Summary
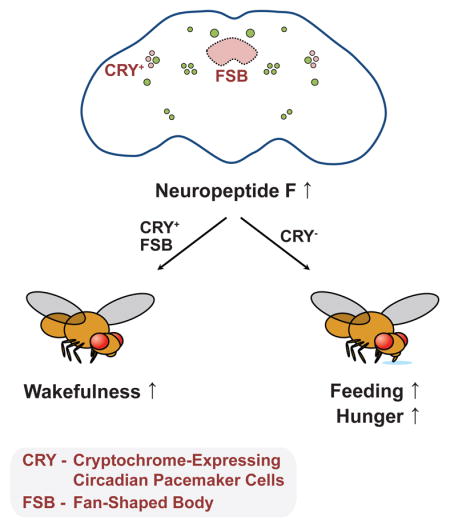
Proper regulation of sleep-wake behavior and feeding is essential for organismal health and survival. While previous studies have isolated discrete neural loci and substrates important for either sleep or feeding, how the brain is organized to coordinate both processes with respect to one another remains poorly understood. Here, we provide evidence that the Drosophila Neuropeptide F (NPF) network forms a critical component of both adult sleep and feeding regulation. Activation of NPF signaling in the brain promotes wakefulness and adult feeding, likely through its cognate receptor NPFR. Flies carrying a loss-of-function NPF allele do not suppress sleep following prolonged starvation conditions, suggesting that NPF acts as a hunger signal to keep the animal awake. NPF-expressing cells, specifically those expressing the circadian photoreceptor cryptochrome, are largely responsible for changes to sleep behavior caused by NPF neuron activation, but not feeding, demonstrating that different NPF neurons separately drive wakefulness and hunger.
eTOC Blurb
For optimal health, sleep and feeding behavior must be coordinated to not conflict with one another. However, it is unclear how the brain accomplishes this. Chung et al. demonstrate that enhanced Neuropeptide F (NPF) signaling promotes wakefulness and feeding behavior, presumably through different subsets of NPF-expressing neurons.
Introduction
Intercellular neuropeptide signaling plays an integral role in controlling and coordinating an ensemble of physiological and behavioral mechanisms to optimize organismal health (Nassel and Winther, 2010). Recently, we reported that neurons expressing the Drosophila Neuropeptide F (NPF), as well as the NPF peptide itself, are important for effects on health and longevity that are induced in adult male fruit flies following their exposure to female pheromones (Gendron et al., 2014, Gendron et al., 2015, Harvanek et al., 2017). Shortly after pheromone exposure, NPF protein levels are increased in the brain and the animals begin to lose triacylglyceride, become susceptible to stress, and age faster (Gendron et al., 2014, Gendron et al., 2015). Both inhibition of NPF-expressing neurons and reduced NPF gene function reverse these effects (Gendron et al., 2014, Harvanek et al., 2017). However, the consequences of increased NPF signaling on physiological and behavioral processes in the adult fly that have long-term ramifications on health and lifespan are largely unknown.
Accumulating evidence posits that NPF, and its mammalian ortholog Neuropeptide Y (NPY), may primarily modulate health through changes in sleep-wake behavior and nutritional state. Intact sleep-wake behavior is vital for optimum well-being and, in turn, healthy aging across taxa, with changes in NPF/NPY signaling known to alter the circadian timing (Hermann et al., 2012, Wiater et al., 2011), amount (He et al., 2013, Szentirmai and Krueger, 2006), or continuity of sleep behavior. Likewise, manipulating the excitability of Drosophila NPF neurons changes the fly’s state of hunger (Krashes et al., 2009) and feeding behavior following starvation (Hergarden et al., 2012), with similar effects observed when NPY signaling is altered in mammals (Luquet et al., 2005). Despite these implications, the role of NPF signaling in the context of steady-state sleep-wake behavior and feeding, such as those experienced when adult flies are aging naturally in nutritionally-replete conditions, is unclear. To address this outstanding issue, we investigated how NPF signaling controls both sleep and feeding behavior, its effects on fly physiology, and how altering nutritional inputs affect NPF control over these behaviors.
Results
Enhanced Neuronal NPF Signaling via NPFR Increases Wake Behavior
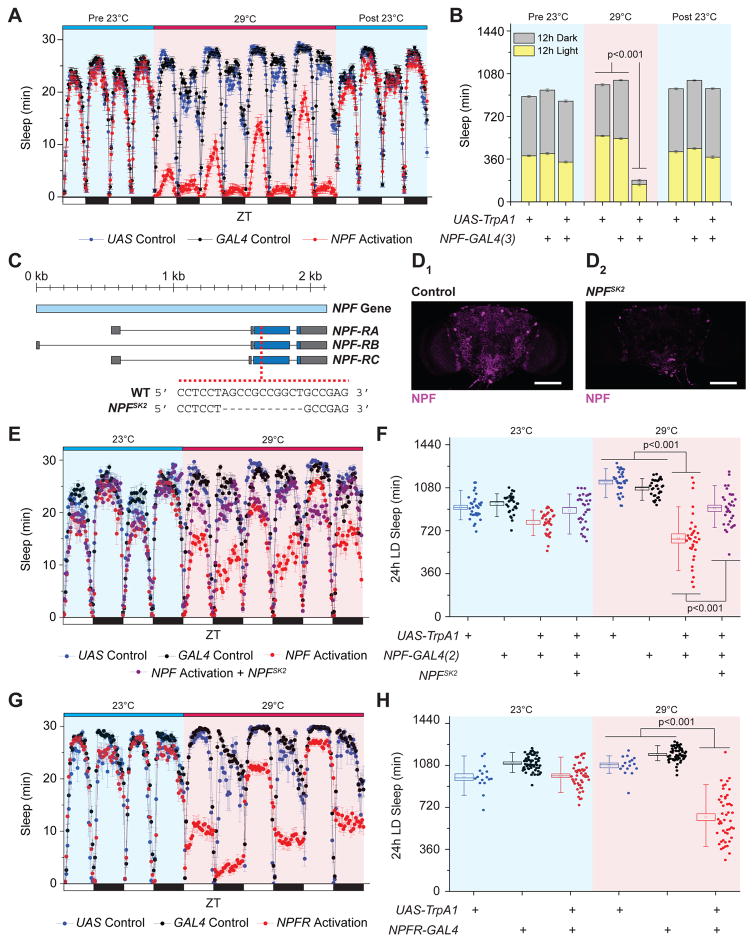
Given that increased NPF/NPY signaling was associated with long-term health consequences and that its role in sleep-wake regulation remains contentious, we investigated whether acute, enhanced NPF signaling disrupts adult fly sleep. This was accomplished by ectopically expressing the Transient Receptor Potential A1 (TrpA1) thermosensitive cation channel in NPF-producing cells, which only promotes neuron depolarization at elevated temperatures (>25°C). While no unusual baseline sleep phenotypes were observed during 23°C non-activation conditions, exposure of experimental genotypes to 29°C activation conditions throughout the day using two different NPF-GAL4 drivers caused a rapid decrease in sleep amount compared to transgene-only controls under 12h light/12h dark cycles, with the largest differences observed during the 12h dark phase (Figure 1A, B, Figure S1A, B). The reduced sleep amount caused by NPF cell activation was accompanied by shorter average sleep bout length and a decrease in the total number of sleep bouts (Figure S1C, D), suggesting an inability for flies to maintain continuous sleep. Interestingly, the short-sleep phenotype declined with prolonged exposure to activation conditions (particularly during the light phase, Figure 1A), perhaps due in part to homeostatic mechanisms that compensate for the significant loss of sleep in prior days. When flies were transitioned back to 23°C inactivation conditions, the short sleep phenotype rapidly reverted to normal sleep levels, which were not different from levels observed prior to activation (Figure 1A, B; Figure S1A, B). These results demonstrate that acute activation of NPF-expressing cells rapidly promotes wakefulness.
Figure 1. Acute Activation of the NPF-NPFR1 Signaling Axis Increases Wakefulness.
(A–B) NPF-expressing cell activation reduces sleep behavior and is reversible, illustrated as (A) a sleep profile depicting sleep amounts in 30 min binned intervals and (B) a stacked bar chart showing total sleep amount. n=80–111. (C–D) Characterization of a loss-of-function NPF allele, NPFSK2. (C) Schematic showing the position of the 11 bp deletion within the NPF coding region. (D) Representative images of (D1) control and (D2) NPFSK2 adult male brains immunostained against NPF (magenta). White scale bars denote 100 μm. (E–F) NPF-expressing cell activation effects on wakefulness depend on intact NPF signaling, illustrated as (E) a sleep profile and (F) a box plot quantifying total sleep amount. n=32. (G–H) NPFR-expressing cell activation increases wake behavior, illustrated as (G) a sleep profile and as (H) a box plot quantifying total sleep amount. n=16–63. For all box plots, squares within the box indicate mean, box range represents standard error the mean (SEM), whiskers denote 10–90 percentiles, and dots indicate individual data values. All error bars indicate SEM. See also Figure S1.
We next determined whether the NPF neuropeptide was responsible for the increased wake behavior caused by NPF-expressing cell activation. To confirm the wake-promoting properties of NPF, we used the CRISPR/Cas9 technique (Kondo and Ueda, 2013) to generate a mutant allele of NPF, NPFSK2, which carries an 11-bp deletion in the NPF open reading frame (ORF; Figure 1C). The mutant is predicted to be a protein null, as the deletion causes a frameshift that truncates most of the ORF. As expected, stereotypical NPF immunostaining patterns were eliminated in NPFSK2 adult brains, indicating the absence of a functional gene product (Figure 1D). Similarly, the expression levels of NPF mRNA were substantially reduced in NPFSK2 homozygotes (Figure S1E). As anticipated, activation of NPF-producing cells in the absence of the NPF peptide failed to promote wakefulness (Figure 1E, F). Furthermore, RNAi-mediated knockdown of NPF expression reduced the effect of TRPA1 activation of NPF-producing cells on wakefulness (Figure S1F). Together, these data implicate NPF as a wake-promoting neuropeptide.
NPF is thought to bind to a single receptor, NPFR, and we therefore asked whether activation of NPFR-expressing cells is sufficient to recapitulate the increased wakefulness observed following activation of NPF-expressing cells. We used an NPFR-GAL4 driver which has been previously shown to successfully ablate NPFR+ immunoreactive neurons in larvae (Wu et al., 2003). Although modestly smaller in magnitude than the effects caused by targeting NPF-expressing cells, we observed a significant decrease in total sleep amount under 29°C activation conditions but not 23°C inactivation conditions (Figure 1G, H). Similar to prolonged NPF effects on sleep behavior (Figure 1A, B; Figure S1A, B), the increased wake phenotype that accompanied NPFR cell activation progressively declined with increasing exposure of 29°C activation conditions (Figure 1G). Taken together, these data reinforce the notion that acute, enhanced NPF signaling promotes wakefulness, primarily through its cognate targets expressing NPFR.
NPF expression can be found in both the central nervous system (CNS) and in a subset of enteroendocrine cells (EECs) of the midgut (Song et al., 2014). To determine whether one or both cell types were responsible for the increased wake phenotype, we examined whether TRPA1-mediated activation of NPF+ EECs alone increased wake behavior. A GAL4 driver containing a fragment upstream of the Tachykinin gene (Tk-gut-GAL4) was found to have prominent expression in NPF+ EECs, but not the CNS (Song et al., 2014). While ectopic expression of TrpA1 in NPF+ EECs caused a slight, but significant, decrease in total sleep amount under 29°C activation conditions throughout the 24h day and the 12h dark period compared to transgene controls (Figure S2A, 2B), the effects were much smaller than those observed using either NPF-GAL4 drivers. Furthermore, co-expression with the pan-neuronal GAL4 repressor nSyb-GAL80 (Rezaval et al., 2012) completely reverted the increased wake phenotype caused by NPF cell activation (Figure S2C). It has been reported that nSyb-GAL80 blocks GAL4 expression both in the nervous system (Rezaval et al., 2012) and AstA-producing EECs (Chen et al., 2016). However, in our hands, nSyb-GAL80 successfully blocked NPF-GAL4 reporter expression in the adult brain, but failed to completely repress expression in the EECs (Figure S2D). Collectively, these data support the notion that NPF effects on wakefulness are primarily caused by activation of NPF+ neurons rather than NPF+ EECs.
NPF Signaling Promotes Wakefulness Under Nutrient Deprivation Conditions
If enhanced NPF signaling is associated with increased wake behavior, we reasoned that reduced NPF signaling should promote sleep. Surprisingly, homozygous NPFSK2 mutant flies did not exhibit an increase in overall sleep amount compared to control animals (Figure S3A). Constitutive overexpression of the potassium channel Kir2.1, which promotes neuron hyperpolarization, in NPF-expressing neurons was also unable to significantly alter sleep behavior compared to single transgene-only controls (Figure S3B). Furthermore, adult-specific overexpression of Kir2.1 in NPF-expressing neurons, which was achieved using the temperature-sensitive tub-GAL80ts TARGET system, failed to produce significant changes to sleep behavior when experimental flies were tested at restrictive temperatures (29°C; Figure S3C).
Because suppression of NPF signaling did not increase sleep behavior under standard environmental conditions, we hypothesized that the effects of reduced NPF function on sleep-wake behavior might manifest only under more severe nutritional environments where NPF signaling is normally promoted. Indeed, enhanced NPF signaling putatively mimics a food deprivation state that promotes appetitive memory formation and retrieval under fed conditions (Krashes et al., 2009). We therefore examined whether starvation-induced sleep loss was affected by loss of NPF. As previously shown (Keene et al., 2010), control animals exhibited a significant increase in wake approximately 12h after starvation onset (Figure 2A, B). However, NPFSK2 mutants did not exhibit a significant change in wake behavior during the same interval compared to fed controls (Figure 2A, B), establishing that NPF is required for starvation-induced sleep loss during this period. Notably, Kir2.1-mediated inhibition of NPF+ neurons either constitutively or exclusively as adults did not recapitulate the NPF mutant phenotype, suggesting either compensatory effects in these neurons or incomplete silencing of neuron function (Retzke et al., 2017). Taken together, these observations suggest that NPF signaling plays a minor role in modulating sleep when nutrients are replete, yet may be recruited to promote wakefulness under limited or challenging nutritional environments.
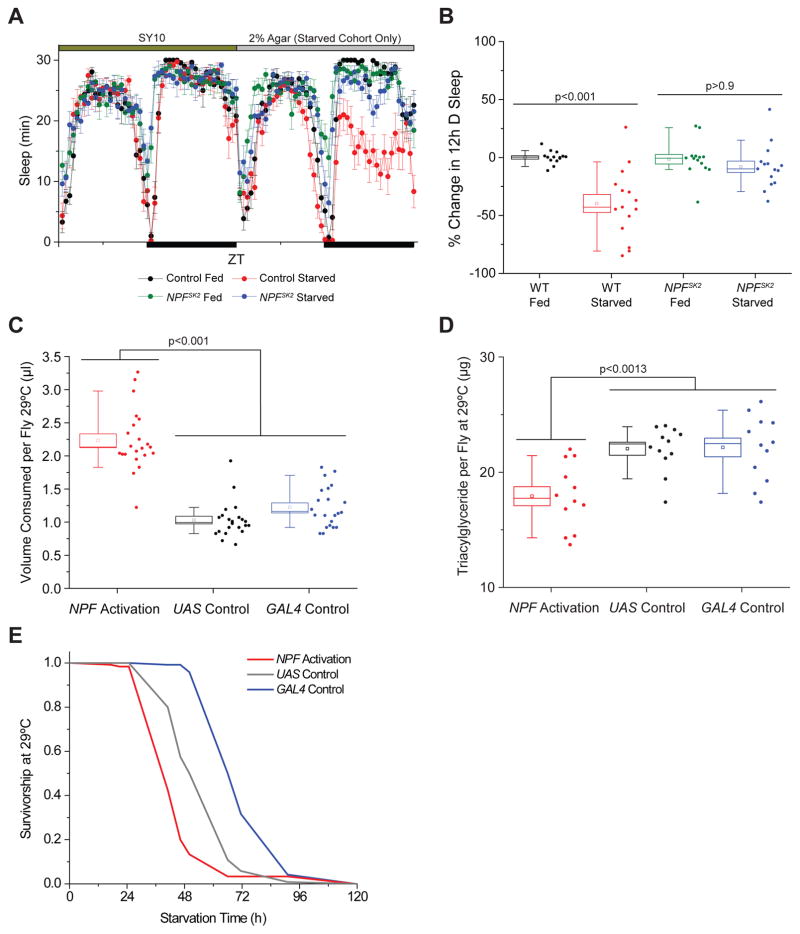
Figure 2. NPF Signaling Regulates the Physiological State of the Fly.
(A–B) Loss of NPF signaling suppresses starvation-induced sleep loss. (A) Sleep profiles of starved and fed control flies carrying the NPFSK2 allele compared to control genotypes. n=14–15. (B) Percent change in 12h dark period sleep during the first day of starvation compared to prior 12h dark fed baseline period. (C) NPF cell activation increases liquid food consumption in adult male flies. n=11 experimental replicates. (D) NPF cell activation decreases whole male adult fly triacylglyceride levels. n=12 experimental replicates. (E) NPF-expressing cell activation renders adult male flies sensitive to starvation. All error bars indicate SEM. See also Figure S2, S3.
Increased NPF Signaling Alters Feeding Behavior and Physiological Status
Published data have indicated that NPF promotes feeding in response to starvation (Hergarden et al., 2012), but it remains unclear whether NPF signaling influences feeding behavior under nutritionally-replete conditions. We therefore monitored grouped fly feeding behavior using the capillary feeder (CAFÉ) assay (Ja et al., 2007). In 23°C inactivation conditions, we did not observe significant differences between NPF activation cohorts and single-transgene controls. However, following the transition to 29°C activation conditions, NPF activation caused a significant increase in liquid food consumption compared to controls (Figure 2C). Similar conclusions were observed when we used the Fly Liquid Interaction Counter System (FLIC, (Ro et al., 2014)) to assess NPF activation effects on feeding behavior in single flies (Figure S2E). We failed to observe significant changes in FLIC-monitored feeding behavior following NPF+ EEC thermoactivation via the Tk-gut-GAL4 driver (Figure S2F), suggesting that NPF effects on feeding are primarily due to neuron activation.
To assess the physiological state of the fly, we examined the consequences of NPF circuit activation on fat storage and starvation response. Activation of NPF-expressing neurons caused a significant decrease in triacylglyceride levels and increased sensitivity to starvation conditions (Figure 2D, E). While it is unusual to see both a reduction in whole-fly triacylglyceride levels accompanied by an increase in food consumption, this relationship is likely due to increased activity and the flies’ perceived starvation state caused by NPF neuron activation.
Discrete NPF Neurons Differentially Control Sleep and Feeding Behavior
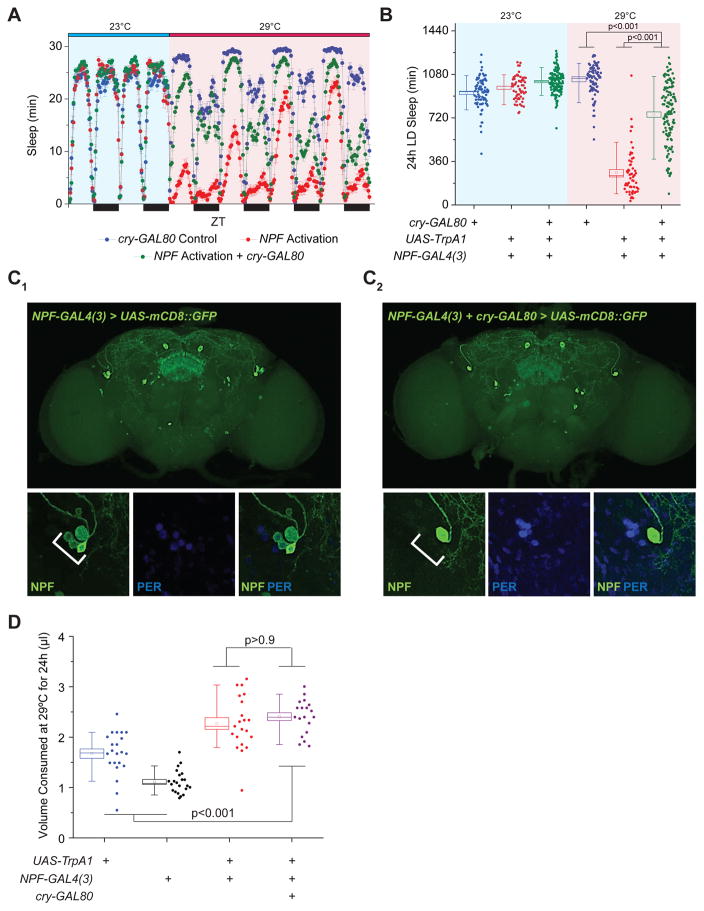
We next sought to investigate how subsets of NPF+ neurons regulate wakefulness and feeding. The NPF network contains cell bodies that either localize or arborize to many regions within the CNS known to control sleep/circadian behavior (e.g., circadian pacemaker neurons, fan-shaped bodies [FSB], pars intercerebralis [PI]), energy homeostasis (e.g., PI), and feeding (e.g., subesophageal ganglion [SOG]; (Lee et al., 2006, Scott et al., 2001, Ueno et al., 2012). We presumed that NPF regulation of feeding and wakefulness originates in different NPF+ neurons. To test this hypothesis, we first asked whether NPF+ circadian pacemaker cells contribute to the regulation of feeding and wakefulness. Using the GAL4-GAL80 intersectional approach, we found that cry-GAL80, which predominantly represses GAL4 function in circadian pacemaker cells (Stoleru et al., 2004), was able to significantly reverse the wake phenotype caused by NPF activation (Figure 3A–C), suggesting that NPF+, cry+ neurons are wake-promoting. We next examined whether NPF+, cry+ neurons similarly control feeding behavior. Unlike sleep-wake behavior, cry-GAL80 was unable to revert the increased feeding behavior caused by NPF-neuron activation (Figure 3D), implying NPF control of feeding extends beyond the NPF+, cry+ neurons that regulate wakefulness.
Figure 3. A Subset of Circadian Pacemaker Cells Largely Regulates NPF Effects on Wakefulness but Not Feeding.
(A–B) Repressing activation of NPF signaling in cry-expressing cells largely revert the sleep loss phenotype, illustrated as (A) a sleep profile and (B) as a box plots quantifying total sleep amount. n=59–128. (C) NPF-GAL4, cry-GAL80 expression pattern reveals NPF expression in a subset of the PER+ LNd circadian pacemaker neurons. (Top) Representative expression pattern of adult brain images for (C1) NPF-GAL4 and (C2) NPF-GAL4, cry-GAL80 using membrane GFP as a reporter. White arrows denote location of LNd neurons in the adult brain. (Bottom) High magnification images of region indicated by white arrows in top panels demonstrating co-localization of NPF (green) and PER (blue) within LNd neurons. White scale bars in images denote 25 μm. (D) Repressing activation of NPF signaling in cry-expressing cells does not alter adult feeding behavior. n=19–21 experimental replicates. All error bars indicate SEM. See also Figure S4.
To distinguish subsets of NPF+ neurons that directly control sleep-wake behavior from those that influence feeding, we carried out a mosaic analysis approach using the MARCM technique (mosaic analysis with a repressible cell marker, (Lee and Luo, 2001)). When MARCM was used, mobilization of a FLP recombinase through heat-shock at specific times during early fly development results in TrpA1 expression in small, random subsets of NPF+ neurons in individual flies. These neurons, which are marked with GFP, will be activated when the flies are placed at 29C. In this way, we correlated sleep patterns in individual flies with specific patterns of neuronal activation.
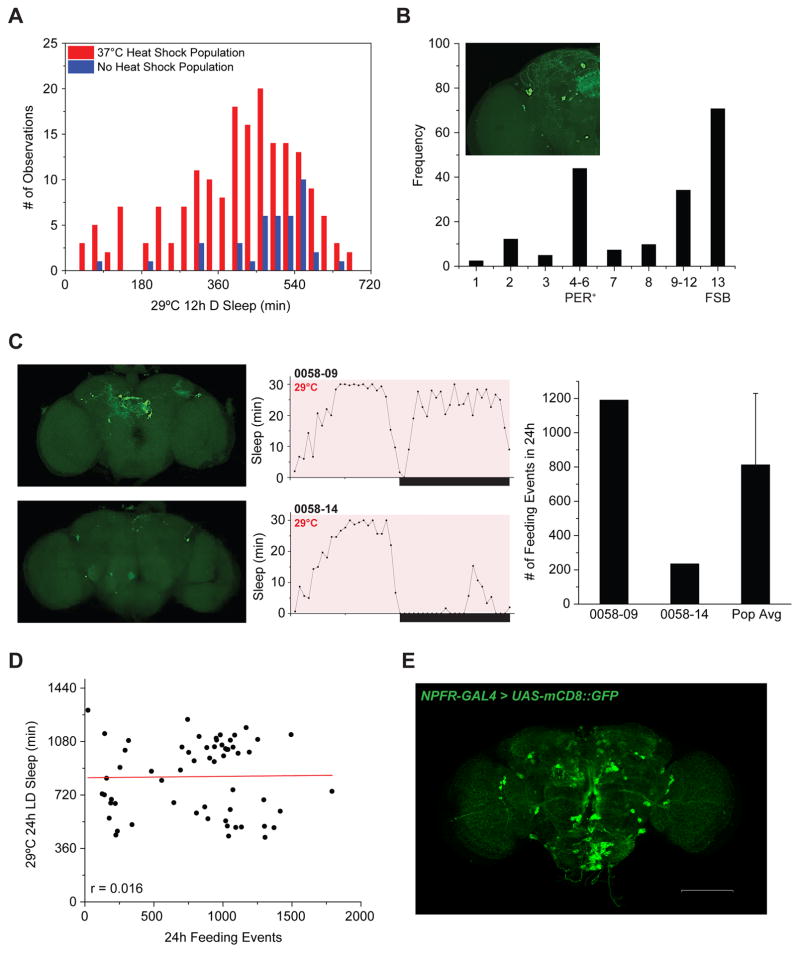
The effectiveness of this approach was evident in our ability to generate a mosaic fly population with greater variation in total sleep times and a substantial increase in the frequency of short-sleeping flies within the heat-shock fly population compared to non-heat shock control flies (Figure 4A). We then examined which subsets of NPF+ neurons were activated in severely short-sleeping flies. Several clusters of NPF-expressing neurons were represented in flies with the increased wake phenotype, although no single neuropil structure could be clearly implicated (Figure 4B). Relevant clusters include a subset of PER+ dorsal lateral circadian neurons (LNds) that were also implicated in the cry-GAL80 experiments, and 4 neuron clusters located in the protocerebrum. Many of the neurons linked to a pronounced effect on sleep made projections to the FSB, which nearly 80% of the time produced a wake phenotype when activated.
Figure 4. Mosaic Population Reveals that NPF Regulation of Feeding and Wake Behaviors Can Be Dissociated.
(A) Increased prevalence of short-sleeping flies in a mosaic NPF activation population (red; n=183) compared to a non-heat shock control population (blue; n=41). (B) Characterization of a mosaic population reveals distinct neuronal centers important for NPF control of wakefulness. The inset image shows the numbering system used to catalog NPF+ loci. Frequency represents the percentage of short-sleeping mosaic flies where GFP expression was observed (brain images characterized, n=41). Some flies exhibited expression in more than one neuronal locus and across both hemispheres. “FSB” is short for fan-shaped body. (C–D) Feeding behavior does not correlate with sleep-wake behavior in a mosaic NPF activation population. (C) Representative image (left), matching sleep profile (middle), and number of feeding events (right) from two individual males from the mosaic population. Bold white arrows highlight location of LNds. Error bar indicates standard deviation. (D) Scatterplot of individual fly phenotypes (n=60), with 29°C LD sleep amount plotted on the y-axis and subsequent 29°C LD feeding events plotted on the x-axis. p=0.9045 as determined by ANOVA. (E) NPFR-GAL4 exhibits prominent expression in established homeostatic centers of the adult brain. White scale bar denotes 100 μm.
We next examined feeding behavior using the FLIC system in individual, short-sleeping flies to determine whether those that slept less also increased their feeding. Similar to our sleep-wake analysis, we were unable to isolate a single neuropil that entirely contributed to an increase in feeding. We also found no correlation between increased feeding behavior and increased wakefulness, which, together with observations from the cry-GAL80 studies, reinforce the idea that the control of sleep and feeding behaviors are regulated by independent subsets of NPF-expressing neurons (Figure 4C, D).
Discussion
To date, the Drosophila NPF-NPFR signaling axis has been implicated in a variety of processes, all of which define how the animal behaves within its environment and coordinates its physiological milieu. Our data demonstrate that acute NPF signaling rapidly promotes wakefulness and is likely invoked under nutrient stressful conditions, thus playing an essential role for determining physiological state.
Enhanced NPF/NPFR expression has been reported to increase Drosophila sleep behavior and reduce homeostatic sleep response (He et al., 2013), contradicting our observations. Contrary to He et al., we found that overexpression of NPF in NPF-expressing neurons was unable to increase total sleep compared to appropriate transgene controls (Figure S3A–C, (He et al., 2013)). Moreover, simultaneous overexpression of NPF while activating NPF-expressing cells caused a further increase in wake behavior when compared to NPF activation controls (Figure S3D). We cannot, at present, explain the discrepancy between these findings, but the established functions of NPF would suggest that differences in dietary conditions and/or nutrient status either before or during behavioral testing may be responsible.
Our data suggest that different neurons within the NPF network modulate sleep-wake preference and feeding behavior. Notably, activation of NPF+, cry+ neurons largely drive wakefulness. These observations are consistent with reports establishing the importance of pacemaker neurons in sleep-wake regulation and also further implicate NPF-NPFR signaling within these neural centers on arousal. The PDF+ ventral lateral circadian neurons (LNvs) comprise a wake-promoting network that may potentially include NPF release from or feedback control via NPFR+ to these cells (Parisky et al., 2008, Chung et al., 2009, Hermann et al., 2012, Kim et al., 2013)). The NPF+ LNd circadian neurons, which may receive wake-promoting signals from the PDF+ LNvs, have been previously implicated in the control of both starvation-induced sleep suppression and night time sleep regulation (Keene et al., 2010, Cong et al., 2015).
While we were unable to identify a single NPF+ neuropil that regulates feeding behavior, our data suggest that NPF control of feeding does not include the NPF+, cry+ clock neurons. The SOG, a well-defined feeding and nutrient evaluation center (Scott et al., 2001, Wang et al., 2004), exhibits prominent NPFR expression and remains a putative target for NPF influence on feeding behavior (Figure 4E). Diet-mediated effects on sleep-wake behavior rely on gustatory receptors that converge onto the SOG (Linford et al., 2012), and NPF activity within the SOG may play a role in this response. Beyond the CNS, midgut NPF+ EEC effects on nutrient evaluation or feeding cannot be completely ruled out, and both published findings and the data presented leaves open the possibility that the gut-brain axis may utilize neuropeptide signaling, which may include NPF, to properly synchronize feeding with wakefulness (Chen et al., 2016).
NPF signaling may control feeding, nutrient status, and arousal at many different levels, especially with respect to dopamine function. Dopaminergic neurons subject to NPF control project to the mushroom bodies, a critical brain region that both integrates hunger status with appetitive-dependent memory performance and regulates sleep (Pitman et al., 2006, Krashes et al., 2009). The FSB, a neuroanatomical region that has already been shown to alter sleep homeostasis (Donlea et al., 2014), also receives dopaminergic arousal inputs via the DA1 receptor capable of regulating sleep behavior (Ueno et al., 2012). Our MARCM analyses largely corroborate these observations and further implicate the FSB in NPF-mediated sleep-wake regulation (Figure 4B, C). Thus, NPF-NPFR signaling in the aforementioned brain regions may serve as an additional control over dopaminergic arousal and feeding inputs in higher-ordered processing centers.
A relationship among nutrient inputs, feeding behavior, and sleep-wake preference in the context of NPF signaling has been observed in rodent models of NPF signaling, suggesting conserved mechanisms of action across taxa. Of particular note, central administration of the NPF mammalian homolog, NPY, into the lateral hypothalamus in rats induced wakefulness and increased feeding behavior (Szentirmai and Krueger, 2006). In addition to nutrients, other environmental inputs that have potentially rewarding effects, such as the presence of pheromones and the availability of mates, rely on NPF signaling to produce changes to fly behavior and physiology that have health consequences (Gendron et al., 2014, Shohat-Ophir et al., 2012). Further dissection of the heterogeneity of NPF-expressing neurons may therefore provide an understanding of how nutrient status converges with sleep-wake behavior, as well as other biological modalities to regulate health across taxa.
Experimental Procedures
Fly Strains
All fly strains and crosses were reared on a standard cornmeal-sugar-yeast diet (CT) at or below specified baseline temperature testing conditions, 12h light:12h dark, and 60% humidity. The NPFSK2 allele was created on a yw background using the CRISPR/Cas9 technique previously described (Kondo and Ueda, 2013) with the following 20-bp gRNA sequence: 5′-GCCCTTGCCCTCCTAGCCGC-3′. All reported transgenic genotypes were tested as heterozygotes unless otherwise noted. For all experiments involving NPFSK2, the allele was tested as homozygotes. Additional details may be found in the Supplemental Experimental Procedures.
Sleep-Wake Behavioral Analysis
Activity recordings and data processing were performed using the Drosophila Activity Monitor System (TriKinetics). 7–12 day old adult flies were individually tested in 5 mm × 65 mm polycarbonate tubes with 10% sugar, 10% yeast (SY10) food at one end of the testing tube. The first day of data was removed from the final analysis in order to allow for acclimation to experimental housing conditions. Sleep-wake data analysis was performed using a custom Excel-based macro suite (Pfeiffenberger et al., 2010) that defined sleep behavior as ≥ 5 min of continuous inactivity. For starvation-induced sleep studies, a 200 μl PCR tube filled with 50 μl of SY10 food was affixed to one end of an empty tube. At the onset of starvation, food-filled PCR tubes were replaced with a 200 μl PCR tube filled with 50 μl of 2% Bacto Agar. For fed controls, the food-filled PCR tube was replaced with a fresh 200 μl PCR tube filled with SY10 to address possible effects caused by experimental handling.
Starvation Response
Equal volumes of eggs from mated females were cultured on CT diet until adulthood. Newly emerged flies were collected within a 24h window to synchronize aging and placed into bottles containing SY10 food for 2 days to allow for mating. Male flies were sorted into groups of 15 flies using light CO2 anesthesia and placed into vials containing SY15 food. At least 8 replicate vials were prepared for each genotype/condition. Cohorts were maintained under specified environmental conditions for 7 additional days prior to starvation treatment. Flies were starved by placing them into vials containing 2% Bacto Agar. A census of dead flies was taken approximately every ~6–8h.
Feeding Behavioral Analysis
Volume of food consumed was measured using a modified version of the Capillary Feeder assay (CAFÉ; (Ja et al., 2007)). 3, 7–14 day old adult flies were placed into a 12.5 mm diameter x 35 mm length cylindrical acrylic chamber. Affixed to the top of the chamber was a rubber stopper with two, 5 μl calibrated glass pipets (Drummond Scientific) filled with approximately 5 μl of 5% liquid sucrose food accessible to the flies. A dab of mineral oil was gently layered over the open end of the calibrated pipet to minimize liquid food evaporation. Liquid displacement was marked at the beginning and end of the data collection period to measure total amount fed. A control chamber with no flies was used to correct for evaporation effects. FLIC feeding behavior was performed using 5% sucrose in 4 mg/L MgCl2 as a food source and analyzed with an R script described in detail previously (Ro et al., 2014) and at www.wikiflic.com.
Triacylglyceride Quantification
2–3 day old adult males were sorted into groups of 8 using light CO2 anesthesia and maintained on SY10 diet for 7 days at 29°C. At least 8 replicates were setup for each genotype/condition. After 7 days, whole flies were quickly frozen, arrayed in a 96-well deep well plate, and homogenized in 200 μl of cold 0.05% PBS-T for 30 sec at 30 Hz using a QIAGEN TissueLyser. Homogenized samples were spun down for 1 min at 1,000 rpm and 4°C. The supernatant was collected and transferred to a flat-bottom 96-well plate (Greiner Bio-One) and spun down again for 1 min at 1,000 rpm and 4°C. 5 μl of each sample was added to 150 μl of warm Infinity Triacylglycerides Reagent (Thermo Scientific) and gently shaken at 37°C for 10 minutes. Absorbance readings were taken at 520 nm using a Synergy 2 96-well microplate reader (BioTek). Total triacylglyceride amount was calculated using a 6-point standard curve constructed from known triacylglyceride values (Pointe Scientific).
MARCM Analysis
Mosaic fly populations were generated using the MARCM principles described by Lee and Luo (Lee and Luo, 2001). Virgin P{hsFLP}1; UAS-TrpA1(2), P{neo-FRT}40A female flies were crossed to male tub-GAL80-2, P{neo-FRT}40A; NPF-GAL4(3), UAS-mCD8::GFP-3 flies. Mated females were allowed to lay eggs in bottles for 6h, 12h, 18h, or 24h on CT food, followed immediately by a 1h 37°C heat pulse treatment to mobilize FLP recombinase. A non-heat shock cohort was set up to assess the extent of phenotypic variability in the heat-shock treated mosaic fly population. Eggs/larvae/pupae were allowed to develop under 23°C, 12h L:12h D conditions until adult emergence. Newly eclosed male flies were allowed to mate with females and aged for 7–10d on SY10 diet. Adult male flies were subsequently tested for sleep-wake behavior for 3 days at 23°C followed by 4 days at 29°C. A subset of the flies tested for sleep-wake behavior was allowed to acclimate for 2 days at 23°C, followed by 2 days of FLIC feeding behavior testing at 29°C. After completion of all behavioral testing, adult brains were examined to determine adult GFP expression.
Brain Immunohistochemistry
Adult Drosophila brain immunostaining was performed using a method adapted from Wu and Luo (Wu and Luo, 2006). Adult brains were dissected and fixed in phosphate-buffered saline (PBS), pH 7.4 containing 3.7% formaldehyde for approximately 1 h. Fixed brains were subjected to 3 quick and 3, 20 min washes in PBS containing 0.1% Triton X-100 (PBS-T). Brains were blocked using 5% normal goat serum solution in 0.1% PBS-T (NGS) for at least 30 min at room temperature and gently rocked in primary antibody diluted in 5% NGS for 1 or 2 nights at 4°C. After primary antibody incubation, brains were washed in PBS-T, and gently rocked in Alexa Fluor secondary antibody diluted 1:500 in 5% NGS for 1 night at 4°C. After a thorough PBS-T wash, stained brains were mounted onto a glass microscope slide in VECTASHIELD Antifade Mounting Medium (Vector Laboratories) using a #1.5 cover glass. Samples were imaged on an Olympus Fluoview 500 confocal microscope using either a 20X air or 60X water lens objective. Image processing was performed using the Fluoview software and ImageJ (NIH). Rabbit-anti-NPF antibody (1:2,000 dilution; P. Shen), rat-anti-PER antibody (1:500 dilution; O.T. Shafer), mouse-anti-nc82 (1:50; Developmental Studies Hybridoma Bank) was used for primary antibody staining. Alexa Fluor 488, 568, and 633 (Life Technologies) were used for secondary antibody staining. All reported images are representative maximum projection images compiled from 1–1.25 μm thick sections.
Statistics
The OriginPro software (OriginLab Corp.) was used to perform statistical tests and prepare graphs. To evaluate differences among group means, p-values were determined using either Student’s t-test or One-Way Analysis of Variance (ANOVA) with a Bonferroni post-hoc test. For starvation response experiments, equality between groups was assessed using the log-rank test described previously (Linford et al., 2013). Pearson’s r was used to report the extent of correlation between wakefulness and feeding and the p-value was determined by ANOVA for the linear regression analysis.
Supplementary Material
Highlights.
Activation of the NPF-NPFR signaling axis in the brain rapidly decreases sleep
NPF signaling conveys hunger status and modulates feeding behavior
Sleep-wake behavior and feeding are regulated by different subsets of NPF+ neurons
NPF+ circadian pacemaker neurons largely control wakefulness, but not feeding
Acknowledgments
We thank members of the Pletcher Lab for experimental advice and critical discussion of the manuscript, especially M. Waterson and Z. Harvanek for help with MARCM experiments. We also would like to thank the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center (VDRC), and the generosity of P.Shen, O.T. Shafer, P. Garrity, R. Baines, L. Buttitta, N. Perrimon, and S. Goodwin for sharing reagents used in this study. Imaging was performed at the University of Michigan Microscopy and Image Analysis Laboratory. This work was supported by the following sources: the Ellison Medical Foundation to S.D.P.; NIH grants R01AG030593 and R01AG023166 from the National Institute on Aging (NIA) to S.D.P.; NIH Institutional Training Grant T32AG000114 to the University of Michigan Geriatrics Center to J.R. and B.Y.C.; NIH Institutional Training Grant T32GM007315 to the Cellular and Molecular Biology Program at University of Michigan and Ruth L. Kirschstein National Research Service Award F31AG047696 from the NIA to J.R.; Ruth L. Kirschstein National Research Service Award F32AG042253 from the NIA to B.Y.C.
Footnotes
Author Contributions:
Conceptualization, B.Y.C. and S.D.P.; Methodology, B.Y.C. and S.D.P.; Validation, B.Y.C., S.H., K.M.M., and L.G.; Formal Analysis, B.Y.C, J.R., and S.D.P.; Investigation, B.Y.C., J.R., S.H., K.M.M., and L.G.; Resources, S.K. and S.D.P.; Writing – Original Draft, B.Y.C.; Writing – Review & Editing, B.Y.C. and S.D.P.; Supervision, B.Y.C. and S.D.P.; Funding Acquisition, B.Y.C., J.R., and S.D.P.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- CHEN J, REIHER W, HERMANN-LUIBL C, SELLAMI A, COGNIGNI P, KONDO S, HELFRICH-FORSTER C, VEENSTRA JA, WEGENER C. Allatostatin A Signalling in Drosophila Regulates Feeding and Sleep and Is Modulated by PDF. PLoS Genet. 2016;12:e1006346. doi: 10.1371/journal.pgen.1006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG BY, KILMAN VL, KEATH JR, PITMAN JL, ALLADA R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–90. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONG X, WANG H, LIU Z, HE C, AN C, ZHAO Z. Regulation of Sleep by Insulin-like Peptide System in Drosophila melanogaster. Sleep. 2015;38:1075–83. doi: 10.5665/sleep.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONLEA JM, PIMENTEL D, MIESENBOCK G. Neuronal machinery of sleep homeostasis in Drosophila. Neuron. 2014;81:860–72. doi: 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENDRON CM, CHUNG BY, PLETCHER SD. The sensory system: More than just a window to the external world. Commun Integr Biol. 2015;8:e1017159. doi: 10.1080/19420889.2015.1017159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENDRON CM, KUO TH, HARVANEK ZM, CHUNG BY, YEW JY, DIERICK HA, PLETCHER SD. Drosophila life span and physiology are modulated by sexual perception and reward. Science. 2014;343:544–8. doi: 10.1126/science.1243339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVANEK ZM, LYU Y, GENDRON CM, JOHNSON JC, KONDO S, PROMISLOW DEL, PLETCHER SD. Perceptive Costs of Reproduction Drive Aging and Physiology in Male Drosophila. Nature Ecology & Evolution. 2017 doi: 10.1038/s41559-017-0152. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE C, YANG Y, ZHANG M, PRICE JL, ZHAO Z. Regulation of sleep by neuropeptide Y-like system in Drosophila melanogaster. PLoS One. 2013;8:e74237. doi: 10.1371/journal.pone.0074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERGARDEN AC, TAYLER TD, ANDERSON DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A. 2012;109:3967–72. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANN C, YOSHII T, DUSIK V, HELFRICH-FORSTER C. Neuropeptide F immunoreactive clock neurons modify evening locomotor activity and free-running period in Drosophila melanogaster. J Comp Neurol. 2012;520:970–87. doi: 10.1002/cne.22742. [DOI] [PubMed] [Google Scholar]
- JA WW, CARVALHO GB, MAK EM, DE LA ROSA NN, FANG AY, LIONG JC, BRUMMEL T, BENZER S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–6. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEENE AC, DUBOUE ER, MCDONALD DM, DUS M, SUH GS, WADDELL S, BLAU J. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20:1209–15. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM WJ, JAN LY, JAN YN. A PDF/NPF neuropeptide signaling circuitry of male Drosophila melanogaster controls rival-induced prolonged mating. Neuron. 2013;80:1190–205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONDO S, UEDA R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195:715–21. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRASHES MJ, DASGUPTA S, VREEDE A, WHITE B, ARMSTRONG JD, WADDELL S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–27. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE G, BAHN JH, PARK JH. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc Natl Acad Sci U S A. 2006;103:12580–5. doi: 10.1073/pnas.0601171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE T, LUO L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- LINFORD NJ, BILGIR C, RO J, PLETCHER SD. Measurement of lifespan in Drosophila melanogaster. J Vis Exp. 2013 doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINFORD NJ, CHAN TP, PLETCHER SD. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 2012;8:e1002668. doi: 10.1371/journal.pgen.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUQUET S, PEREZ FA, HNASKO TS, PALMITER RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- NASSEL DR, WINTHER AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- PARISKY KM, AGOSTO J, PULVER SR, SHANG Y, KUKLIN E, HODGE JJ, KANG K, LIU X, GARRITY PA, ROSBASH M, GRIFFITH LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFFENBERGER C, LEAR BC, KEEGAN KP, ALLADA R. Processing sleep data created with the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010 doi: 10.1101/pdb.prot5520. pdb prot5520. [DOI] [PubMed] [Google Scholar]
- PITMAN JL, MCGILL JJ, KEEGAN KP, ALLADA R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–6. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- RETZKE T, THOMA M, HANSSON BS, KNADEN M. Potencies of effector genes in silencing odor-guided behavior in Drosophila melanogaster. J Exp Biol. 2017 doi: 10.1242/jeb.156232. [DOI] [PubMed] [Google Scholar]
- REZAVAL C, PAVLOU HJ, DORNAN AJ, CHAN YB, KRAVITZ EA, GOODWIN SF. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 2012;22:1155–65. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RO J, HARVANEK ZM, PLETCHER SD. FLIC: high-throughput, continuous analysis of feeding behaviors in Drosophila. PLoS One. 2014;9:e101107. doi: 10.1371/journal.pone.0101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT K, BRADY R, JR, CRAVCHIK A, MOROZOV P, RZHETSKY A, ZUKER C, AXEL R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–73. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- SHOHAT-OPHIR G, KAUN KR, AZANCHI R, MOHAMMED H, HEBERLEIN U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–5. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG W, VEENSTRA JA, PERRIMON N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9:40–7. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLERU D, PENG Y, AGOSTO J, ROSBASH M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–8. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- SZENTIRMAI E, KRUEGER JM. Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R473–80. doi: 10.1152/ajpregu.00919.2005. [DOI] [PubMed] [Google Scholar]
- UENO T, TOMITA J, TANIMOTO H, ENDO K, ITO K, KUME S, KUME K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 2012;15:1516–23. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- WANG Z, SINGHVI A, KONG P, SCOTT K. Taste representations in the Drosophila brain. Cell. 2004;117:981–91. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- WIATER MF, MUKHERJEE S, LI AJ, DINH TT, ROONEY EM, SIMASKO SM, RITTER S. Circadian integration of sleep-wake and feeding requires NPY receptor-expressing neurons in the mediobasal hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1569–83. doi: 10.1152/ajpregu.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU JS, LUO L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc. 2006;1:2110–5. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- WU Q, WEN T, LEE G, PARK JH, CAI HN, SHEN P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–61. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.