Abstract
Chemokines are increasingly recognised as playing a role in depression. Here we meta-analyse the data on concentrations of all chemokines in patients diagnosed with a major depression versus healthy controls. We included studies which utilised Diagnostic and Statistical Manual (DSM)-IV diagnostic criteria for major depression, participants free from major medical conditions, studies with healthy controls, and unstimulated measurements of chemokines. We only included chemokines which had ≥3 studies performed. Two chemokines and 15 studies in total met criteria for this meta-analysis; 8 for Monocyte Chemotactic Protein (MCP)-1/CCL2 (n = 747), and 7 for Interleukin (IL)-8/CXCL8 (n = 560). There were significantly higher concentrations of CCL2/MCP-1 in depressed subjects compared with control subjects – overall mean difference of 36.43 pg/mL (95% CI: 2.43 to 70.42). There was significant heterogeneity across these studies (I2 = 98.5%). The estimates of mean difference between the control and depression groups did not remain significant when the trim-and-fill procedure was used to correct for publication bias. There was no significant difference in concentrations of IL-8/CXCL8 in depressed subjects compared with control subjects. Significant heterogeneity was found across these studies (I2 = 96.7%). The estimates of mean difference between the control and depression groups remained non-significant when the trim-and-fill procedure was used to correct for publication bias. This meta-analysis reports significantly heterogeneity in this field among studies. There are higher concentrations of the chemokine MCP-1/CCL2 in depressed subjects compared with control subjects, and no differences for IL-8/CXCL8. More high quality research and consistent methodologies are needed in this important area of enquiry.
Keywords: Chemokine, Depression, Diagnosis, Cytokine, Inflammation, Mood disorder, Meta-analysis
1. Introduction
Novel diagnostic and treatment strategies for depression are urgently needed. Recent global data suggests unipolar depression currently ranks 11th for disability adjusted life years, a 37% increase since 1990 (Murray et al., 2012). The burden is expected to continue to grow into the 21st century (Holtzheimer et al., 2008; Murray et al., 2012). Hence, this is an unprecedented burden of depressive illness requiring increased effort to find novel therapeutic agents for treatment (Licinio, 2011).
In the field of psychiatric immunology, much of the focus on the role of the immune system in depression has been placed on the innate immune response and inflammation. Innate immune cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β,IL-6 and interferon (IFN)-γ have been repeatedly shown to exert effects on key processes such as neuroplasticity, neurotransmission, oxidative stress and neuro-endocrinological functions that are considered to be central to the development of depression (Dantzer et al., 2008; Eyre and Baune, 2012; Haroon et al., 2012; McAfoose and Baune, 2009; Miller et al., 2009). The seminal meta-analysis (Dowlati et al., 2010) of 24 studies found significantly higher concentrations of the proinflammatory cytokines, TNF-alpha and IL-6, in depressed subjects compared with control subjects. An updated meta-analysis (Haapakoski et al., 2015) of IL-6, C-reactive protein and TNF-α found higher levels of IL-6 and CRP in depressed patients versus controls (29 studies for IL-6 and 20 for CRP). These studies strengthen evidence that depression is accompanied by activation of the inflammatory response system (Dowlati et al., 2010).
An involvement of immune factors in the pathophysiology of depression is now considered to be far greater than that of only the innate immune system, inflammation and glia (Eyre et al., 2015). Indeed, a complex interaction is suspected to occur in the CNS between parts of the innate and adaptive immune system (Ransohoff and Brown, 2012). For example, chemokines are increasingly believed to be involved in depression pathophysiology, likely through neuromodulatory and neurotransmitter-like effects, as well as regulation of neurogenesis and axon sprouting (Stuart and Baune, 2014; Stuart et al., 2015).
Recent advances in basic neuroscience have begun to describe novel roles for chemokines in neurobiological processes relevant to depression (Stuart and Baune, 2014; Stuart et al., 2015). In looking beyond traditional roles in chemotaxis of immune cells, these novel processes may include regulating the migration, proliferation, and differentiation of neural stem/progenitor cells; regulation of axon sprouting and elongation; regulating the infiltration and activation states of central and peripheral immune cells; control of blood–brain barrier permeability; regulation of neuroendocrine functions; pre- and post-synaptic modulation of traditional neurotransmitter systems; and possibly direct neurotransmitter-like effects (Reaux-Le Goazigo et al., 2013; Rostene et al., 2011a, 2011b, 2007). For systematic reviews from this group see (Stuart and Baune, 2014; Stuart et al., 2015). The disruption of these functions in vital neurodevelopmental periods or in later life may be mechanistically relevant to the pathogenesis and pathophysiology of depression, while restoration of homeostasis in these functions may be relevant to recovery (Stuart and Baune, 2014; Stuart et al., 2015). From a clinical study perspective, we are aware of a number of cross-sectional studies (monocyte chemoattractant protein (MCP)-1/CCL2 (Domenici et al., 2010; Grassi-Oliveira et al., 2012; Jonsdottir et al., 2009; Lehto et al., 2010; Shen et al., 2010; Suarez et al., 2004; Sutcigil et al., 2007) (Bai et al., 2014; Carvalho et al., 2014; Motivala et al., 2005; Piletz et al., 2009; Simon et al., 2008); macrophage inflammatory protein (MIP)-1α/CCL3 (Merendino et al., 2004; Suarez et al., 2004); CXCL1 (Lee et al., 2009; Merendino et al., 2004); IL-8/CXCL8 (Baune et al., 2012a; Domenici et al., 2010; Jonsdottir et al., 2009; Marsland et al., 2007; O'Brien et al., 2007; Podlipny et al., 2010; Suarez et al., 2004) (Carvalho et al., 2014; Hallberg et al., 2010; Hocaoglu et al., 2012; Lehto et al., 2010; Simon et al., 2008; Song et al., 1998); MCP-3 (Lee et al., 2009); TNF-β (Lee et al., 2009); IL-16 (Lee et al., 2009); CTACK (Lee et al., 2009); macrophages migration inhibitory factor (MIF) (Lee et al., 2009); CCL11 (Grassi-Oliveira et al., 2012; Magalhaes et al., 2014); CXCL11/I-TAC (Lu et al., 2013); MEC/CCL28 (Lu et al., 2013);TECK/CCL25 (Lu et al., 2013); Interferon gamma-induced protein (IP)-10/CXCL10 (Simon et al., 2008; Wong et al., 2008); RANTES/CCL5 (Grassi-Oliveira et al., 2012; Shen et al., 2010). There are only two prospective studies exploring associations between chemokines and depression (Baune et al., 2012b; Eller et al., 2008). In one study, the associations between serum CXCL8 (IL-8) and depressive symptoms were explored in a large cohort of population-based, elderly participants for two years (age 70–90 years) (Baune et al., 2012b). Results indicated that serum IL-8/CXCL8 was positively associated with depressive symptoms on the geriatric depression scale (GDS) at baseline (P = 0.025), at two years follow-up (P = 0.038), and an increase in depressive symptoms from baseline to two years (P = 0.021). Further prospective research, across age groups, is required to understand the role of chemokines as biomarkers and neurobiological factors in depression.
The association between chemokine dysfunction and depression has been documented in individual studies of various chemokines, however the association is not consistently significant in all studies or for all chemokines. Thus a generalizable pattern of chemokine dysfunction in depression remains to be defined. Fortunately the results from individual studies can be combined quantitatively using meta-analytical techniques to improve the strength of the evidence. Taken together, this study reports the results of a meta-analysis conducted to determine whether the concentrations of specific cytokines differs quantitatively between patients diagnosed with a major depressive episode and control subjects.
2. Methods and materials
2.1. Data sources
We (AP and JJ) searched Embase, PsycINFO, Ovid Medline, ScienceDirect, Google Scholar and the Cochrane Central Register of Controlled Trials database up to September 2015. We also manually scrutinized references cited in the systematically searched articles. To optimize sensitivity in searching clinical studies, we used the following basic terms: XCL*, CX3C*, CCL*, CXCL*, IL-*, MCP, scya, scyb, NAP, GCP, depression, major depressive disorder and depressive symptoms.
2.2. Study selection
Studies were selected for data extraction and analysis based on the following inclusion criteria: (a) original research studies measuring chemokine concentrations in depressed and non-depressed subjects; (b) subjects met DSM-IV criteria for major depression; (c) studies were in English; (d) participants were free from major medical co-morbidities (e.g. cancer, heart disease, arthritis); (e) psychiatrically healthy subjects were used as controls; (f) unstimulated chemokine analyses were used. We excluded studies including participants with stimulated chemokine-based analyses or non-serum/plasma markers.
2.3. Data extraction
Two independent reviewers (AP and JJ) used a custom data extraction template to summarize the selected articles. Abstracted information included age, gender, sample size, depression metrics, comorbidities, chemokines analysed, method and source, and concomitant drug use. Where possible, we also sought key data that were missing from the original reports through correspondence with the investigators.
2.4. Quality assessment
This meta-analysis was carried out according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines (Liberati et al., 2009). Weused the Newcastle-Ottawa Quality Assessment Scale (Hartling et al., 2013) for observational studies to assess quality; this scale is recommended by the Cochrane Collaboration. With this method, each study can obtain a maximum of 9 points in three categories: selection of study participants (adequate definition, validation and representativeness of cases and controls), comparability of cases and controls, and the ascertainment of exposure. For this study, our quality points for exposure were adapted from Haapakoski et al. (Haapakoski et al., 2015). These included method of assay, consistency of assay used, three or more immune markers analysed. Given the small number of studies in this field, we included all in our meta-analysis (see Table 1).
Table 1.
Quality assessment of studies included in the meta-analysis.
| Study | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|
| Song et al. (1998) | * | * | *** | 5 |
| Motivala et al. (2005) | *** | ** | ** | 7 |
| O'Brien et al. (2007) | ** | *** | 5 | |
| Sutcigil et al. (2007) | ** | * | *** | 6 |
| Simon et al. (2008) | ** | * | ** | 5 |
| Eller et al. (2008) | ** | * | *** | 6 |
| Jonsdottir et al. (2009) | ** | ** | *** | 7 |
| Piletz et al. (2009) | *** | * | ** | 6 |
| Lehto et al. (2010) | *** | * | *** | 7 |
| Hocaoglu et al. (2012) | ** | ** | *** | 7 |
| Bai et al. (2014) | ** | ** | *** | 7 |
| Carvalho et al. (2014) | *** | ** | ** | 7 |
2.5. Statistical analysis
Weighted mean differences between controls and cases were used to calculate the effect size (ES) of each study. A spreadsheet containing the extracted study data and the calculated ES was imported into Stata 12.0 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) to perform the additional analyses. Random-effects models were used to estimate the overall ES. We measured statistical heterogeneity using the I2 statistic for statistical variation across studies; values of 25% are low, 50% moderate and 75% high (Higgins and Flicker, 2003). In addition, we used the metareg command in Stata 12.0, to conduct random-effects meta-regression analyses to assess for the source of heterogeneity. Univariate models were used to examine for the effects for the following study characteristics: mean age of the sample, gender (% male) and assay type (CBA vs. ELISA). If 2 or more of these analyses were significant, multivariate meta-regressions were planned. The possibility of publication bias was assessed through a Begg funnel plot graph and testing for asymmetry using the Egger weighted regression test. The nonparametric “trim and fill” method was used to estimate the number of hypothetical studies that were missing due to possible publication bias using the metatrim command in STATA.
3. Results
3.1. Study inclusion
The utilization of the PRISMA guidelines and a systematic search of electronic databases and manual searching through literature reviews yielded a total of 504 studies. A total of 358 duplicates were removed leaving 146 studies. After reviewing titles and abstracts, 118 studies were again excluded. Twenty-eight studies were then examined via review of full-text articles. Thirteen studies were excluded given the following. Five studies were excluded as the chemokines analysed were not explored in ≥3 studies (Lee et al., 2009; Lu et al., 2013; Magalhaes et al., 2014; Merendino et al., 2004; Wong et al., 2008); 1 study was excluded due to a lack of control group (Marsland et al., 2007); 1 study was excluded as the authors did not respond to requests for data, and standardized means or standard deviations (SDs) were not available (Hallberg et al., 2010); 5 studies were excluded as they didn't include a DSM-IV-based clinician assessment of depression (Baune et al., 2012a; Grassi-Oliveira et al., 2012; Podlipny et al., 2010; Suarez et al., 2003, 2004); 1 study was excluded as genetic markers for chemokines were utilised (Domenici et al., 2010). Finally, 15 studies were included in the meta-analysis and these studies included only 2 chemokines, MCP-1/CCL2 and IL-8/CXCL8. See Fig. 1 and Tables 2, 3, 4 and 5 for details.
Fig. 1.
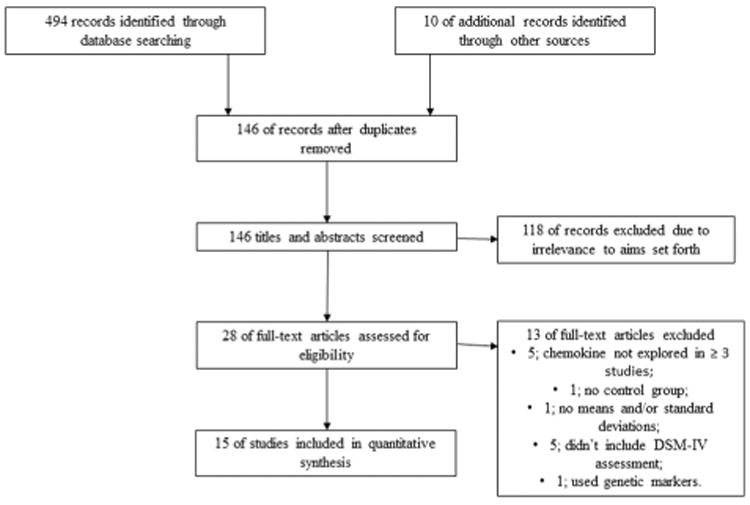
Study selection and inclusion process for meta-analyses.
Table 2.
Sociodemographic characteristics of included studies of looking at MCP-1/CCL2 concentrations in depression.
| Study/year | N (D, ND) | Gender (% male) (D, ND) | Age (D, ND) | Comorbidities |
|---|---|---|---|---|
| Motivala et al. (2005) | 40 (22/18) | 100%/100% | 44.4 ± 7.5 and 40.3 ± 9.1 | No underlying medical condition that might influence sleep disturbance or depression. No recent viral infections (past 10 days). No chronic medical conditions (diabetes mellitus, cancer, COPD). No hypertension or antihypertensives being taken. |
| Sutcigil et al. (2007) | 55 (30/25) | 52.2%/52% | 34.78 ± 7.42 and 34.32 ± 7.8 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Simon et al. (2008) | 98 (49/49) | 59.18%/57.14% | 41.65 ± 11.07 and 41.69 ± 11.28 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Piletz et al. (2009) | 39 (22/17) | 14%/17% | 39.4 ±1.9 and 39 ± 2.1 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Jonsdottir et al. (2009) | 84 (42/42) | 0/0 | 42.1 ± 9.4 and 42.7 ± 7.5 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Lehto et al. (2010) | 122 (61/61) | 31.3%/31.3% | 53.74 ± 1.19 and 53.77 ± 1.2 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Bai et al. (2014) | 235 (109/126) | 22.9%, 31% | 41.96 ± 13.8,41.88 ± 10.0 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Carvalho et al. (2014) | 89 (47/42) | 43%/50% | 54 (32–82) and 49 (31–74) | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
Abbreviations: D, depressed; ND, non-depressed; DSM, Diagnostic and Statistical Manual; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; SCID, Structured Clinical Interview for Depression; MADRS, Montgomery Asberg Depression Rating Scale; IL, interleukin; MCP, monocyte chemoattractant protein; DSSS, Depression and Somatic Symptom Scale.
Table 3.
Clinical characteristics of included studies of looking at MCP-1/CCL2 concentrations in depression.
| Study/year | Inpatient or outpatient | Depression diagnosis (scales) | Depression score (D/ND) | Duration of illness | Study type | Medication status | Assay: source, kit, fasting conditions, time of day |
|---|---|---|---|---|---|---|---|
| Motivala et al. (2005) | Outpatient | DSM-IV/SCID; HDRS-17 | 19.3 ± 4.2/1.2 ±1/3 | n/a | Cross-sectional | Nil | Serum; 9 pm; not fasting; ELISA. |
| Sutcigil et al. (2007) | Outpatients | DSM-IV/SCID; HDRS-17 | 28.39 ± 4.53/4.2 ±1.8 | n/a | Open label trial | Sertraline | Serum; fasting; 9 am; ELISA. |
| Simon et al. (2008) | Outpatient | DSM-IV/SCID/HDRS.17 | 19.3 ± 5.3/0 | 6.22 ± 8.81/0 | Cross-sectional | None for ≥1 wk | Serum; multiplex; time not given; unclear if fasted |
| Piletz et al. (2009) | Outpatient | DSM-IV/SCID; HDRS/21 | 26.2 ± 1.0/1.0 ± 0.4 | n/a | Open label trial | Venlafaxine | Serum; fasting; ELISA; time not given. |
| Jonsdottir et al. (2009) | Outpatient | DSM-IV/SCID; HAD | 32% had HDRS > 10 | n/a | Cross-sectional | 31% on antidepressants | Serum; before 9:30 am; fasting; multiplex. |
| Lehto et al. (2010) | Outpatient | DSM-IV/SCID; HDRS-29 | 14.79 ± 0.95/3.59 ± 0.40 (HDRS-29) | n/a | Cross-sectional | Mixed antidepressants | Serum; multiplex; 8 am; 12 h fasting; |
| Bai et al. (2014) | Unclear | DSM-IV/SCID; BDI-II/DSSS-22 | 27 ± 12.1 ± 3.3 ± 3.3 (BDI) | n/a | Cross-sectional | Mixed antidepressant | Serum; ELISA; fasting; time not given. |
| Carvalho et al. (2014) | Inpatient | DSM-IV/SCID; HDRS-17 | 24.4 (range 18–30)/0 | 1 episode (range 0–4)/o | Cross-sectional | Nil for ≥1 wk | Serum; 8–10 am; cytometric bead assay; unclear if fasted. |
Abbreviations: D, depressed; ND, non-depressed; DSM, Diagnostic and Statistical Manual; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; SCID, Structured Clinical Interview for Depression; MADRS, Montgomery Asberg Depression Rating Scale; IL, interleukin; MCP, monocyte chemoattractant protein; DSSS, Depression and Somatic Symptom Scale.
Table 4.
Sociodemographic characteristics of included studies of looking at IL-8/CXCL8 concentrations in depression.
| Study/year | N (D, ND) | Gender (% male) (D, ND) | Age, years (D, ND) | Comorbidities |
|---|---|---|---|---|
| Song et al. (1998) | 20 (6/14) | 33.3%/57% | 50.3 ± 15.3 and 45.5 ± 15.5 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| O'Brien et al. (2007) | 52 (28/24) | 32%/41.7% | 44.15 ± 13.20 and 35.58 ± 8.98 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Simon et al. (2008) | 98 (49/49) | 59.18%/57.14% | 41.65 ± 11.07 and 41.69 ± 11.28 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Eller et al. (2008) | 145 (100/45) | 35%/42.2% | 32.1 ± 11.9 and 32.9 ± 14.1 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Jonsdottir et al. (2009) | 84 (42/42) | 0/0 | 42.1 ± 9.4 and 42.7 ± 7.5 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Lehto et al. (2010) | 122 (61/61) | 31.3%/31.3% | 53.74 ± 1.19 and 53.77 ± 1.2 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
| Hocaoglu et al. (2012) | 60 (30/30) | 20%/53.3% | 38 ± 13 and 30 ± 9 | No endocrinological or general medical conditions (e.g. diabetes, heart disease). |
Abbreviations: D,depressed; ND, non-depressed; DSM, Diagnostic and Statistical Manual; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; HAD, Hospital Anxiety and Depression Scale; SCID, Structured Clinical Interview for Depression; MADRS, Montgomery Asberg Depression Rating Scale; IL, interleukin; MCP, monocyte chemoattractant protein.
Table 5.
Clinical characteristics of included studies of looking at IL-8/CXCL8 concentrations in depression.
| Study/year | Inpatient or outpatient | Depression diagnosis (scales) | Depression scale score, years(D/ND) | Duration of illness, months | Study type | Medication status | Assay: source, kit, fasting conditions, time of day |
|---|---|---|---|---|---|---|---|
| Song et al. (1998) | Not mentioned | DSM-IV/SCID | n/a | n/a | Cross-sectional | None for 6 wks prior | Serum; 7:45 am; fasting; ELISA |
| O'Brien et al. (2007) | Outpatient | DSM-IV/SCID; HDRS-17 | 25.44/0 | n/a | Cross-sectional | SSRI, SNRI, lithium + SSRI | Serum; 9–11 am; ELISA; unclear if fasted |
| Simon et al. (2008) | Outpatient | DSM-IV/SCID/HDRS-17 | 19.3 ± 5.3/0 | 6.22 ± 8.81/0 | Cross-sectional | None for ≥1 wk | Serum; multiplex; time not given; unclear if fasted |
| Eller et al. (2008) | Outpatient | DSM-IV/SCID; MADRS | 28.5 ± 5.9/0 | 10.8 ± 14.3 | Prospective | Citalopram | Serum; 9–11:30 am; enzyme labelled, chemiluminescent sequential immunometric assay |
| Jonsdottir et al. (2009) | Outpatient | DSM-IV/SCID; HAD | 32% had HDRS > 10 | n/a | Cross-sectional | Mixed antidepressants | Serum; before 9:30 am; fasting; multiplex. |
| Lehto et al. (2010) | Outpatient (population based sample) | DSM-IV/SCID; HDRS 29 | 14.79 ± 0.95/3.59 ± 0.40 (HDRS-29) | n/a | Cross-sectional | Mixed antidepressants | Serum; multiplex; 8 am; 12 h fasting; |
| Hocaoglu et al. (2012) | Outpatient | DSM-IV/SCID; HDRS-17 | n/a | n/a | Cross-sectional | Treatment as usual | Serum; fasting; no time given (morning); ELISA. |
Abbreviations: D,depressed; ND, non-depressed; DSM, Diagnostic and Statistical Manual; BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; HAD, Hospital Anxiety and Depression Scale; SCID, Structured Clinical Interview for Depression; MADRS, Montgomery Asberg Depression Rating Scale; IL, interleukin; MCP, monocyte chemoattractant protein.
3.2. Studies of MCP-1/CCL2
Eight studies, involving 747 participants, were included in the MCP-1/CCL2 meta-analysis. There were significantly higher concentrations of MCP-1/CCL2 in depressed subjects compared with control subjects with an overall mean difference of 36.43 pg/mL (95% CI: 2.43 to 70.42; P = 0.036) (see Fig. 2). There was significant heterogeneity across studies (I2 = 98.5%). Our meta-regression explored heterogeneity in included studies with regard to the mean age of the sample, gender (% male), and assay used (CBA vs ELISA). None of these variables significantly influenced our estimates of the ES (P = 0.599, P = 0.485, P = 0.603 respectively). Funnel plots showed evidence of asymmetry (not shown here), and there was evidence of bias using the Egger (weighted regression) method (P for bias = 0·05). The estimates of mean difference between the control and depression groups did not remain significant when the trim-and-fill procedure was used to correct for publication bias. Adjustment for publication bias according to Duval & Tweedie's trim and fill procedure resulted in a mean difference of −8.98 (95% CI −38.27–20.30; P = 0.548) with 4 studies imputed.
Fig. 2.
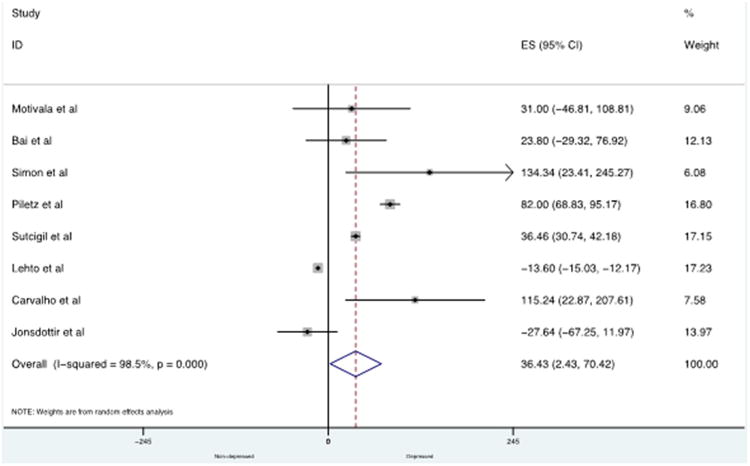
Forest plot showing individual and combined effect size estimates and 95% CIs for all trials in the analysis for MCP-1/CCL2.
3.3. Studies of IL-8/CXCL8
Seven studies, involving 560 participants, were included in the IL-8/CXCL8 meta-analysis. There was no significant difference in concentrations of IL-8/CXCL8 in depressed subjects compared with control subjects with an overall mean difference of −0.58 pg/mL (95% CI: −1.53 to 0.36, P = 0.228) (see Fig. 3). There was significant heterogeneity across studies (I2 = 96.7%). Our meta-regression explored heterogeneity in included studies with regard to the mean age of the sample, gender (% male), and assay used (CBA vs ELISA). None of these variables significantly influenced our estimates of the ES (P = 0.984, P = 0.374, P = 0.865 respectively). There was evidence of asymmetry in funnel plots (not shown here), however the Egger test (weighted regression) was not significant (P for bias = 0.69). The estimates of mean difference between the control and depression groups remained nonsignificant when the trim-and-fill procedure was used to correct for publication bias. Adjustment for publication bias according to Duval & Tweedie's trim and fill procedure resulted in a mean difference of −0.594 (95% CI −1.56–0.37; P = 0.227) with 9 studies imputed.
Fig. 3.
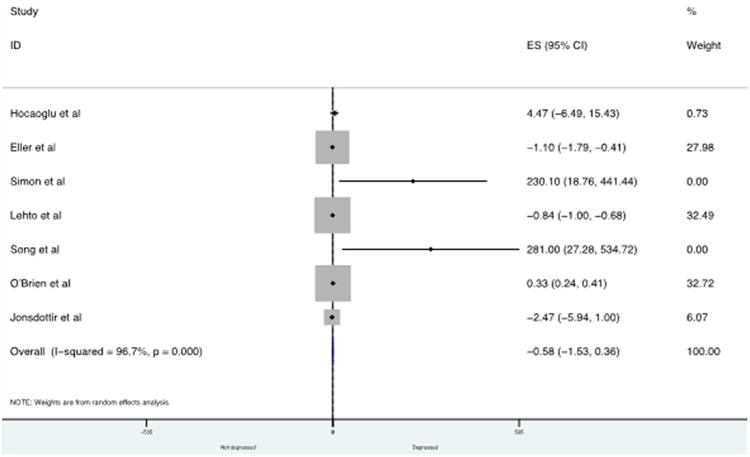
Forest plot showing individual and combined effect size estimates and 95% CIs for all trials in the analysis for IL-8/CXCL8.
4. Discussion
After a number of narrative and systematic reviews, this study is the first quantitative analysis of chemokines in depression. After exploring all chemokines, the meta-analysis was only possible for two, MCP-1/CCL2 and IL-8/CXCL8, given stringent inclusion and exclusion criteria. The analysis reports higher concentrations of MCP-1/CCL2 in depressed subjects compared with control subjects. It highlights the state of this field and outlines both positive and negative findings hence providing direction for future research.
MCP-1/CCL2 was found to be elevated in depressed vs non-depressed individuals, however significant heterogeneity was found and levels were non-significant when publication bias was corrected for. When considering the clinical significance of chemokines, it is important to also appreciate their background neurobiological functions and associated research initiatives. MCP-1/CCL2 is known for its pleiotrophic actions with functions in chemotaxis, activating function on monocytes/macrophages, T lymphocytes and dendritic cells and CNS-specific functions (Stuart and Baune, 2014). In the CNS, the functions are only beginning to be understood. MCP-1/CCL2 and its receptor (CCR2) are expressed on astrocytes, microglia, neurons and neural stem cells (Banisadr et al., 2005; Tran et al., 2007). The expression is under both basal conditions and upregulated in response to inflammatory cytokines (Stuart and Baune, 2014). MCP-1/CCL2 has been found in pre-clinical models to regulate the inflammatory activation state of CNS resident microglia (Hinojosa et al., 2011); neuromodulation in electrophysiological models (Melik-Parsadaniantz and Rostene, 2008); and mediating the migration and proliferation of neural stem cells after release from blood–brain barrier cells (Gordon et al., 2009). A recent study highlighted the importance of CCL2/CCR2 signaling in a pre-clinical depression model (Cazareth et al., 2014). In this study mice were provided peripheral immune challenge (lipopolysaccharide), then a detailed neuroimmune assessment was conducted including analysis of brain cytokines, chemokines, immune cells and neurons. LPS caused a pro-inflammatory state in the brain, and caused microglia and CNS-associated phagocytic activation characterized by a marked overproduction of CCL2, TLR4/CD14, CD80 and IL-4Rα. The LPS administration also caused a selective increase of CCR2+ inflammatory monocytes within the brain. Finally, CCL2 hyperpolarised serotonergic raphe neurons in the midbrain possibly suggesting a reduced serotonin tone in projection areas.
IL-8 was not found to be elevated in depressed vs. non-depressed individuals, and studies were found to carry significant heterogeneity. IL-8/CXCL8 is also known for pleiotropic actions given functions in chemotaxis and pro-inflammatory effects supporting activation and degranulation of neutrophils, basophils and monocytes/macrophages (Murphy et al., 2000; Stuart and Baune, 2014). In recent times, the CNS-specific effects of IL-8/CXCL8 have begun to be explored (Stuart and Baune, 2014). In the CNS, it appears IL-8/CXCL8 has both immune and non-immune functions. It is constitutively expressed on neurons, astrocytes, microglia, oligodendrocytes, BBB endothelial cells and neural stem cells (Subileau et al., 2009; Weiss et al., 2010). From an immune perspective, IL-8/CXCL8 is thought to be involved in regulating the activity of infiltrating peripheral immune cells in states of significant BBB compromise (Subileau et al., 2009). From a non-immune perspective, it has been shown to enhance neurotransmitter release and inhibit LTD in cultured rodent Purkinje neurons (Giovannelli et al., 1998). Finally, Kelland et al. (Kelland et al., 2011) has found a role for IL-8/CXCL8 on stem cell biology in vitro. IL-8/CXCL8 was found to cause CXCR-1-mediated death of neural stem cells, but not oligodendrocyte progenitor cells. IL-8/CXCL8 also acted as a potent chemoattractant to both cell types. This suggests a role for IL-8/CXCL8 in the CNS for recruiting these cells types to sites of inflammation which may have an impact in depression pathophysiology.
The relative lack of significant findings in this study is useful to consider. One explanation for this may be due to the small sample size of these studies. The average was around 60 participants (minimum 20 and maximum 245). Another reason is the relatively inconsistent time of day chemokines were collected across these studies, most in the morning and after fasting, however some without fasting and at least one in the evening. This is critical given the circadian based expression of cytokines and chemokines (Nakao, 2014). From a mechanistic perspective, there is emerging data to suggest a significant role for chemokines in depression-related pathophysiological processes and depression-like behaviour (Harrison et al., 2014; Jaehne and Baune, 2014). These results from mechanistic studies may not be translating into human studies for a number of reasons. The CNS immune milieu in human depression may not be adequately sampled from peripheral chemokine levels. This may be due to chemokines being expressed a very low levels in the CNS, particularly under basal conditions (Rostene et al., 2007). Deranged levels of chemokines may not be detected in the periphery if their function is autocrine or paracrine within the CNS (Rostene et al., 2011b). This is an argument for measuring chemokine levels via cerebrospinal fluid sampling, and via human CNS cell lines from induced pluripotent stem cells (iPS) derived from peripheral somatic cells of depressed subjects (Brennand et al., 2011).
A recent systematic review explored the role of chemokines in clinical and pre-clinical populations in depression (Stuart and Baune, 2014), and another explores the pathophysiology mechanisms in depression (Stuart et al., 2015). These reviews demonstrate the majority of published studies including measurement of chemokines in these psychiatric disorders have concerned the prototypical ‘pro-inflammatory’ chemokines IL-8/CXCL8 and MCP-1/CCL2. There is, however, evidence to suggest a wide range of chemokines are involved in the pathophysiology of depression and depression-like behaviour. The chemokine CXCL12 has effects of depression-related pathophysiological mechanisms as it may enhance the activity of GABA and glutamate on serotonergic neurons, enhance the proliferation and direct the migration of human neural progenitor cells (Reaux-Le Goazigo et al., 2013). CX3CL1 also has several non-immune mechanisms which may be relevant to depression including inhibition of serotonergic neurotransmission by enhancement of the activity of GABA on serotonergic neurons, inhibition of glutamatergic activity in hippocampal neurons, and regulation processes of neuroplasticity such as long term potentiation (LTP) (Reaux-Le Goazigo et al., 2013). It is important to note that evidence from systematic reviews shows early mechanistic evidence does associate select chemokines with the neurobiological processes (neurogenesis, neuroinflammation, HPA axis, neuro-transmission), however, as with the current clinical evidence, this early evidence does not clearly demonstrate any specificity for a certain psychiatric disorder, but is primarily relevant to mechanisms which are shared across disorders i.e. bipolar disorder, schizophrenia (Stuart et al., 2015).
This study has a number of limitations which should be outlined, and these limitations come from the field being quantitatively assessed. Firstly, only two chemokines could be analysed as there were only two chemokines which had ≥3 studies conducted on them, were from unstimulated serum/plasma chemokine markers and had participants free of major medical comorbidities. This may play a factor in our relative lack of significant findings. Secondly, there were limited options to control for covariates. Covariates which should have been controlled for, but which was not possible due to a lack of information from available studies, included body mass index, smoking states, chronic disease burden, anti-inflammatory drug usage and antidepressant usage. There were a number of studies outlining their antidepressant use, and these varied widely. The wide variation is concerning given various antidepressant classes are likely to influence the immune system in differing ways. For example, a recent meta-analysis of cross-sectional studies on serum inflammatory cytokines reported SSRIs as having larger anti-inflammatory effects than other antidepressants (Hannestad et al., 2011). The influence of antidepressant classes on chemokines is very poorly known. Thirdly, sub-analysis of mitogen stimulated chemokines assays or other non-serum/plasma chemokine markers (i.e. genetics, cerebrospinal fluid) could not be performed as there were no chemokines with ≥ 3 studies conducted on them. Fourthly, there are a variety of depression subtypes which haven't been parsed out in this study e.g. melancholic, non-melancholic, atypical. Therefore, this is an important area for further study given there is some evidence to suggest inflammatory profiles differ among depression subtypes (Stuart and Baune, 2012), hence one could hypothesise there may be differences in chemokine levels. When aiming to parse out chemokine level associations with depression subtypes, another approach may be to parse out associations with specific symptoms associated with depression, or among psychiatric disorders. Such an approach would be in keeping with the Research Domain Criteria (RDoC) approach e.g. negative and positive valence systems, as well as cognitive systems (Cuthbert, 2014). Finally, in addition to this pilot meta-analysis exploring associations between chemokine levels and MDD diagnosis, future directions should explore the role of chemokines as treatment response biomarkers. For example, in a study by Rethorst et al. (Rethorst et al., 2013), in subjects with treatment resistant depression, only subjects with baseline high levels of TNF-α were shown to benefit from the antidepressant effects of aerobic exercise, as compared with those who had low levels of TNF-α.
We have a number of recommendations for the future of this research field. Longitudinal studies in this field are urgently needed to better understand correlation versus causation, and how chemokine levels may change with the fluctuation of depressive symptoms. We believe levels of chemokines in depressed vs. non-depressed subjects should be explored in a range of age groups. We speculate the chemokine levels in depressed subjects of varying ages may change depending on the pathophysiology driving depression in various age groups. For example, in late-life depression there is a greater burden of vascular-related changes in the brain (Taylor et al., 2013). More chemokines should be assessed for behavioural effects in in vivo models. There are few studies in this field exploring the behavioural outcomes of chemokine-based transgenic modified rodents (e.g. knockout or over-expression). Our group has begun to explore this topic (Harrison et al., 2014; Jaehne and Baune, 2014). Finally, care must be taken to ensure chemokine ligand and receptors are similar in their biological activity and pharmacology as compared with humans; there is significant variability between species.
5. Conclusion
This meta-analysis reports significantly heterogeneity in this field among studies. There are higher concentrations of the chemokine MCP-1/CCL2 in depressed subjects compared with control subjects, and no differences for IL-8/CXCL8. More high quality research and consistent methodologies are needed in this important area of enquiry given the growing evidence accruing from clinical and pre-clinical models.
Acknowledgments
Disclosures: HAE, TA, MJS, AP, JJ and BTB have no disclosures to report.
Footnotes
Disclosures: All authors declare that there are no conflicts of interest or financial disclosures.
References
- Bai YM, Chiou WF, Su TP, Li CT, Chen MH. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J Affect Disord. 2014;155:28–34. doi: 10.1016/j.jad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012a;37:1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012b doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK, Drexhage HA. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry. 2014;4:e344. doi: 10.1038/tp.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J Neuroinflammation. 2014;11:132. doi: 10.1186/1742-2094-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology. 2014;51:1205–1206. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici E, Wille DR, Tozzi F, Prokopenko I, Miller S, McKeown A, Brittain C, Rujescu D, Giegling I, Turck CW, Holsboer F, Bullmore ET, Middleton L, Merlo-Pich E, Alexander RC, Muglia P. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case–control collections. PLoS One. 2010;5:e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Eyre H, Baune BT. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Eyre H, Air T, Proctor S, Rositano S, Baune BT. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57:11–16. doi: 10.1016/j.pnpbp.2014.10.003. http://dx.doi.org/10.1016/j.pnpbp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Giovannelli A, Limatola C, Ragozzino D, Mileo AM, Ruggieri A, Ciotti MT, Mercanti D, Santoni A, Eusebi F. CXC chemokines interleukin-8 (IL-8) and growth-related gene product alpha (GROalpha) modulate Purkinje neuron activity in mouse cerebellum. J Neuroimmunol. 1998;92:122–132. doi: 10.1016/s0165-5728(98)00192-1. [DOI] [PubMed] [Google Scholar]
- Gordon RJ, McGregor AL, Connor B. Chemokines direct neural progenitor cell migration following striatal cell loss. Mol Cell Neurosci. 2009;41:219–232. doi: 10.1016/j.mcn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012;34:71–75. doi: 10.1590/s1516-44462012000100013. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg L, Janelidze S, Engstrom G, Wisen AG, Westrin A, Brundin L. Exercise-induced release of cytokines in patients with major depressive disorder. J Affect Disord. 2010;126:262–267. doi: 10.1016/j.jad.2010.02.133. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EL, Jaehne EJ, Jawahar MC, Corrigan F, Baune BT. Maternal separation modifies behavioural and neuroendocrine responses to stress in CCR7 deficient mice. Behav Brain Res. 2014;263:169–175. doi: 10.1016/j.bbr.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, Dryden DM. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 2013;66:982–993. doi: 10.1016/j.jclinepi.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Flicker L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst Rev. 2003:CD001015. doi: 10.1002/14651858.CD001015. [DOI] [PubMed] [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocaoglu C, Kural B, Aliyazicioglu R, Deger O, Cengiz S. IL-1beta, IL-6, IL-8, IL-10, IFN-gamma, TNF-alpha and its relationship with lipid parameters in patients with major depression. Metab Brain Dis. 2012;27:425–430. doi: 10.1007/s11011-012-9323-9. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Meeks TW, Kelley ME, Mufti M, Young R, McWhorter K, Vito N, Chismar R, Quinn S, Dey S, Byrd EH, McDonald WM. A double blind, placebo-controlled pilot study of galantamine augmentation of antidepressant treatment in older adults with major depression. Int J Geriatr Psychiatry. 2008;23:625–631. doi: 10.1002/gps.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaehne EJ, Baune BT. Effects of chemokine receptor signalling on cognition-like, emotion-like and sociability behaviours of CCR6 and CCR7 knockout mice. Behav Brain Res. 2014;261:31–39. doi: 10.1016/j.bbr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Jonsdottir IH, Hagg DA, Glise K, Ekman R. Monocyte chemotactic protein-1 (MCP-1) and growth factors called into question as markers of prolonged psychosocial stress. PLoS One. 2009;4:e7659. doi: 10.1371/journal.pone.0007659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland EE, Gilmore W, Weiner LP, Lund BT. The dual role of CXCL8 in human CNS stem cell function: multipotent neural stem cell death and oligodendrocyte progenitor cell chemotaxis. Glia. 2011;59:1864–1878. doi: 10.1002/glia.21230. [DOI] [PubMed] [Google Scholar]
- Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Lee SH, Hong CH. Simultaneous measurement of 23 plasma cytokines in late-life depression. Neurol Sci. 2009;30:435–438. doi: 10.1007/s10072-009-0091-1. [DOI] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Herzig KH, Tolmunen T, Huotari A, Viinamaki H, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Hintikka J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35:226–232. doi: 10.1016/j.psyneuen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J. Translational psychiatry: leading the transition from the cesspool of devastation to a place where the grass is really greener. Transl Psychiatry. 2011;1:e1. doi: 10.1038/tp.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, Wu W, Liao M, Wang M, Tang H, Li W, Li W, Li Z, Zhou J, Zhang Z, Li L. Elevated specific peripheral cyto-kines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry. 2013;54:953–961. doi: 10.1016/j.comppsych.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48:13–15. doi: 10.1016/j.jpsychires.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behav Immun. 2007;21:218–228. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Melik-Parsadaniantz S, Rostene W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198:62–68. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Merendino RA, Di Pasquale G, De Luca F, Di Pasquale L, Ferlazzo E, Martino G, Palumbo MC, Morabito F, Gangemi S. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate–severe depression. Mediat Inflamm. 2004;13:205–207. doi: 10.1080/09511920410001713484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cy-tokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Nakao A. Temporal regulation of cytokines by the circadian clock. J Immunol Res. 2014;2014:614529. doi: 10.1155/2014/614529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scully P, Fitzgerald P, Scott LV, Dinan TG. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J Psychiatr Res. 2007;41:326–331. doi: 10.1016/j.jpsychires.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Halaris A, Iqbal O, Hoppensteadt D, Fareed J, Zhu H, Sinacore J, Devane CL. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J Biol Psychiatry. 2009;10:313–323. doi: 10.3109/15622970802573246. [DOI] [PubMed] [Google Scholar]
- Podlipny J, Hess Z, Vrzalova J, Rosolova H, Beran J, Petrlova B. Lower serum levels of interleukin-6 in a population sample with symptoms of depression than in a population sample without symptoms of depression. Physiol Res/Acad Sci Bohemoslov. 2010;59:121–126. doi: 10.33549/physiolres.931695. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;8:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD, Huebinger RM, Barber RC, Trivedi MH. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013;18:1119–1124. doi: 10.1038/mp.2012.125. http://dx.doi.org/10.1038/mp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostene W, Dansereau MA, Godefroy D, Van Steenwinckel J, Reaux-Le Goazigo A, Melik-Parsadaniantz S, Apartis E, Hunot S, Beaudet N, Sarret P. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011a;118:680–694. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- Rostene W, Guyon A, Kular L, Godefroy D, Barbieri F, Bajetto A, Banisadr G, Callewaere C, Conductier G, Rovere C, Melik-Parsadaniantz S, Florio T. Chemokines and chemokine receptors: new actors in neuroendocrine regulations. Front Neuroendocrinol. 2011b;32:10–24. doi: 10.1016/j.yfrne.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lu P, Wei L, Cai I, Hu X, Chen W. Fluoxetine treatment for major depression decreases the plasma levels of cytokines. Afr J Biotechnol. 2010;9:7346–7351. [Google Scholar]
- Simon NM, McNamara K, Chow CW, Maser RS, Papakostas GI, Pollack MH, Nierenberg AA, Fava M, Wong KK. A detailed examination of cytokine abnormalitiesinMajor Depressive Disorder. Eur Neuropsychopharmacol. 2008;18:230–233. doi: 10.1016/j.euroneuro.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, de Jongh R, Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev. 2012;36:658–676. doi: 10.1016/j.neubiorev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Singhal G, Baune BT. Systematic review of the neurobiological relevance of chemokines to psychiatric disorders. Front Cell Neurosci. 2015;9:357. doi: 10.3389/fncel.2015.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Subileau EA, Rezaie P, Davies HA, Colyer FM, Greenwood J, Male DK, Romero IA. Expression of chemokines and their receptors by human brain endothelium: implications for multiple sclerosis. J Neuropathol Exp Neurol. 2009;68:227–240. doi: 10.1097/NEN.0b013e318197eca7. [DOI] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;2007:76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Deboux C, Chaverot N, Miller F, Baron-Van Evercooren A, Couraud PO, Cazaubon S. IL8 and CXCL13 are potent chemokines for the recruitment of human neural precursor cells across brain endothelial cells. J Neuroimmunol. 2010;223:131–134. doi: 10.1016/j.jneuroim.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]


