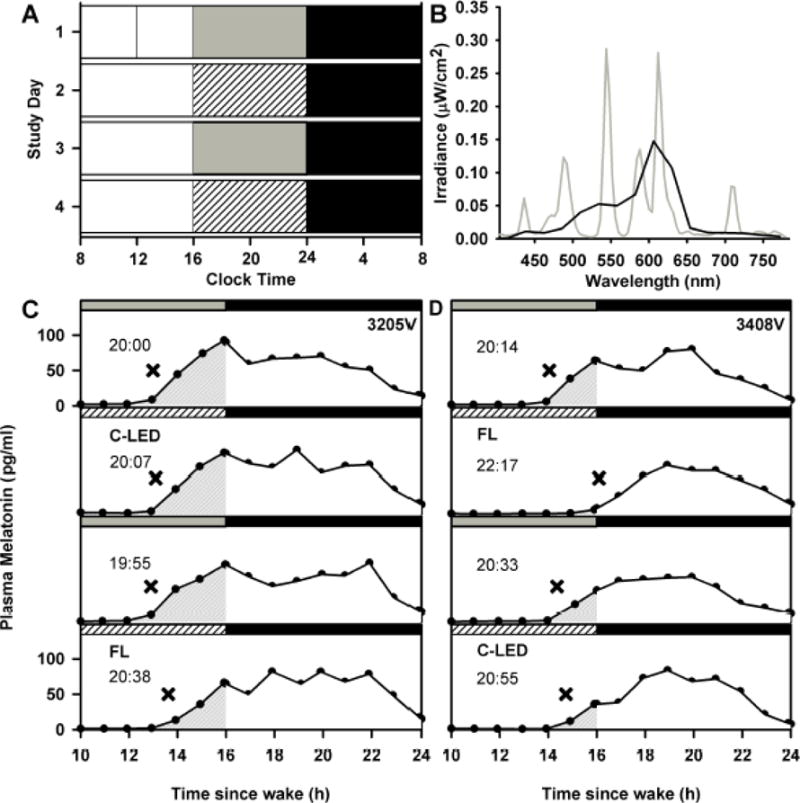
Representative study raster of a participant maintaining a sleep schedule from midnight to 0800 h (A). White bars represent awake under ambient ~90 lux fluorescent light; gray bars represent awake under ambient dim (<3 lux) light; black bars represent sleep in darkness; white hashed bars represent experimental light exposure. Spectral irradiance profile of the FL (gray line) and C-LED (black line) sources (B). Data were collected in 4 nm bins and are expressed as averages in 24 nm bins for the C-LED. Representative 14 h melatonin profiles are shown from participants 3205V (C) and 3408V (D) across the four consecutive study days. Shaded areas show the area under the curve. For each condition, the time (EST) at which the melatonin profile crossed the DLMO threshold is reported (hh:mm) and marked as X.
2.3. Study Lighting Conditions
During the first 8 h of each wake episode, maximum ambient light was ~190 lux (48 μW/cm2) when measured in the horizontal plane at 187 cm and ~88 lux (23 μW/cm2) in the vertical plane at 137 cm. From midway through days 1 and 3 until bedtime, maximum ambient light was decreased to <3 lux (0.4 μW/cm2, ~1.5 lux) in the horizontal plane at 187 cm and <1 lux (0.1 μW/cm2) in the vertical plane at 137 cm). Ambient lighting was generated using ceiling-mounted 4100K fluorescent lamps (F96T12/41U/HO/EW, 95W; F32T8/ADV841/A, 32W; F25T8/TL841, 25W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA, USA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA, USA).
For 8 hours from midway through days 2 and 4 until bedtime, participants were exposed to light from the FL or C-LED sources in randomized balanced order. We chose an eight hour light exposure duration to ensure that all individuals started their light exposure prior to dim light melatonin onset. Ceiling lights were turned off. During the first 2 hours, participants were ambulatory but restricted to a 5 m2 (~50 sq. ft.) central area within the 21 m2 (~225 ft2) suite, which allowed a maximum corneal illuminance of 50 lux in the vertical plane from the center of the wall-mounted lighting units. During the next 6 h, all participants remained in a constant seated posture (CP) in the same location with continuous staff supervision.
The FL and C-LED lighting were emitted from identical light box units (24 × 24 × 12 in.) The FL lamps were 4100K commercially-available U-bent lamps (FB32T8/TL741/6; Philips Lighting, The Netherlands) with digital ballasts (GE232MAX90-V60 Ultra Max High Efficiency Electronic Ballast, General Electric Corp., Fairfield, CT, USA). The C-LED units were provided by Lighting Science Group Corporation (Satellite Beach, FL, USA). Four units of each light type were placed in a north, south, east, and west orientation in the room, and remained throughout the study.
Neutral density filters (Rosco Laboratories Inc., Stamford, CT, USA) were used to achieve an average room illuminance of 28.6 ± 2.2 lux (FL) and 28.9 ± 2.2 lux (C-LED) (average from 64 readings at a height of 54 in. in the horizontal plane across the entire room), which ensured a maximum corneal exposure of 50 lux in the horizontal plane (91 cm from the lamp, 137 cm from the ground, oriented directly to the center of the lamp) while participants were ambulatory but restricted to their ~5 m2 area. During the 6 h CP, participants were seated ~91 cm from the light source, adjusted for each individual to receive a corneal illumination of 50 lux in the vertical plane [FL: 48.4 ± 0.6 lux (13.2 ± 0.2 μW/cm2); C-LED: 48.9 ± 0.5 lux (13.5 ± 0.2 μW/cm2)]. Participants were not told which units were being used. The lamps were turned on and off automatically at preset times (SmartLinc device-automation module, Insteon Inc., Irvine, CA, USA) based on each individual’s inpatient schedule. The lamps were turned on 2 minutes prior to the scheduled start time and before the overhead lights were turned off to ensure that participants did not receive a dark pulse.
Routine illuminance/irradiance measures were conducted using an IL1400 radiometer/powermeter with an SEL-033/Y/W or SEL-033/F/W detector, respectively (International Light, Inc., Newburyport, MA, USA). Spectrophotometry recordings were made using a PR-650 SpectraScan Colorimeter (CR-650, PhotoResearch Inc., Chatsworth, CA, USA) 91 cm from the lamp at 137 cm from the ground oriented directly to the center of the lamp.
Spectral readings for all light sources and conditions were analyzed using the Toolbox for calculating melanopic lux as provided by Lucas et al. (18). Table 1 shows the spectral characteristics of the three light sources.
Table 1.
Spectral characteristics of the light sources studied.
| Radiometric and Photometric Values (380–780 nm inclusive) | Retinal Photopigment Weighted Illuminances (α-opic lux)47 | |||||||
|---|---|---|---|---|---|---|---|---|
| Photon Flux (photons/cm2/s) | Irradiance (μW/cm2) | Photopic Illuminance (lux) | S Cone | Melanopsin ipRGC | Rod | M Cone | L Cone | |
| FL (Experimental) | 4.29 × 1013 | 14.82 | 49.37 | 17.18 | 31.18 | 35.35 | 43.79 | 48.82 |
| C-LED (Experimental) | 3.93 × 1013 | 13.26 | 47.65 | 6.73 | 18.59 | 24.35 | 36.82 | 48.12 |
| FL (Dim light) | 1.30 × 1012 | 0.46 | 1.45 | 0.63 | 1.01 | 1.13 | 1.32 | 1.42 |
| FL (Ambient) | 1.81 × 1014 | 64.71 | 214.77 | 136.49 | 147.22 | 168.00 | 194.16 | 208.56 |
Fluorescent (FL) and circadian-sensitive LED (C-LED) lights were measured 91 cm from the lamp, at 137 cm from the ground in the vertical plane, oriented directly to the center of the lamp. Overhead fluorescent lamps used to generate ambient and dim lighting conditions were measured at a height of 187 cm from the ground in the horizontal plane. Refer to text for lamp details.
2.4. Farnworth-Munsell 100 hue chromatic discriminaition
The Farnsworth-Munsell 100-Hue test was administered as per the manufacturer’s instructions (Richmond Products, Albuquerque, NM, USA). Briefly, subjects were presented with colored caps in a random order and were instructed to sort the caps in order to minimize the difference in hue between neighboring caps, starting from a fixed anchor cap (11). Four trays of colored caps were administered binocularly under each light condition, and standard scoring procedures were followed to compute the total error score (TES) (11, 15).
2.5. Sleepiness and Performance Assessments
Subjective sleepiness was assessed using an auditory version of the Karolinska Sleepiness Scale (KSS), a 9-point scale from 1-“very alert” to 9-“very sleepy, fighting sleep.” Objective performance was measured with an auditory 10-minute psychomotor vigilance task (PVT-10A), during which the participant was instructed to respond via button press as soon as possible to an auditory signal presented at random intervals (1–9 seconds). The KSS and PVT-10A were administered every 60 min throughout the 16-h wake episode starting 2.5 h after wake (21).
2.6. Sleep and Waking EEG Recordings
Polysomnography [electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), and a 2-lead electrocardiogram (ECG)] was recorded continuously during scheduled sleep using a portable, modular, battery-operated, ambulatory, digital polysomnographic recorder (Vitaport-3 digital recorder, TEMEC Instruments B.V., Kerkrade, The Netherlands) using the International 10–20 System for electrode placement with linked mastoid references (Mx). Wake EEG signals included the frontal (Fz,) central (Cz), parietal (Pz) and occipetal (Oz) derivations. Sleep EEG signals included the central 3 (C3), and central 4 (C4) derivations. All EEG signals were high-pass filtered (time constant: 1.0 seconds), low-pass filtered (−6 dB at 30 Hz, 12 dB/octave), and digitized (resolution: 12-bit, sampling rate: 256 Hz, storage rate: 256 Hz). The raw signals were stored on a Flash RAM Card (SanDisk, Sunnyvale, CA, USA) and downloaded off-line. Electrode impedances were checked using an OhmMate impedance meter (TEMEC Instruments B.V., Kerkrade, The Netherlands) at the beginning and end of each sleep episode. Electrode impedances were documented, and applications repeated until the impedances were all <10 kΩ. Participants were asked to complete the Karolinska Drowsiness Test (KDT) hourly between the KSS and PVT-10A and instructed to relax and fixate on a 2.0-in black dot at a distance of ~39 in for 3 minutes with their eyes open. For KDTs during the light exposure, participants were asked to focus on the center of the illuminated surface at a distance of ~36 in.
2.7. Melatonin Assays
Plasma melatonin was collected hourly starting 8 h before bedtime on Day 1 until study end. Salivary melatonin was collected hourly for 8 h before bedtime on all days. Melatonin concentration was determined by double-antibody radioimmunoassay (Specialty Assay Research Core Laboratory, Brigham and Women’s Hospital, Boston, MA, USA). The plasma melatonin inter-assay coefficient of variation (%CV) was 16.2% at 2.3 pg/mL and 7.2% at 18.7 pg/mL, and the intra-assay %CV was 14.0% at 1.6 pg/mL and 4.5% at 19.5 pg/mL. The saliva melatonin inter-assay coefficient of variation (%CV) was 6.6% at 9.00 pg/mL and 8.4% at 25.6 pg/mL, and the intra-assay %CV was 4.1% at 3.6 pg/mL and 4.8% at 24.4 pg/mL.
2.8. Data Analysis and Statistics
All analyses and graphical presentations were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA) and SigmaPlot for Windows Version 11.0 (Systat Software Inc., San Jose, CA, USA), respectively. Outcome measures as described below were compared between C-LED and FL conditions using linear mixed model analysis with duration, spectral condition or EEG frequency as fixed effects, as appropriate and subject as the random effect. Two additional fixed effects, order and sequence were included in all analyses to account for the (1) order in which the light conditions were presented (C-LED/FL vs. FL/C-LED on the second and fourth nights, respectively) and the chronological sequence of light condition (day in the study).
Melatonin secretion was compared between the C-LED and FL condition using three different outcome measures, which included (1) the time course of melatonin secretion (2) total secretion during the 6-h CP calculated as the area under the curve (AUC), and (3) suppression, which was calculated as the difference in the AUC during the 6-h CP interval of the light exposure adjusted to the corresponding dim-light control interval 24 h earlier.
Alertness under the different lighting conditions was assessed using three different outcome measures, which included (1) subjective sleepiness, (2) PVT reaction times, and (3) EEG power density derived from Fz-, Cz-, Pz-, and Oz-Mx during the 6 hours of constant posture under the C-LED and FL conditions and expressed relative (percentage) to the power density assessed 30 minutes before bedtime under the first dim-light control condition (21).
The effects on sleep were assessed using three outcome measures, which included (1) total sleep time (TST), (2) % sleep efficiency (TST relative to the total time in bed), and (3) slow wave activity (power density in the 0.5–4.5 Hz band) derived from C3-, and C4-Mx during non-rapid eye movement (NREM) sleep during the first 90 minutes of sleep.
Saliva was assayed for 2/16 participants (31A2V, 3407V) when no blood was available. The same medium (plasma or saliva) was used for all analyses within an individual. Cognitive test batteries were missed once during the C-LED and once during the dim-light control in the same participant (31A2V) due to schedule interruption caused by IV failure (0.01% data loss). One additional (3373V) participant had a late sleep onset due to a technical error after the C-LED, and PSG data from that sleep episode was removed (1.56% data loss).
3. RESULTS
3.1. FM-100 Chromatic Discrimination
Binocular color discrimination was significantly worse under C-LED compared to FL [Farnsworth Munsell D-100 (11) median (±SD) score, FL: 42 ± 52.3; C-LED: 96 ± 57.5 units; p<0.05 Mann-Whitney Test], although both median scores were within average range for color discrimination ability (11, 25).
3.2. Melatonin Suppression
The mean melatonin profiles showed significant (p<0.01) increase in melatonin levels with time under the dim-control, C-LED and FL condition. Levels were highest under dim light conditions followed by C-LED and then FL (Figure 2A) (p<0.01). The mean melatonin AUC was significantly (p<0.05) higher under C-LED (72.4 ± 19.9 pg/ml) compared to FL (41.4 ± 7.7 pg/ml) (Figure 2B). Moreover, melatonin suppression was significantly (p<0.01) reduced under C-LED (C-LED: 24.6 ± 10.3%) compared to FL (56.5 ± 4.9%;) (Figure 2C). Individual data on melatonin suppression are presented in Table 2. Post-hoc analyses showed that the 6-h constant posture interval during light exposure started between −5.7 to −0.9 h prior to DLMO [mean (± SD) for FL: −2.8 ± 1.0 h; C-LED: −3.0 ± 1.1 h]. There was no significant effect of exposure order on any of these three outcome measures.
Figure 2. Effects of spectral tuning on pre-bed melatonin suppression.
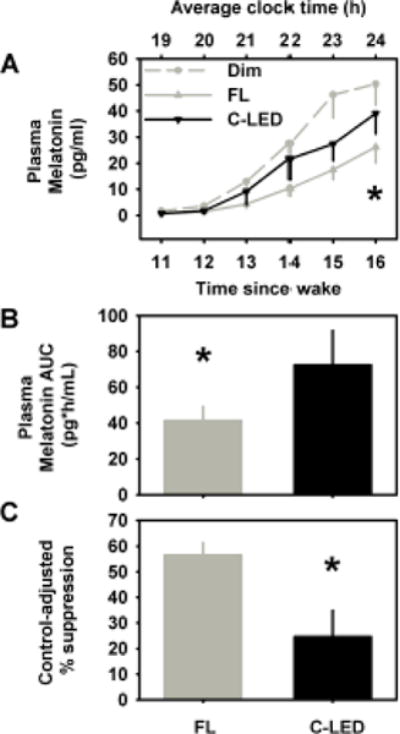
Melatonin secretion related outcome measures included the time course of melatonin secretion profiles during the 6-h constant posture interval under C-LED (black line), FL (gray line) and dim light (gray dashed line) (A), melatonin area under the curve (AUC) secretion during the 6-h CP under C-LED and FL conditions (B), and melatonin suppression calculated as the difference in the AUC during the 6-h CP interval of the light exposure adjusted to the corresponding dim-light control interval 24 h earlier (C). Data are expressed as group means ± SEM. * p<0.05; between lighting conditions.
Table 2.
Individual data for melatonin suppression under the different lighting conditions.
| FL | C-LED | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | ORDER OF EXPOSURE | Recovery to DLMO (hh:mm) | Time to recovery to DLMO (h)* | AUC | Control adjusted Δ in AUC (%)‡ | Clock Time of Recovery to DLMO (hh:mm) | Time to recovery to DLMO (h)* | AUC | Control adjusted Δ in AUC (%)‡ |
| 30J3V | FL/C-LED | 19:47 | −0.62 | 74.05 | 42.10 | 19:25 | −0.12 | 98.72 | 29.59 |
| 3113V | FL/C-LED | 20:36 | −0.20 | 109.15 | 37.80 | 20:32 | −0.90 | 103.79 | 45.47 |
| 31A2V | C-LED/FL | 20:40 | −0.87 | 37.07 | 12.23 | 20:25 | 0.18 | 46.91 | −19.03 |
| 3205V | C-LED/FL | 20:12 | −0.88 | 92.18 | 44.85 | 19:57 | −0.59 | 159.01 | 11.96 |
| 3308V | FL/C-LED | 18:39 | −0.19 | 93.87 | 30.05 | 18:23 | −0.14 | 177.18 | −3.00 |
| 3358V | FL/C-LED | 20:34 | −0.28 | 84.30 | 40.66 | 20:54 | −0.68 | 65.40 | 56.78 |
| 3363V | C-LED/FL | 00:16 | −0.41 | 13.31 | 23.73 | 00:35 | 0.30 | 9.42 | −13.57 |
| 3368V | FL/C-LED | 20:06 | −1.32 | 88.08 | 84.48 | 19:51 | −0.49 | 301.12 | 19.53 |
| 3373V | C-LED/FL | 21:02 | −0.63 | 78.04 | 33.84 | 20:38 | −0.11 | 99.16 | 9.36 |
| 3374V | C-LED/FL | 21:35 | −0.95 | 19.81 | 69.88 | 21:23 | −0.31 | 26.55 | 51.82 |
| 3380V | FL/C-LED | 21:41 | −1.39 | 20.14 | 71.68 | 21:45 | −0.63 | 15.81 | 62.13 |
| 3381V | C-LED/FL | 00:02 | −1.91 | 12.09 | 79.99 | 23:01 | −0.94 | 26.34 | 54.02 |
| 3383V | FL/C-LED | 23:59 | −2.16 | 8.53 | 78.04 | 23:22 | −1.64 | 10.35 | 74.62 |
| 3402V | FL/C-LED | 21:36 | −1.18 | 58.75 | 59.33 | 21:26 | −0.23 | 69.38 | 11.97 |
| 3407V | C-LED/FL | 21:45 | −2.19 | 17.85 | 76.31 | 21:28 | −1.58 | 23.84 | 71.44 |
| 3408V | C-LED/FL | 21:49 | −1.52 | 8.06 | 84.04 | 20:27 | −0.54 | 38.01 | 56.69 |
| Average | 21:24 | −1.04 | 50.96 | 54.31 | 21:06 | −0.53 | 79.44 | 32.49 | |
| SEM | 0:23 | 0.17 | 9.25 | 5.94 | 0:23 | 0.14 | 19.57 | 7.62 | |
A positive value signifies a delayed time-to-recovery to DLMO threshold relative to the corresponding dim-light control condition.
A positive value signifies suppression.
3.3. Sleepiness and Cognitive Performance
Subjective sleepiness was not different (p=0.96) between conditions (Figure 3A), whereas reaction times on the auditory psychomotor vigilance test was significantly (p<0.01) slower under the C-LED compared to the FL condition (Figure 3B). EEG power density profiles for all four derivations (Fz, Cz, Pz, Oz) were significantly (p<0.01) different between the two lighting conditions (Figure 3C–F). Compared to C-LED, delta-theta (0.5–8.0 Hz) bands were significantly increased under C-LED compared to FL conditions, consistently across all four EEG channels that were measured (Figure 3C–F). There was no significant effect of exposure order on any of these three outcome measures.
Figure 3. Effects of spectral tuning on pre-bed alertness and sleep.
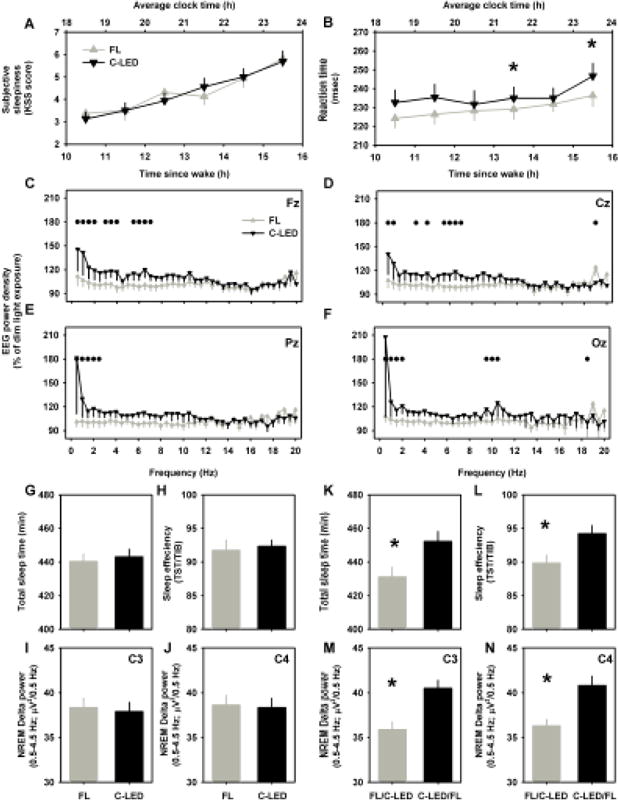
Pre-bedtime alertness was assessed using subjective sleepiness (A), reaction time on a 10-min psychomotor vigilance task (B), and EEG spectral profiles from Fz- (C), Cz- (D), Pz- (E) and Oz- (F) derivations. Sleep related outcome measures were assessed based on the lighting condition (C-LED vs. FL; G, H, I, J) and based on the order of presentation of the lighting condition (C-LED/FL vs. FL/C-LED; K, L, M, N). Sleep related outcome measures included total sleep time (G, K), % sleep efficiency [(TST/TIB)*100] (H, L), and slow wave activity during the first 90-min of non-rapid eye movement sleep calculated from the C3- (I, M) and C4- (J, N) derivations. Data are expressed as group means ± SEM. * and ● p<0.05 between lighting conditions.
3.4. Sleep Structure
A statistically significant effect of lighting condition was not observed on any of the three sleep related outcome measures (Figure 3G–J). The order of the light exposure i.e., C-LED/FL vs. FL/C-LED did significantly affect all three outcome measures, however (Figure 3K–N). Compared to the two-night average of the FL/C-LED order of presentation there was a significant increase in TST (p<0.05) by ~20 min (Figure 3K), a significant increase in sleep efficiency (p<0.05) by ~5% (Figure 3L), and a significant increase in SWA (p<0.05) by ~5% (Figure 3M and N) in the two-night average under the C-LED/FL order of presentation, after adjusting for inter-individual differences in phase angle of sleep and melatonin levels.
4. DISCUSSION
Our results support our hypothesis that exposure to a 50-lux C-LED white light source spectrally tuned to reduce melanopic lux specifically will attenuate melatonin suppression between DLMO and bedtime compared to a 50-lux standard FL white light source. These results are consistent with previous studies from ours and other laboratories that have reduced melatonin suppression as well as disruption in other circadian phase markers by either entirely or almost entirely filtering short wavelengths between 420–530 nm from white light (22, 23, 29, 35) or changing the color temperature of the light source (7, 9, 19) or designing LED lights with reduced blue light content (27). The current study is the first demonstration that a more selective approach by reducing a narrower range of short-wavelength light (~470–500 nm), which corresponds to the peak response range of melanopsin, effectively attenuates evening melatonin suppression in humans at typical indoor bedroom intensities. Additionally, the results support using selective blocking instead of entirely removing the blue portion of the visual spectrum, which can sustain normal visual function. This is critical for designing light systems that incorporate such technology because the color distortion caused by completely filtering all short wavelengths may render the technology impractical. We did find that color discrimination was significantly impaired under C-LED compared to the FL exposure, but within normal ranges under both conditions.
Our exploratory aims also showed differential effects of C-LED light, as compared to standard fluorescent light on alertness and sleep. Light has a strong short-wavelength sensitive alerting effect, improving cognitive performance (21, 26), enhancing EEG-based correlates of alertness (5, 21), and activating brain areas involved in arousal, executive function and mood [thalamus, hippocampus, amygdala (37)], during both the day and night. We did not observe differences in subjective sleepiness between the C-LED and FL conditions, despite differences in melatonin suppression. We did, however, find significantly slower RTs under the C-LED immediately before bedtime compared to the FL. Discrepancy between subjective and objective ratings of alertness have been reported previously (21, 36). Overall, the neurocognitive data suggest only a modest reduction in the alerting effects of the C-LED prior to bedtime in these well-rested individuals compared to the illuminance-matched FL.
We did not detect a statistically significant effect of light condition on sleep-related outcome measures, although receiving C-LED prior to FL condition was associated with significant improvements in TST, sleep efficiency and SWA averaged across both nights as compared to receiving FL before C-LED. This finding suggests that any potential disruptive effects of pre-bedtime fluorescent light exposure on sleep can carry over to subsequent nights. While the possible order effect observed on sleep related outcome measures may theoretically be due to differential phase delay shifts between the C-LED and FL condition, we did not observe an order effect on any of the melatonin-suppression related outcomes, suggesting a lack of differential phase shifts. It is also possible that sleep homeostasis was altered progressively throughout the study, independent of the experimental lighting intervention, which masked differences in sleep architecture that could be attributed solely to the experimental lighting interventions. Therefore, future studies with appropriate statistical power will need to explore this relationship further. Additional studies are required to characterize the effects of these two light conditions on sleep and to examine the time course of sleep disruption caused by pre-bedtime fluorescent light exposure across successive nights. Previous studies have reported either no difference in sleep architecture after receiving blue-enriched or blue-depleted light in the evening (20, 35) or reduced SWA up to several hours after the light offset (8, 20). Compared to dim light (~6 lux), bright light exposure (~2500 lux) prior to bed increases sleep latency but induces a modest reduction in SWA and no change in slow-wave sleep during subsequent sleep (3, 10), which may explain the lack of robust changes in sleep architecture following only a 50-lux exposure in the current study.
While further work is required to understand the precise spectral, dose and temporal relationships between melanopic lux and functional impact, exposure to bedroom-intensity white light increases alertness, likely disrupts sleep following the light exposure and shifts circadian rhythms. Therefore, the goal of the customized lighting intervention was to reduce melatonin suppression and reduce pre-sleep alertness to create a more appropriate environmental and physiological environment for sleep night after night. The ultimate reduction in pre-sleep light, i.e., darkness, can have a dramatic impact on sleep, circadian phase and circadian phase angle of entrainment in a relatively short time (32, 38). While the LED lighting used here was not the biological equivalent of darkness, melanopic lux was 67.7% higher for the FL (FL: 31.2 melanopic lux, C-LED 18.6 melanopic lux; Table 1), and therefore closer than the fluorescent source, as indicated in reduced melatonin suppression, whilst maintaining the same visual photopic lux (50 lux) and therefore practical for modern living when darkness is not convenient. It is important to note that the increasingly popular standard energy-efficient white LED lamps that have not been designed to reduce melanopic lux, are even more blue enriched than the fluorescent sources used in the current study and therefore would likely induce stronger responses. Pre-bedtime exposure to ~30 lux blue-enriched LED light from a personal electronic device for 5 consecutive nights induces significant melatonin suppression, ~1.5 h of circadian phase delay and significantly increases the time required to fall asleep, as compared to being exposed to dim light. We propose therefore that melanopic lux is taken into account when designing and comparing different light sources. Biologically-sensitive lighting may reduce the downside of industrialization on sleep and health.
Highlight.
Pre-bedtime light exposure disrupts sleep and circadian rhythms.
We tested whether these disruptions are attenuated under dim blue-depleted light.
Blue-depleted ambient light reduced melatonin suppression and alertness.
Acknowledgments
We thank Alicia Foote, Wendy Chan, research staff, and research participants at the Division of Sleep Medicine, BWH; the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at the Brigham and Women’s Hospital; Jonathan Williams M.D. for medical supervision; Core Laboratory staff (BWH) for melatonin assays; Robert Soler and Fred Maxik, Lighting Science Group Corporation for designing and providing the light sources.
Funding: This work was supported by an investigator-initiated grant from Biological Illuminations LLC, a subsidiary of Lighting Science Group Corporation (LSGC), who also provided the study lights. SAR and MSH were supported in part by NIH/NHLBI T32-HL007901. The project described was supported by Grant Number 1 UL1 TR 001102 and Grant Number 8 UL1 TR000170-05, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health.
Competing interests: SAR has received research funding from Government of Ontario/Pharmacia Canada Inc./ Genesis Research Foundation/OBGYN Graduate Scholarship in Science and Technology at the University of Toronto, Faculty of Medicine and the Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award from Canadian Institutes of Health Research, Standard Life (Canada) Inc.; SAR holds a patent for Prevention of Circadian Rhythm Disruption by Using Optical Filters and Improving sleep performance in subject exposed to light at night; SAR owns equity in Melcort Inc., and Circadian ZircLight Inc., Inc.; SAR is a co-investigator on studies sponsored by Biological Illuminations, LLC; Vanda Pharmaceuticals Inc. MSH has been a co-investigator on studies sponsored by Biological Illuminations, LLC and Philips HealthCare Solutions. SWL holds a consulting contract with Pegasus Capital Advisors which owns a stake in LSGC. The companies did not play any role in study design, data collection or data analysis. SWL holds current consulting contracts with Akili Interactive Labs, Inc.; Consumer Sleep Solutions; Delos Living LLC; Environmental Light Sciences LLC; Focal Point LLC; Headwaters Inc.; Hintsa Performance AG; Mental Workout; OpTerra Energy Services Inc; Pegasus Capital Advisors LP; PlanLED; and Wyle Integrated Science and Engineering. In the past 5 years, he has received consulting fees related to lighting work from Carbon Limiting Technologies Ltd for work conducted with PhotonStar LED. He has also received consulting fees from Naturebright and Thomas Jefferson University. He has received unrestricted equipment gifts from Biological Illuminations LLC; Bionetics Corporation; F. Lux Software LLC; and Philips Lighting. SWL receives royalties from Oxford University Press. In the past 5 years, SWL has received honoraria plus support for travel, accommodation or meals for invited seminars, conference presentations or teaching related to lighting from Brown University; Harvard University (CME); MediCom Worldwide, Inc (CME); North East Sleep Society; Portland General Electric; and Teague. SWL has completed investigator-initiated research grants from Alcon Inc, and Vanda Pharmaceuticals Inc., and has ongoing investigator-initiated research grants from Biological Illumination LLC, F. Lux Software, LLC; Philips Lighting, and Philips Respironics Inc. Dr. Lockley has served as a paid expert on in a legal case related to sleep and lighting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: a field study. Photochem Photobiol. 2014;90:723–726. doi: 10.1111/php.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cajochen C, Dijk DJ, Borbély AA. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 1992;15:337–343. [PubMed] [Google Scholar]
- 4.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 5.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 6.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chellappa SL, Steiner R, Oelhafen P, Lang D, Gotz T, Krebs J, Cajochen C. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013 doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- 9.Chellappa SL, Viola AU, Schmidt C, Bachmann V, Gabel V, Maire M, Reichert CF, Valomon A, Gotz T, Landolt HP, Cajochen C. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–437. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Cajochen C, Borbély AA. Effect of a single 3-hour exposure to bright light on core body temperature and sleep in humans. Neurosci Lett. 1991;121:59–62. doi: 10.1016/0304-3940(91)90649-e. [DOI] [PubMed] [Google Scholar]
- 11.Farnsworth D. Munsell Color Co Inc. Baltimore: Munsell Color Co Inc; 1957. The Farnsworth Munsell 100 Hue test for the examination of colour discrimination. [Google Scholar]
- 12.Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van RE, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayumov L, Casper RF, Hawa RJ, Perelman B, Chung SA, Sokalsky S, Shapiro CM. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J Clin Endocrinol Metab. 2005;90:2755–2761. doi: 10.1210/jc.2004-2062. [DOI] [PubMed] [Google Scholar]
- 15.Kinnear PR, Sahraie A. New Farnsworth-Munsell 100 hue test norms of normal observers for each year of age 5–22 and for age decades 30–70. Br J Ophthalmol. 2002;86:1408–1411. doi: 10.1136/bjo.86.12.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 17.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 18.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, Brainard GC. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita T, Tokura H. Effects of lights of different color temperature on the nocturnal changes in core temperature and melatonin in humans. Appl Human Sci. 1996;15:243–246. doi: 10.2114/jpa.15.243. [DOI] [PubMed] [Google Scholar]
- 20.Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1421–R1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 21.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman SA, Kollara A, Brown TJ, Casper RF. Selectively filtering short wavelengths attenuates the disruptive effects of nocturnal light on endocrine and molecular circadian phase markers in rats. Endocrinology. 2008;149:6125–6135. doi: 10.1210/en.2007-1742. [DOI] [PubMed] [Google Scholar]
- 23.Rahman SA, Marcu S, Shapiro CM, Brown TJ, Casper RF. Spectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposure. Am J Physiol. 2011;300:E518–527. doi: 10.1152/ajpendo.00597.2010. [DOI] [PubMed] [Google Scholar]
- 24.Rahman SA, Shapiro CM, Wang F, Ainlay H, Kazmi S, Brown TJ, Casper RF. Effects of filtering visual short wavelengths during nocturnal shiftwork on sleep and performance. Chronobiol Int. 2013;30:951–962. doi: 10.3109/07420528.2013.789894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigby HS, Warren BF, Diamond J, Carter C, Bradfield JW. Colour perception in pathologists: the Farnsworth-Munsell 100-hue test. J Clin Pathol. 1991;44:745–748. doi: 10.1136/jcp.44.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–R1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 27.Santhi N, Thorne HC, van der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJ, Archer SN, Dijk DJ. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2011;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasseville A, Benhaberou-Brun D, Fontaine C, Charon MC, Hebert M. Wearing blue-blockers in the morning could improve sleep of workers on a permanent night schedule: a pilot study. Chronobiol Int. 2009;26:913–925. doi: 10.1080/07420520903044398. [DOI] [PubMed] [Google Scholar]
- 29.Sasseville A, Paquet N, Sevigny J, Hebert M. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. J Pineal Res. 2006;41:73–78. doi: 10.1111/j.1600-079X.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheuermaier K, Laffan AM, Duffy JF. Light exposure patterns in healthy older and young adults. J Biol Rhythms. 2010;25:113–122. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 32.Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J, Wright KP., Jr Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Curr Biol. 2017;27:508–514. doi: 10.1016/j.cub.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Werken M, Gimenez MC, de Vries B, Beersma DG, Gordijn MC. Short-wavelength attenuated polychromatic white light during work at night: limited melatonin suppression without substantial decline of alertness. Chronobiol Int. 2013;30:843–854. doi: 10.3109/07420528.2013.773440. [DOI] [PubMed] [Google Scholar]
- 35.van der Lely S, Frey S, Garbazza C, Wirz-Justice A, Jenni OG, Steiner R, Wolf S, Cajochen C, Bromundt V, Schmidt C. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J Adolesc Health. 2015;56:113–119. doi: 10.1016/j.jadohealth.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 37.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009 doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


