Abstract
Objective
To evaluate the prognostic value and test characteristics of coronary artery calcium (CAC) score for the identification of obstructive coronary artery disease (CAD) in comparison with coronary computed tomography angiography (CCTA) among symptomatic patients.
Methods
Retrospective cohort study at two large hospitals, including all symptomatic patients without prior CAD who underwent both CCTA and CAC. Accuracy of CAC for the identification of ≥50% and ≥70% stenosis by CCTA was evaluated. Prognostic value of CAC and CCTA were compared for prediction of major adverse cardiovascular events (MACE, defined as non-fatal myocardial infarction, cardiovascular death, late coronary revascularization (>90 days), and unstable angina requiring hospitalization).
Results
Among 1145 included patients, the mean age was 55 ± 12 years and median follow up 2.4 (IQR: 1.5–3.5) years. Overall, 406 (35%) CCTA were normal, 454 (40%) had <50% stenosis, and 285 (25%) had ≥50% stenosis. The prevalence of ≥70% stenosis was 16%. Among 483 (42%) patients with CAC zero, 395 (82%) had normal CCTA, 81 (17%) <50% stenosis, and 7 (1.5%) ≥ 50% stenosis. 2 (0.4%) patients had ≥70% stenosis. For diagnosis of ≥50% stenosis, CAC had a sensitivity of 98% and specificity of 55%. The negative predictive value (NPV) for CAC was 99% for ≥50% stenosis and 99.6% for ≥70% stenosis by CCTA. There were no adverse events among the 7 patients with zero calcium and ≥50% CAD. For prediction of MACE, the c-statistic for clinical risk factors of 0.62 increased to 0.73 (p < 0.001) with CAC versus 0.77 (p = 0.02) with CCTA.
Conclusion
Among symptomatic patients with CAC zero, a 1–2% prevalence of potentially obstructive CAD occurs, although this finding was not associated with future coronary revascularization or adverse prognosis within 2 years.
Keywords: Coronary computed tomography, angiography, Coronary artery calcium score, Atherosclerosis, Epidemiology
Coronary artery disease (CAD) and its complications remain the leading cause of morbidity and mortality in most industrialized nations [1]. For decades, triage has begun with an assessment of pretest probability of obstructive CAD [2], based upon known cardiovascular risk factors such as hypertension, dyslipidemia, family history of CAD, and smoking [3,4]. Additionally, the presence of anginal symptoms increases the clinical likelihood of significant CAD [2]. In spite of the progress made and efforts put into clinical assessment of pretest probability, this remains an imperfect science [5], thus promoting interest in imaging tests as a means of diagnosing CAD and identifying sub-groups of patients who have a favorable prognosis.
Contrast enhanced coronary CT angiography (CCTA) provides high resolution images of the coronary arteries, and due to its high negative predictive value, has an excellent accuracy for excluding the presence of coronary stenosis or ischemia [6]. On the other hand, coronary artery calcium (CAC) scanning is more widely available, simpler to perform (e.g., not heart rate dependent), does not require contrast, less expensive, and offers highly reproducible results. However, this test cannot determine whether stenosis is present or absent and cannot identify the presence or extent of exclusively non-calcified plaque. While the role of CAC has been extensively studied among asymptomatic patients, recently there has been increasing debate [7,8] regarding the potential role of CAC among low risk symptomatic patients. Central to this debate is whether the prevalence and clinical significance of non-calcified plaque and stenosis in the setting of zero calcium is low enough to allow for exclusion of obstructive CAD.
In recognition of recent data regarding CAC testing in symptomatic patients, the United Kingdom National Institute for Health and Clinical Excellence (NICE) guidelines for assessment of possible angina have incorporated the use of CAC as part of the recommended algorithm [9]. Although major American guidelines for unstable angina do not identify CAC as part of any diagnostic algorithm [3], the 2007 expert consensus document on calcium scoring published by the American College of Cardiology and the American Heart Association states that CAC may serve “as a filter prior to invasive coronary angiography or stress nuclear imaging” but acknowledges that the “prognostic studies of CAC in symptomatic patients have generally been limited by biased samples (e.g., patients referred for invasive coronary angiography) and small numbers of hard outcome events. Future studies should include larger numbers of patients and should allow for adequate length of follow-up and assessment of larger numbers of hard endpoint events, especially all-cause mortality and myocardial infarction” [10].
Therefore, our objective was to evaluate the diagnostic accuracy of CAC for excluding coronary stenosis among symptomatic patients also having CCTA and to investigate the prognostic value of each test.
1. Methods
1.1. Study population
We included all consecutive subjects, 18 years or older, who underwent both a non-contrast CAC score and a contrast enhanced CCTA at the Massachusetts General Hospital or the Brigham and Women’s Hospital from 2004 – 2011. We included in and outpatients. All CAC and CTA scans were performed with 64 detector (or newer generation) scanner. We excluded patients without symptoms referred for screening purposes or research protocols and patients with prior known CAD (defined as prior percutaneous coronary intervention (PCI)), coronary artery bypass graft surgery (CABG), or MI. The Partners’ Healthcare Institution Review Board approved the study.
1.2. CCTA exam acquisition and interpretation
CAC and CCTA scans were performed according to established guidelines [11,12] and institutional protocol at the time of the scan. CAC scans were performed and read according to the Agatston method [13]. All CCTA exams were categorized as having no (0%), non-obstructive (<50%), or obstructive (≥50%) coronary artery disease (CAD). Vessels smaller than 2 mm were not evaluated. We used the Society of Cardiovascular Computed Tomography 18 segment coronary model based upon the original American Heart Association model [12], to categorize CAD presence and severity for each segment. Extent of CAD was also assessed by the number of vessels with CAD [left anterior descending (LAD), left circumflex (LCX), and right coronary artery (RCA)] as 1-vessel, 2-vessel and 3-vessel/left main (LM) disease. More detailed analysis of the extent and severity of CAD were performed using previously validated scores:
Segment involvement score (SIS): the sum of the number of segments with disease, which ranges from 0 to 16. [14]
Segment severity score (SSS): each segment receives a value according the amount of disease present in that vessel (0: no CAD, 1: non-obstructive CAD, 2: 50–70% stenosis, 3: >70% stenosis). The final score is the sum of each individual score, and ranges from 0 to 48. [15]
1.3. Baseline risk factors
We defined systemic arterial hypertension as a systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or diagnosis/treatment of hypertension. Dyslipidemia was defined as total cholesterol >240 mg/dL or serum triglycerides >150 mg/dL or high density lipoprotein cholesterol (HDL) < 40 mg/dL (male) or <50 mg/dL (women) or diagnosis/treatment of dyslipidemia. Diabetes was defined by a hemoglobin A1C ≥ 6.5% [16], physician-based diagnosis, or use of anti-diabetic medications. Smoking was defined as current (tobacco products used within the last month), former or never. Family history of premature CAD was defined as any first-degree family member with a history of clinical CAD prior to age 60. The pretest probability of CAD was calculated using the Morise score [17], which includes age, gender, risk factors and symptoms to predict the probability of obstructive CAD.
1.4. Cardiovascular outcomes
All patient medical records were reviewed by two cardiologists who were blinded to CTA results for adjudication of cardiovascular events. A standardized questionnaire was mailed to each patient in order to ensure that events outside of our healthcare network are captured. For patients who did not reply to the questionnaire upon repeated mailings, scripted phone interviews were performed. In addition, patients had the option of completing a web-based version of the questionnaire via the REDCap (Research Electronic Data Capture) system [18], which is encrypted, secure, and HIPAA compliant. All self-reported events were verified via outside medical record review by two cardiologists, blinded to CTA results with discordant events adjudicated by consensus.
Major adverse cardiovascular events (MACE) was defined as a composite of non-fatal myocardial infarction, late coronary revascularization (>90 days), unstable angina, and cardiovascular mortality. We additionally evaluated the outcome of cardiovascular mortality and non-fatal MI to avoid inherent bias of softer outcomes (e.g., angina, coronary revascularization). Diagnosis of MI was confirmed by two of three: chest pain or equivalent symptom complex; positive cardiac biomarkers; ECG changes typical of MI [19]. Time to the first coronary revascularization procedure (PCI or CABG) was evaluated. Early revascularizations (≤90 days post CCTA) were removed from survival analysis to minimize verification bias [20–22]. That is, patients with ≥50% stenosis by CCTA more frequently undergo invasive angiography and revascularization early after the CCTA, whereas late revascularizations are less likely to be associated with the CCTA and more associated with CAD progression and prognosis. Unstable angina without revascularization (USA) was defined as chest pain or chest pain equivalent with dynamic electrocardiogram (ECG) changes such as ST depression or T wave inversion but without abnormal cardiac biomarkers and characterized by: 1) rest symptoms; 2) new onset angina (less than 2 months duration); or, 3) increasing duration or severity of previously stable anginal symptoms [23].
We confirmed all deaths using the Social Security Death Index. For all patients who died, the cause of death was obtained using the National Death Index as well as the Massachusetts Department of Vital Statistics, when applicable. In addition, other relevant clinical records (e.g., death notes, autopsy findings, hospice notes) related to mortality were reviewed. Using all available data, the causes of deaths were adjudicated by two cardiologists blinded to the CTA results with cardiovascular mortality defined as a primary cause of acute MI, atherosclerotic coronary vascular disease, congestive heart failure, valvular heart disease, arrhythmic heart disease, stroke, or other structural or primary cardiac cause of death.
1.5. Statistical analysis
Continuous variables are reported as mean +/− standard deviation. Categorical variables are reported as counts and proportions. Continuous variables were compared between groups using analysis of variance techniques. Median segment scores were compared between groups using the Kruskal–Wallis test. Categorical variables were compared using chi-squared test or Fisher’s exact test, where appropriate. The Kaplan Meier method was used to assess event-free survival for all outcomes of interest. Univariable and multivariable Cox proportional hazards models were constructed to compare risk between strata. The incremental prognostic value was compared for four different Cox models for prediction of MACE and CV death or MI. These four models included baseline risk factors and symptoms (Morise score), Morise score + CAC, Morise score + CCTA, and Morise Score + CAC + CCTA. Predictors with a p < 0.10 in univariable analysis were included in the multivariable model. All statistics were performed using Stata version 12.1 (Statacorp, College Station, TX).
2. Results
1145 patients with mean age 55 ± 12 years age were included, and followed over a median of 2.4 (IQR: 1.5–3.5) years. Demographics are reported on Table 1.
Table 1.
Baseline demographics.
| Demographic | Mean or n | sd or % |
|---|---|---|
| Age, years | 55 | 12 |
| Male gender | 720 | (63%) |
| Hypertension | 502 | (52%) |
| Hyperlipidemia | 533 | (55%) |
| Diabetes | 150 | (16%) |
| Current smoking | 138 | (15%) |
| Family history of clinical CAD | 577 | (61%) |
| Chest pain, typical | 101 | (9%) |
| Chest pain, atypical | 404 | (35%) |
| Chest pain, nonanginal | 415 | (36%) |
| Dyspnea | 220 | (19%) |
| Equivocal stress | 104 | (9%) |
| Positive stress | 213 | (19%) |
| Pretest probabilitya | 47 | 21 |
As determined by age, symptoms, and risk factors. [17].
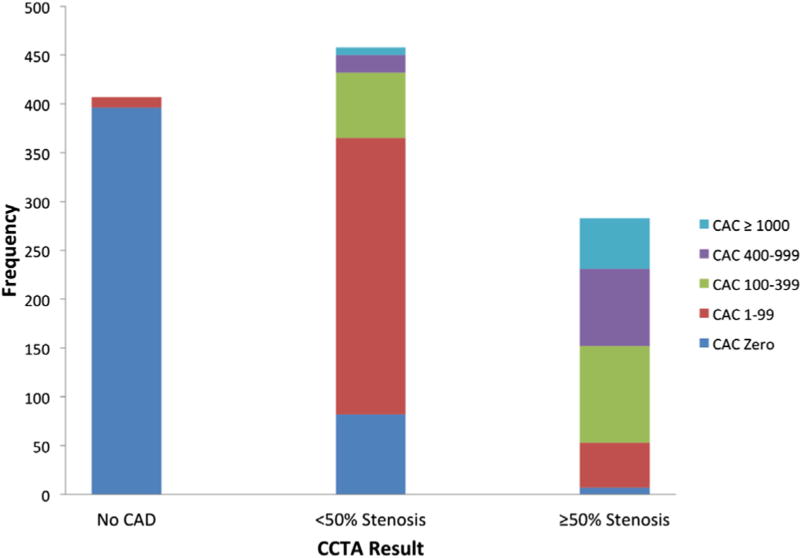
When examining the CTA results, 406 (35%) patients were found to have no CAD, 454 (40%) had <50% stenosis, and 285 (25%) ≥50% stenosis, of whom 178 (16%) patients had ≥70% stenosis (Supplemental Table 1 and Fig. 1). Increasing calcium score was associated with increasing extent and severity of CAD (Fig. 1).
Fig. 1.
Coronary arterial calcium (CAC) according to coronary computed tomography angiography (CCTA) findings.
Among 662 (58%) subjects with a positive calcium score, 11 (1.6%) had no CAD by CCTA, 373 (56%) had <50% stenosis, and 278 (42%) had ≥50% stenosis (176/278 with ≥70% stenosis). There were 483 (42%) patients with a calcium score of zero, of which, 395 (82%) had no CAD, 81 (17%) had <50% stenosis, 7 (1.5%) had ≥50% stenosis, and 2 (0.4%) had ≥70% stenosis.
The median SIS, SSS, and Duke CAD index was 0 for those with CAC of zero (Supplemental Table 2). The median SIS and SSS for those with positive CAC were 4 and the median Duke CAD index was 2. Among the 483 subjects with CAC of zero, 6 (1.2%) had 1 vessel obstructive CAD, 1 (0.2%) 2-vessel, and 0 (0%) 3-vessel. For those with positive CAC,149 (23%) had 1-vessel obstructive CAD, 79 (12%) 2-vessel, and 50 (8%) 3-vessel.
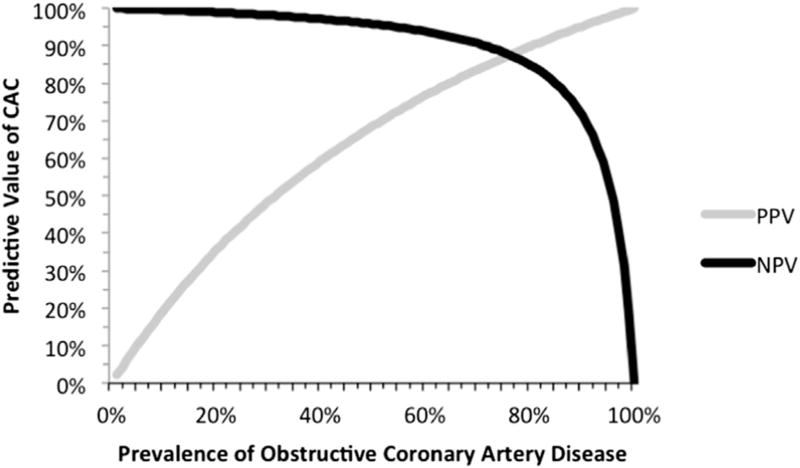
For detection of ≥50% stenosis, CAC had a sensitivity of 98% and specificity of 55%, corresponding to a negative LR of 0.04 and positive LR of 2.18 (Supplemental Fig. 1). The negative predictive value (NPV) was 99%. For detection of ≥70% stenosis, CAC had a sensitivity of 99% and specificity of 50%, corresponding to a negative LR of 0.02 and positive LR of 1.97. The NPV was 99.6%. Fig. 2 depicts how varying the pretest probability of ≥50% stenosis affects the NPV and PPV in different populations. For example, for a population with a disease prevalence of 10% (low pre-test probability), the PPV is 20% and the NPV 100%. As the pre-test probability for the population increases to 57% (a high-intermediate value), the NPV drops below 95% and the PPV increases to 74%. When the pre-test probability is high, the NPV drops further (e.g., at pre-test probability of 73% the NPV drops below 90% and the PPV is 86%). This concept is further illustrated using the Bayesian technique of a Fagan’s nomogram on Supplemental Fig. 2.
Fig. 2.
Increasing pre-test probability of disease decreases the negative predictive value (NPV) of coronary artery calcium (CAC) for prediction of obstructive coronary artery disease (≥50% stenosis) by coronary computed tomography angiography (CCTA) among patients with stable symptoms of possible angina. In low clinical risk populations (low pre-test probability), the NPV approaches 100%. In intermediate pre-test probability populations, the NPV remains higher than 95%. In high clinical risk populations, the NPV decreases, although remains above 90% until the pre-test probability exceeds 73%. PPV = positive predictive value.
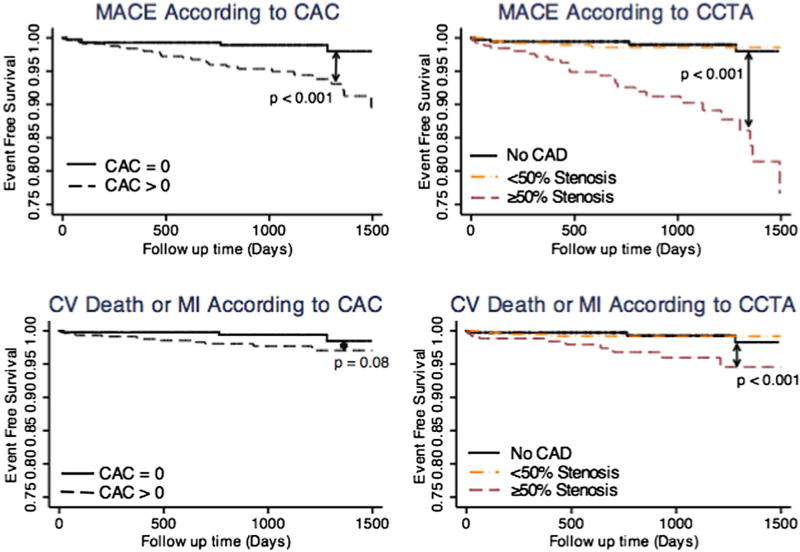
Among all 1145 patients, there were 38 deaths, 9 of those of cardiovascular cause, 8 non-fatal MI, 11 late-revascularizations, and 7 hospital re-admissions for unstable angina without revascularization. The annualized MACE rate was 2.1% for those with positive CAC versus 0.5% for CAC zero (Fig. 3 upper panel, log-rank p-value <0.001). The annualized incidence of CV death or MI was 0.8% for those with positive CAC versus 0.3% for CAC zero (Fig. 3 lower panel, log-rank p-value <0.001).
Fig. 3.
Kaplan Meier curve for prediction of major adverse cardiovascular events (MACE: unstable angina requiring hospitalization, late coronary revascularization, non-fatal MI, or CV death) according to coronary arterial calcium (top left panel) and CCTA (top right panel). Kaplan Meier curves for CV Death or MI according to CAC (bottom left panel) and CCTA (bottom right panel). CAC = coronary arterial calcium; CAD = coronary artery disease; CCTA = coronary CT angiography; CV = cardiovascular; MI = myocardial infarction.
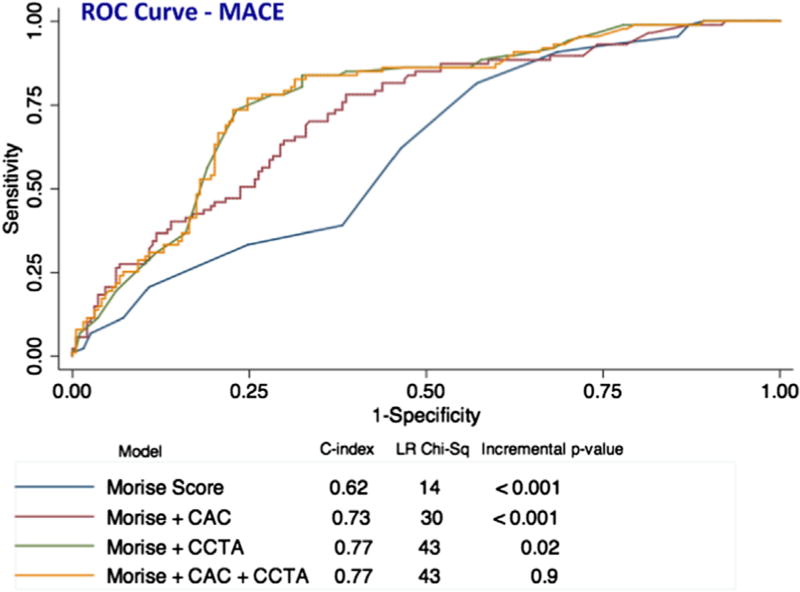
The prognostic value of symptoms combined with clinical risk factors (Morise score) was evaluated in a Cox proportional hazards model, and then compared to a model that included the Morise score plus CAC, a third model that included the Morise score plus CCTA, and a fourth model that included the Morise score plus both CAC and CCTA findings. (Fig. 4). From a Cox model for prediction of MACE using Morise score (c-statistic 0.62), the addition of CAC improved the c-statistic to 0.73 (p < 0.001). Morise + CCTA more significantly improved the c-statistic to 0.77 (p = 0.02). However, addition of both tests to a predictive model (Morise + CAC + CCTA) did not further improve the c-statistic beyond 0.77 (Fig. 4). Thus, the most parsimonious best fit model included Morise Score + CCTA. For prediction of CV death or MI, addition of either CAC or CCTA or CAC + CCTA to Morise score resulted in a similar change in prognostic value versus Morise Score alone that did not reach statistical significance (Supplemental Fig. 3). When considering only the CAC zero subgroup for analysis, such a comparison of predictive models (Morise score versus Morise score plus CCTA findings) was not powered due to no events in the small subgroup with CAC zero and stenosis.
Fig. 4.
ROC curves for prediction of major adverse cardiovascular events (MACE: cardiovascular death, non-fatal myocardial infarction, and coronary revascularization >90 days post-CCTA). For the prediction of MACE, CAC added prognostic value to Morise Score but CCTA in addition to Morise Score was superior to CAC. The combination of both CAC and CCTA in addition to Morise score was not of further benefit. Incremental p-value compares the c-statistic to the preceding row. CAC = coronary artery calcium; CCTA = coronary computed tomography angiography.
We conducted detailed clinical follow-up of the 7 patients with CAC zero and potentially obstructive CAD as defined by ≥50% worst coronary stenosis by CCTA. Over a median follow-up of 1.8 years for this subgroup, no adverse outcomes occurred. Among 3 patients that also underwent nuclear perfusion imaging, 2 patients had abnormal and 1 had equivocal stress results. One patient had abnormal exercise treadmill testing in addition to the CCTA. In spite of these findings, only 1 patient underwent subsequent invasive catheter angiography (ICA), which showed a 35% LAD stenosis (false positive CCTA). 4 patients declined ICA due to improved symptoms. When examining the impact of CAC and CTA testing on medical therapy, 4 patients had intensification of medical (statin) or lifestyle (smoking cessation) interventions.
3. Discussion
Our study demonstrates that among a large population of symptomatic patients, CAC alone was highly sensitive for exclusion of potentially obstructive CAD. Among the small number (n = 7, 1.5%) of patients with zero CAC who were found to have ≥50% stenosis, no patient required early or late coronary revascularization or suffered an adverse clinical event. Moreover, the presence of ≥70% stenosis was only identified in 2 (0.4%) patients, 1 of which was determined to be a false positive upon follow-up. Thus, the negative predictive value of CAC for prediction of obstructive CAD was high and the prognostic value of zero calcium reassuring.
Our findings are similar to those of an international multicenter CONFIRM registry study of 10,037 patients published by Villines et al., [20] who showed that although a low prevalence (3.5%) of potentially obstructive CAD exists among those with zero calcium, such patients did not have an increased risk of all-cause mortality. However, these patients did have an increased risk of late coronary revascularization compared to those without stenosis, and there were 2 MI among 177 patients with CAC zero and ≥50% stenosis. Consequently, among the population of CAC zero patients the number needed to scan (NNS) by CCTA to identify one patient at higher risk of MI over 2.1 year follow-up would be 100. Similarly, the NNS for late coronary revascularization would be 43. For combined events (all-cause mortality, MI, or late revascularization), the NNS would be 30. By comparison in our study, the NNS for CV death or MI would be 200 and for combined MACE (CV death, MI, late revascularization) 63. These very low adverse event incidences underscore the low clinical risk of CAC zero patients.
In contrast to our findings and those of most other similar analyses (that a low prevalence of 2–3% obstructive CAD may exist among symptomatic patients with calcium zero that appears to have low clinical risk), the CORE-64 authors found a very high prevalence (19%) of obstructive CAD among patients with zero CAC [24]. However, the baseline pretest probability of the patients in this study was higher than in ours and other studies, since patients recruited into CORE-64 were referred for invasive coronary angiography. These differences illustrate how underlying population characteristics that influence pretest probability may affect test sensitivity and specificity [25,26].
Because of encouraging results that CAC may perform diagnostically at least as well as alternative diagnostic tests for CAD among patients with a pretest probability of obstructive CAD from10 to 29%, recent guideline updates have incorporated CAC into a diagnostic algorithm in the UK NICE recommendations for assessment of chest pain [9]. Our finding that CAC zero has a high NPV throughout a range of low and intermediate pre-test probabilities (Figs. 3 and 4) supports this recent guideline update. Similarly, a focused update [27] on calcium scoring by the American College of Cardiology and American Heart Association has recommended that CAC may be a reasonable test before considering further testing among low-intermediate risk chest pain patients. Further supporting this strategy, an economic analysis performed based upon the utilization of CAC according to the NICE guideline, has concluded that CAC would be cost-effective compared with stress-ECG for low-intermediate pretest probability symptomatic patients [28].
While the use of CTA provides a more detailed analysis of plaque and can more accurately ensure the absence of coronary stenosis, this test is more expensive than CAC and cannot be performed in patients who cannot tolerate intravenous contrast. Thus, an important question is how much does CTA add beyond CAC, and does the incremental benefit justify the additional cost of this exam. To answer this question, one might ask how many patients with CAC zero need to be scanned with CTA to identify one patient who has ≥50% stenosis. The number needed to scan in this scenario would be 69. However, since our data and others indicate that the prognosis of such patients who have exclusively non-calcified plaque and stenosis ≥50% is favorable, it is also relevant that the number needed to scan to potentially prevent 1 MI would be much higher and depends upon the MI risk reduction for those with CAC zero who are diagnosed by CCTA with stenosis, and treated with revascularization and/or intensified medical therapy.
Given the increased cost and complexity of CCTA, and the fact that CAC has a high accuracy to exclude patients who have no disease, a potential role for CAC is to serve as a gatekeeper for low risk symptomatic patients. However several barriers may exist for such an approach. A practical question is whether performing CAC testing of symptomatic patients may delay diagnosis or increase testing, since up to 50% of patients will have a positive CAC, a result that could require further testing for assessment of possible angina. Additionally, given that failure to diagnose symptomatic coronary artery disease is a leading cause of malpractice litigation [29], some clinicians may favor the more detailed information offered by CCTA versus CAC, in spite of the reassuring value of a negative CAC.
Due to these potential barriers, it seems prudent that a randomized trial of CAC versus CCTA in low to intermediate risk symptomatic patients is warranted in order to determine the comparative cost-effectiveness of CAC versus CCTA. Such a trial would help determine the safety of using CAC versus CCTA for symptomatic patients by comparing post-test MI incidence. Also, it could help determine test efficiency since some have argued that use of CAC could increase testing and delay diagnosis due to the high prevalence of positive CAC. One potential design would be to obtain a blinded CCTA among patients randomized to the CAC group. Such a design may clarify if intensified medical therapy among patients with purely non-calcified obstructive CAD benefit from revascularization and intensified medical therapy. However, such a trial would likely require a large sample size with long term follow-up. In addition to a prospective randomized study comparing CAC versus CTA, further data is needed to evaluate the role of CAC for selecting the need and type of downstream testing, as is currently suggested by the NICE guidelines [9].
Our study results should be viewed in the context of important limitations. First, since all patients underwent both CAC and CCTA, the CCTA results may influence the prognosis of patients who have zero calcium who may have undergone intensified medical therapy that reduces downstream cardiovascular risk. Next, not all patients referred for CCTA in our centers underwent calcium score, and thus this analysis represents a subgroup of patients who had both tests. However, the characteristics of patients who were included in this study and underwent both CCTA and CAC was similar to those who underwent only CCTA. This is due to the fact that the decisions to perform both CAC and CCTA was largely a random one and had to do with the clinical protocol that was used at the time of testing rather than on any patient characteristics. Due to increased concern to minimize radiation in our centers [30], and due to increased recognition regarding the diagnostic/prognostic overlap of both tests, the use of combined CAC and CTA was far less frequent in recent years. Finally, like most cohorts referred to CCTA, the overall event rate is not high and thus the results should not be extrapolated to higher risk populations in whom a higher prevalence of significant CAD and adverse clinical event rate could be anticipated.
4. Conclusion
In conclusion, among symptomatic patients with CAC zero, a small prevalence of potentially obstructive CAD occurs, although this finding was not associated with a need for coronary revascularization or adverse prognosis. These findings, together with the potential reduction in cost associated by using CAC instead of CTA, suggest that a trial to assess the potential role of CAC versus CTA in low risk symptomatic patients is needed.
Supplementary Material
Acknowledgments
Disclosures
Dr. Hoffmann reports research support from Siemens Medical Systems. Dr. Rybicki reports research support from Toshiba Medical Systems Corporation. The opinions and assertions contained herein are the authors’ alone and do not represent the views of the Walter Reed National Military Medical Center, the US Army, or the Department of Defense.
Funding sources
None.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2013.12.029.
References
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part i: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, et al. Acc/aha 2007 guidelines for the management of patients with unstable angina/non st-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non st-elevation myocardial infarction): developed in collaboration with the American college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons: endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. Circulation. 2007;116:e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 4.Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the acc/aha 2002 guidelines for the management of patients with chronic stable angina: a report of the american college of cardiology/american heart association task force on practice guidelines writing group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–72. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
- 5.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary ct angiography evaluation for clinical outcomes: an international multicenter registry (confirm) Circulation. 2011;124:2423–32. 2421–2428. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mowatt G, Cook JA, Hillis GS, et al. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–93. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 7.Villines TC, Carbonaro S, Hulten E. Calcium scoring and chest pain: is it dead on arrival? J Cardiovasc Comput Tomogr. 2011;5:30–4. doi: 10.1016/j.jcct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Joshi P, Blaha M, Blumenthal R, Blankstein R, Nasir K. What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol. 2012;19:1226–35. doi: 10.1007/s12350-012-9626-6. [DOI] [PubMed] [Google Scholar]
- 9.Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A. Nice guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974–8. doi: 10.1136/hrt.2009.190066. [DOI] [PubMed] [Google Scholar]
- 10.Greenland P, Bonow RO, Brundage BH, et al. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the society of atherosclerosis imaging and prevention and the society of cardiovascular computed tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Abbara S, Arbab-Zadeh A, Callister TQ, et al. Scct guidelines for performance of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Raff GL, Abidov A, Achenbach S, et al. Scct guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–36. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KM, Dowe DA, Brink JA. Traditional clinical risk assessment tools do not accurately predict coronary atherosclerotic plaque burden: a ct angiography study. Am J Roentgenol. 2009;192:235–43. doi: 10.2214/AJR.08.1056. [DOI] [PubMed] [Google Scholar]
- 16.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med. 1997;102:350–6. doi: 10.1016/s0002-9343(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaca MP, Wiviott SD, Braunwald E, et al. American college of cardiology/american heart association/european society of cardiology/world heart federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the triton-timi 38 trial (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38) Circulation. 2012;125:577–83. doi: 10.1161/CIRCULATIONAHA.111.041160. [DOI] [PubMed] [Google Scholar]
- 20.Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the confirm (coronary ct angiography evaluation for clinical outcomes: an international multicenter) registry. J Am Coll Cardiol. 2011;58:2533–40. doi: 10.1016/j.jacc.2011.10.851. [DOI] [PubMed] [Google Scholar]
- 21.Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging. 2009;2:404–11. doi: 10.1016/j.jcmg.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Min JK, Dunning A, Lin FY, et al. Rationale and design of the confirm (coronary ct angiography evaluation for clinical outcomes: an international multicenter) registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JL, Adams CD, Antman EM, et al. Acc/aha 2007 guidelines for the management of patients with unstable angina/non-st-elevation myocardial infarction: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-st-elevation myocardial infarction) developed in collaboration with the american college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons endorsed by the american association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. J Am Coll Cardiol. 2007;50:e1–157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb I, Miller JM, Arbab-Zadeh A, et al. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–34. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16:981–91. doi: 10.1002/(sici)1097-0258(19970515)16:9<981::aid-sim510>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: a bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–6. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 27.Greenland P, Bonow RO, Brundage BH, et al. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography) Circulation. 2007;115:402–26. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 28.Raman V, McWilliams ET, Holmberg SR, Miles K. Economic analysis of the use of coronary calcium scoring as an alternative to stress ecg in the non-invasive diagnosis of coronary artery disease. Eur Radiol. 2012;22:579–87. doi: 10.1007/s00330-011-2304-2. [DOI] [PubMed] [Google Scholar]
- 29.Wallace E, Lowry J, Smith SM, Fahey T. The epidemiology of malpractice claims in primary care: a systematic review. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoshhajra BB, Engel LC, Major GP, et al. Evolution of coronary computed tomography radiation dose reduction at a tertiary referral center. Am J Med. 2012;125:764–72. doi: 10.1016/j.amjmed.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.