Abstract
Mesenchymal stem cells (MSCs) have emerged as a potentially powerful cellular therapy for autoimmune diseases including multiple sclerosis (MS). Based on their success in treating animal models of MS like experimental autoimmune encephalomyelitis (EAE), MSCs have moved rapidly into clinical trials for MS. The majority of these trials use autologous MSCs derived from MS patients, although it remains unclear how CNS disease may affect these cells. Here, we report that bone marrow MSCs derived from EAE mice lack therapeutic efficacy compared to naïve MSCs in their ability to ameliorate EAE. Treatment with conditioned medium from EAE-MSCs also fails to modulate EAE, and EAE-MSCs secrete higher levels of many pro-inflammatory cytokines compared to naïve MSCs. Similarly, MSCs derived from MS patients have less therapeutic efficacy than naïve MSCs in treating EAE and secrete higher levels of some of the same pro-inflammatory cytokines. Thus diseases like EAE and MS diminish the therapeutic functionality of bone marrow MSCs, prompting reevaluation about the ongoing use of autologous MSCs as a treatment for MS.
Keywords: mesenchymal stem cells (MSCs), multiple sclerosis, experimental autoimmune encephalomyelitis
Introduction
Transplantation of mesenchymal stem cells (MSCs) has recently emerged as a promising new therapeutic approach for the treatment of both autoimmune and neurological diseases including multiple sclerosis (MS) (Cohen, 2013). MSCs are a multipotent, non-hematopoietic class of stem cell that can be isolated from a variety of tissues, including the bone marrow (Kolf et al., 2007). MSCs possess strong immunomodulatory and regenerative properties that are derived from their ability to secrete a wide array of diverse chemokines, cytokines, and trophic factors (Caplan and Dennis, 2006; Chen et al., 2006), and have been shown to be effective in modulating disease progression in a number of different conditions, including graft vs host disease and rheumatoid arthritis (English et al., 2010; Sargent and Miller, 2016).
Based on their immunomodulatory properties and success in treating other diseases, bone marrow-derived MSCs (BM-MSCs) were seen as a strong candidate for a cell-based therapy for MS (Miller et al., 2010), and have been tested in a number of animal models of MS, including experimental autoimmune encephalomyelitis (EAE). Several independent laboratories have shown that when transplanted systemically into mice with ongoing EAE, BM-MSCs rapidly halt disease progression and improve recovery (Bai et al., 2009; Zappia et al., 2005; Zhang et al., 2005). BM-MSCs appear to mediate recovery in EAE not by replacing cells lost to disease but rather by suppressing the immune response and inhibiting CNS inflammation while also promoting remyelination and neural repair (Morando et al., 2012). At the cellular level, transplanted BM-MSCs are thought to modulate disease progression by secreting multiple factors that inhibit inflammation and/or promote tissue repair (Jumah and Abumaree, 2012), including immunomodulatory cytokines like prostaglandin e2 and TGFB and trophic factors like hepatocyte growth factor (HGF) (Bai et al., 2012; Liu et al., 2009; Matysiak et al., 2011).
Due to their success in animal models of MS, BM-MSCs have moved into clinical trials in MS patients. The majority of these trials utilize autologous MSCs (derived from MS patients) (Meamar et al., 2016), in contrast to previous work in animal models that utilized BM-MSCs from healthy animal or human donors. While preliminary clinical trials using autologous MSCs report good safety data most report limited therapeutic efficacy (Connick et al., 2012; Karussis et al., 2010; Mohyeddin Bonab et al., 2007; Yamout et al., 2010). One possible explanation for the limited effects of autologous BM-MSCs in clinical trials is that autoimmune diseases like MS alter the functionality of MSCs and reduce their therapeutic potential.
In order to determine if inflammatory diseases like MS might alter MSC functionality, we cultured MSCs from the bone marrow of MOG35–55 induced EAE mice at different phases of the disease and compared them to naïve MSCs in terms of their therapeutic efficacy. We found that EAE-MSCs fail to improve recovery when transplanted into EAE mice, in contrast to the strong therapeutic effect observed following transplantation of naïve MSCs. Our data suggests this lack of therapeutic functionality of EAE-MSCs stems from differences in the paracrine factors they secrete relative to naïve MSCs, as EAE-MSCs secrete higher levels of many pro-inflammatory cytokines, including IL-6 and CCL2. Similarly, we found that MSCs derived from patients with MS (MS-MSCs) also lack therapeutic efficacy in treating EAE and secrete higher levels of the same pro-inflammatory cytokines. Our results show that diseases like EAE and MS diminish the therapeutic functionality of BM-MSCs, raising concern about the continued use of autologous BM-MSCs in the treatment of MS.
Materials and Methods
EAE induction and scoring
EAE was induced in 10–12 week-old-female C57BL/6 mice (Jackson Laboratory: 000664) using Hooke Labs MOG35–55 EAE Induction kits according to the manufacturer’s protocol. Briefly, mice were immunized via subcutaneous injection of 200ul of MOG35–55 peptide in complete Freund’s adjuvant. Pertussis toxin (250 ug) was injected intraperitoneally at 2 and 24 hours post immunization. Animals began showing signs of paralysis 9–11 days post immunization, and were graded by blinded observers according to a previously described clinical index (Bai et al., 2012): 1 = limp tail, 2 = hind limb weakness, 3 = plegia of one limb, 4 = plegia of two limbs, 5 = moribund or dead.
MSC culture and treatment protocols
Mouse MSC isolation and culture
Mouse mesenchymal stem cells were isolated and cultured from the bone marrow of MOG35–55 – induced EAE mice at either 14 days (peak EAE-MSCs) or 28 days (chronic EAE-MSCs) after immunization, with the animals having a clinical score of 4 or higher. Naïve MSCs were cultured from non-immunized, age-matched C57BL/6 mice. Each culture preparation consisted of MSCs derived from 4–6 mice, and a different culture preparation was used for each experiment. Growth medium for all mouse cultures consisted of α-MEM with GLUTAMAX (Gibco) supplemented with 10% MSC-qualified fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco).
MSCs were isolated according to a previously published protocol (Soleimani and Nadri, 2009). Briefly, bone marrow from the tibias and fibulas was collected by flushing out each bone’s central canal with a 26 × g syringe containing fresh growth medium. Bone marrow cells were then seeded in P75 flasks (Corning) at a concentration of 2 × 10^5 cells/cm^2, with cells grown in a 37°C with 5% CO2. Flasks were washed twice with media 48 hours later to remove non-adherent cells, with medium changed every 2–3 days. Cells were then passaged using 0.25% Trypsin/EDTA (Gibco) for 2 minutes at 37°C and re-plated in P75 flasks at a concentration of 1 × 10^4 cells/cm^2. Cells derived from both naïve and EAE mice were identified through immunolabeling as a purified population of MSCs (Figure 5), and were subsequently expanded with cells from passages 2–4 (21–30 days in-vitro) used for all experiments. For cell transplantation experiments, 0.8 × 10^6 MSCs in saline were delivered intravenously into EAE mice via tail vein injection at 15 days post immunization. In experiments where MSCs were first labeled with CMTPX (ThermoFisher), cells were incubated with 20 uM dye for 2 hours immediately prior to infusion according to the manufacturer’s protocol.
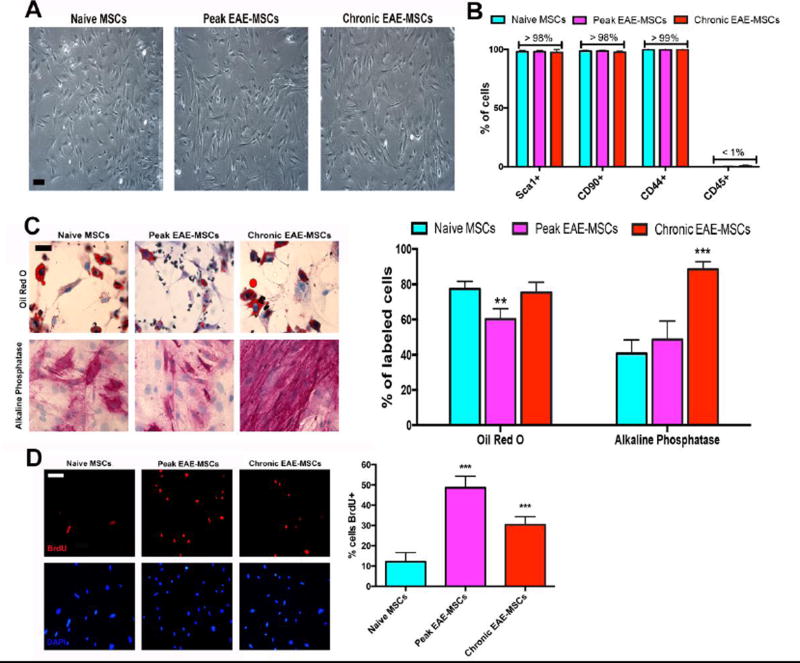
Figure 5. Comparison of cultured EAE-MSCs with naïve MSCs.
(A) Phase contrast images of passage 3 naïve MSCs versus peak and chronic EAE-MSCs showing cells have similar morphologies. (B) Both peak and chronic EAE-MSCs express common MSC markers and are CD45 negative like naïve MSCs. Data shown = mean + SEM, n = 3 experiments. (C) (Left) Representative images of naïve and EAE-MSCs differentiated into Oil-Red O positive adipocytes and Alkaline Phosphatase (ALP) positive osteoblasts. (Right) Slightly fewer peak EAE-MSCs differentiate into Oil-Red O stained adipocytes compared to naïve or chronic EAE MSCs, whereas differentiation of chronic EAE-MSCs into ALP-positive osteoblasts is significantly increased. Data shown = mean + SEM, n = 2 experiments. **P<0.01, ***P<0.005, One-way ANOVA. (D) (Left) Representative images of BrdU labeled naïve MSCs versus peak and chronic EAE-MSCs after a 16 hour BrdU pulse. (Right) The proportion of cells labeled with BrdU (16 hour pulse) is significantly higher for peak and chronic EAE-MSCs versus naïve MSCs. Data shown = mean + SEM, n = 3 experiments, ***P<0.005, One-way ANOVA. Scale bar in (A) = 50um, (C) and (D) = 20 um.
Human MSC isolation and culture
For functional studies, bone marrow aspirates were obtained from the iliac crest of patients at the Southwest Hospital of the Third Military Medical University. For molecular studies, marrow aspirates were obtained from the posterior superior iliac crest of patients at University Hospitals Case Medical Center. MS-MSCs were obtained from bone marrow aspirates derived from 3 separate female donors diagnosed with relapsing-remitting MS, while naïve MSCs were obtained from bone marrow aspirates from female age-matched donors. Both MS-MSCs and naïve MSCs were isolated from the bone marrow aspirates and expanded under identical culture conditions at Case Western Reserve School of Medicine according to standard protocols used in the Cellular Therapy Laboratory at Case Western Reserve University (Romieu-Mourez et al., 2009). Growth medium consisted of low glucose DMEM (Invitrogen), 10% fetal bovine serum [FBS] (Gibco), 1% antibiotic/antimycotic, 1% Glutamax (Gibco), and 10 ng/ml fibroblast growth factor (Peprotech). After being expanded for 2–4 passages, 4 × 106 hMSCs per milliliter were frozen in cryovials using Plasma-Lyte A, containing 10% dimethyl sulfoxide and 5% human serum albumin as the freezing medium.
Prior to transplantation or collection of conditioned medium, hMSCs were thawed, reseeded into P75 flasks at a concentration of 7.5×10^ 3 cells / cm^ 2, and grown for 1–2 additional passages (4–7 days) in complete growth medium. 1×10^6 hMSCs in saline were then intravenously infused via the tail vein into MOG35–55 – induced EAE mice at 14 days post immunization.
Tissue processing & histopathology
For immunohistochemistry, mice were perfused transcardially, and tissue was dissected out and processed as previously described (Bai et al., 2012). Antibodies used including rat anti-CD45 (BioLegend; 103101) and rat anti-CD3 (BioLegend; 100201). To assess the amount of myelin loss, sections were also stained for Eriochrome Cyanine (EC) using a previously published protocol (DePaul et al., 2015). Lesion load was calculated from six serial sections of the lumbar spinal cord by measuring lesion area and dividing it by the total area of white matter. Observers blinded to sample treatment performed cell counting and lesion load analysis. For electron microscopy, animals were perfused with 2.5% glutaraldehyde (Electron Microscopy Sciences). Spinal cords were then post-fixed in 1% osmium tetroxide (Sigma) for 1 hour, dehydrated and embedded in epoxy resin. One-micrometer epoxy sections were stained with toluidine blue and examined by light microscopy.
Collection and analysis of MSC CM
Conditioned medium was collected from naïve or EAE MSCs grown in P75 flasks that contained 1.5 × 10^ 6 cells; medium was collected between 21 and 24 days in-vitro, with unconditioned medium collected as a control. Conditioned medium was collected from a different culture preparation for each experiment. Conditioned medium was likewise collected from passage 3 MS-MSCs and DN-MSC between 21–25 DIV. Analysis of murine MSC conditioned medium was performed using the RayBio Mouse Inflammation Antibody Array C1 (Raybiotech), while analysis of human MSC conditioned medium was performed using the RayBio Human Cytokine Antibody Array C5. Arrays were treated and analyzed according to the manufacturer’s instructions. Densitometric quantification of spot intensities for each cytokine was performed using ImageJ, with spot intensity values normalized to cell number. To account for any signal in the arrays that may have been due to serum proteins present in the growth medium, arrays of unconditioned medium were also analyzed and any spot intensity values observed were subtracted from values obtained for MSC conditioned medium. HGF levels were quantified via ELISA (R&D Systems).
For experiments where mice were treated with MSC conditioned medium, aliquots were thawed and the total amount of protein was quantified using a Pierce Bradford Protein Assay Kit (Thermofisher Scientific) according to the manufacturer’s instructions. Then 0.5 mg of total protein (approximately 100ul of medium) was delivered intravenously into EAE mice via tail vein injection at 16 days post immunization.
Splenocyte culture and recall assay
Mouse splenocytes were cultured from MOG35–55 induced EAE mice at 14 days post induction using a previous protocol (Zappia et al., 2005). Briefly, spleens were dissected out and manually dissociated in culture medium (10% FBS, 90% RPMI 1640 medium, 1% sodium pyruvate, 1%, non-essential amino acids, 1% penicillin/streptomycin), and passed through a 70 um cell strainer to generate a single cell suspension. After centrifugation cells were re-suspended in 2 mL BD Pharm Lyse Solution (BD Biosciences) for 2 minutes to lyse out red blood cells, and the purified splenocytes were re-suspended in fresh culture medium before being plated in 96 well plates, 2×10^5 cells per well.
To test the effects of MSC CM on MOG35–55 induced recall, cells were cultured in medium that contained 50ul splenocyte culture medium and 50ul MSC CM or unconditioned medium (controls). MOG35–55 peptide dissolved in culture medium (Hooke Labs) was then added to each well to a final concentration 20uM MOG35–55 per well. Twenty-four hours later, BrdU reagent (10uM) was added to each well, and twenty-four hours after that the plates were fixed and processed according to the manufacture’ protocol for BrdU Elisa (Abcam). The Stimulation Index (SI) for each treatment was calculated as SI = (Absorbance value of MOG stimulated cells) / (Absorbance value of un-stimulated cells). Experiments were replicated three times.
MSC characterization
For characterization of mouse MSCs, cells were stained for surface markers using a previously described protocol (Bai et al., 2009). Antibodies used include: anti-CD45 (Abcam: ab25386), anti-CD44 (Abcam: ab25340), anti-CD90 (BioLegend: 206101), and anti Sca-1 (BioLegend: 108101). For characterization of human MSCs, cells were stained and analyzed via flow cytometry as previously described (Bai et al., 2009), with antibodies used including anti-CD90 (Abcam: ab11155), anti-CD105 (Abcam: ab2529), and anti-STRO1 (Abcam: ab190282). Differentiation of MSCs was carried out using a MSC Functional Identification Kit (R&D Systems) according to the manufacturer’s instructions. Coverslips were stained with a 0.5% Oil-Red O solution (Sigma) to assess differentiation into adipocytes or stained with an Alkaline Phosphatase detection kit (EMD Millipore) to assess differentiation into osteoblasts. To assess proliferation, MSC treated with 10 uM BrdU (Sigma) for sixteen hours. Coverslips were then fixed for 10 minutes with 4% PFA and stained for rat anti-BrdU (Abcam: ab6326) using a previously described protocol (Zacharaki et al., 2013).
Statistical Analysis
All statistical tests were performed using GraphPad Prism 6. All statistical tests are indicated in text or figure legends, with Dunnett’s multiple comparison tests performed post-hoc for one-way ANOVAs. P values of ≤0.05 were considered statistically significant.
Study Approval
All animal experiments were approved by Case Western Reserve University School of Medicine’s IACUC with adherence to the NIH Guide for the Care and Use of Laboratory Animals. Human bone marrow cells were obtained after informed written consent from patients in accordance with a protocol approved by the institutional review board at the Cleveland Clinic or Third Military Medical University.
Results
Transplanted EAE-MSCs fail to significantly improve recovery in EAE
In order to determine if disease affects the therapeutic functionality of BM-MSCs, and to better model transplantation of autologous MSCs as a therapy for MS, MSCs were derived from the bone marrow of MOG35–55 EAE mice at two distinct phases of the disease: at 14 days post induction during the peak of the disease (“peak EAE-MSCs”), and later at 28 days post induction during the chronic phase of EAE (“chronic EAE-MSCs”). Both peak and chronic EAE-MSCs derived from multiple donors were then expanded and systemically delivered into cohorts of MOG35–55 - induced EAE mice and compared to naïve MSCs in their therapeutic efficacy.
While intravenous infusion of naïve MSCs improved functional recovery in EAE mice, infusion of EAE-MSCs produced no significant improvement in clinical recovery. Infusion of naïve MSCs (0.8×10^6) into MOG35–55 EAE mice 15 days after EAE induction resulted in a rapid improvement in clinical score. The improvement in functional recovery in mice treated with naïve MSCs was sustained for up to 1 month after infusion (Figure 1A). Cumulative disease score after 30 days (measured as area under the curve) was significantly lower in EAE mice treated with naïve MSCs relative to controls (116 ± 6 naïve MSCs: 190 ± 9 controls; p<0.001, n =12 mice per group from 3 independent experiments, one-way ANOVA). By contrast, infusion of peak or chronic EAE-MSCs (0.8×10^6) into MOG35–55 EAE mice 15 days after EAE induction failed to produce comparable improvements in functional recovery. Though infusion of peak EAE-MSCs resulted in a small improvement in average clinical score 6 days after treatment (2.8 ± 0.28 compared to 3.3 ± 0.28 for controls), this improvement was transient, as the average clinical score returned to that of controls within a few days (Figure 1A). Consistent with these observations, mice treated with peak EAE-MSCs showed no significant difference from controls in cumulative disease score (183 ± 13 compared to 190 ± 9 for controls; p>0.05, n =12 mice per group from 3 independent experiments, one-way ANOVA). Infusion of chronic EAE-MSCs failed to produce any improvement in functional recovery (Figure 1A), with mice receiving chronic EAE-MSCs showing no significant difference from controls in cumulative disease score after 30 days of treatment (194 ± 5 compared to 190 ± 9 for controls; p>0.05, n =12 mice per group from 3 independent experiments, one-way ANOVA).
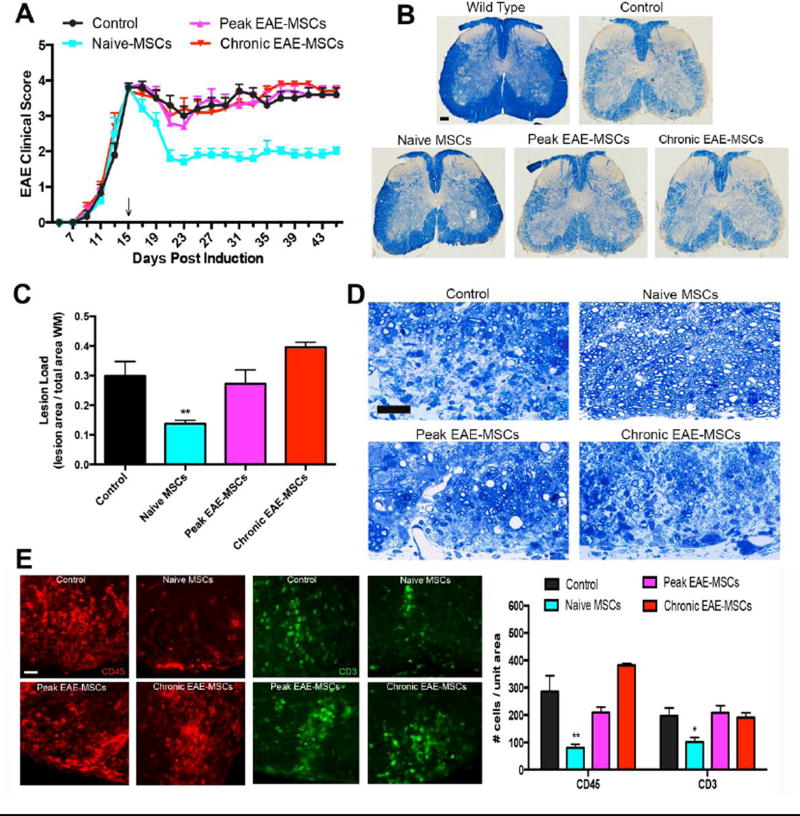
Figure 1. Transplanted EAE-MSCs fail to improve functional recovery or CNS pathology in EAE mice.
(A) Infusion of naïve MSCs leads to improved functional recovery in MOG35–55 EAE-mice, while treatment with peak or chronic EAE-MSCs does not improve functional recovery versus saline-treated controls. 0.8 × 10^6 MSCs were intravenously injected into mice 15 days after EAE induction (arrow). Data shown represents mean + SEM, n=12 mice per group, from 3 independent experiments. (B) Representative images of solochrome cyanine stained spinal cord sections showing myelin loss in EAE mice 30 days after treatment with naïve MSCs versus peak or chronic EAE-MSCs. (C) EAE mice treated with naïve MSCs have less myelin loss and lower lesion load, while mice treated with EAE-MSCs have a higher lesion load that is comparable to controls. Data shown = mean + SEM, with lesion load quantified from 6 solochrome cyanine stained sections per mouse, 3 mice per group; **P<0.01, One-way ANOVA. (D) Representative images of Toluidine blue stained spinal cord sections from EAE mice 30 days after treatment confirms that mice treated with naïve MSCs have more myelinated axons and less inflammatory infiltrates than controls. By contrast, animals treated with peak or chronic EAE-MSCs show no appreciable difference from controls. (E) EAE-mice treated with naïve MSCs have significantly lower numbers of CD45 positive inflammatory cells (red) and CD3 positive T-cells (green) in their spinal cords 30 days after treatment, while mice treated with peak or chronic EAE-MSCs show no significant difference from controls. Data shown in graph = mean + SEM, quantified from 4 sections per animal, 3 animals per group. *P<0.05, **P<0.01, One-way ANOVA. Scale bars in (B) = 500um, (D) = 25 um, (E) = 20um.
This lack of functional recovery in mice treated with EAE-MSCs also correlated with a lack of improvement in CNS histology. Solochrome cyanine staining of spinal cord sections showed that mice infused with naïve MSCs had less white matter loss at 30 days post treatment compared to saline treated controls, whereas mice treated with peak or chronic EAE-MSCs had higher levels of myelin loss similar to controls (Figure 1B). Quantification of lesion load from solochrome cyanine stained spinal cord sections 30 days after treatment demonstrated that mice treated with naïve MSCs had a significantly lower lesion load compared to controls, while mice treated with peak or chronic EAE-MSCs had no significant difference from controls (Figure 1C). Toluidine blue staining of spinal cord sections from EAE mice 30 days after treatment confirmed that mice treated with naïve MSCs had more myelinated axons and less inflammatory infiltrates compared to controls, in contrast to mice treated with peak or chronic EAE-MSCs, which showed no appreciable difference (Figure 1D). There was a significant reduction in the number of CD45 positive immune cells and CD3 positive T-cells in spinal cord sections of EAE mice treated with naïve MSCs, while mice treated with either peak or chronic EAE-MSCs had higher numbers of inflammatory cells and T-cells similar to controls (Figure 1E).
Overall, mice treated with EAE-MSCs failed to show any substantial or sustained improvement in clinical recovery or neuropathology, in contrast to mice treated with naïve MSCs. One possible explanation for these results is that EAE-MSCs might fail to migrate and engraft into their hosts at equivalent levels to naïve MSCs. To test this hypothesis, peak EAE-MSCs, chronic EAE-MSCs, and naïve MSCs were labeled with the fluorescent cell tracking dye CMTPX (Supplemental Figure 1A). CMTPX-labeled MSCs were then intravenously infused into MOG35–55 EAE mice (0.5×10^6 cells/animal) at 18 days after EAE induction, to assess if EAE-MSCs were any different than naïve MSCs in where they engrafted. One day after infusion, naïve MSCs and EAE-MSCs had migrated into the liver, spleen, and lungs (Supplemental Figure 1B). No CMTPX-labeled MSCs were observed in the heart, kidney, brain or spinal cord (data not shown), consistent with previous studies demonstrating that MSCs do not engraft into the CNS of EAE mice (Gerdoni et al., 2007; Zappia et al., 2005). No difference in the number of peak or chronic EAE-MSCs compared to the number of naïve MSCs was observed in any of these tissues at one or seven days after infusion (Supplemental Figure 1D), suggesting the lack of therapeutic efficacy observed in transplanted EAE-MSCs is not due to lack of cell engraftment or survival.
Conditioned medium from EAE-MSCs fails to ameliorate EAE
Previous studies suggest that naïve MSCs mediate recovery in EAE by secreting various cytokines and growth factors that suppress inflammation and promote remyelination and neural repair (Morando et al., 2012). Much of the therapeutic benefits of transplanting naïve MSCs into EAE mice can be recapitulated by treating mice with conditioned medium (CM) from MSCs (Bai et al., 2012). To determine if the diminished therapeutic functionality of EAE-MSCs was due to differences in paracrine factors they secreted relative to naïve MSCs, conditioned medium was collected from naïve MSCs (naïve MSC CM), peak EAE-MSCs (peak EAE-MSC CM), and chronic EAE-MSCs (chronic EAE-MSC CM), and intravenously infused into MOG35–55 - induced EAE mice.
While treatment with conditioned medium from naïve MSCs improved functional recovery in EAE mice, treatment with CM from EAE-MSCs produced little or no improvement in clinical recovery. Mice treated with naïve MSC CM (0.5 mg total protein, approximately 0.1 mL) at 16 days post EAE induction showed substantial improvement in clinical score compared to control mice treated with unconditioned medium (Figure 2A). This improvement in functional recovery persisted for approximately one week, with the cumulative disease score of naïve MSC CM treated mice being significantly lower than controls (61 ± 2 compared to 89 ± 4 for controls; p<0.01, n=11–12 mice per group from 3 independent experiments, one-way ANOVA). By contrast, mice treated with CM from EAE-MSCs failed to show comparable improvements in functional recovery. While mice treated with peak EAE-MSC CM did show a small improvement in average clinical score starting at 11 days post treatment (3.0 ± 0.23 compared to 3.5 ± 0.26 for controls), this persisted for only a few days (Figure 2A), and the cumulative disease score of mice that received peak EAE-MSC CM was not significantly different from controls (84 ± 4 compared to 89 ± 4 for controls; p>0.05, n=11–12 mice per group from 3 independent experiments, one-way ANOVA). Mice infused with CM from chronic EAE-MSCs showed no improvement in clinical score versus controls at any time up to two weeks post-treatment, and had no significant difference in cumulative disease score versus controls (92 ± 4 compared to 89 ± 4 for controls; p>0.05, n=11–12 mice per group from 3 independent experiments, one-way ANOVA).
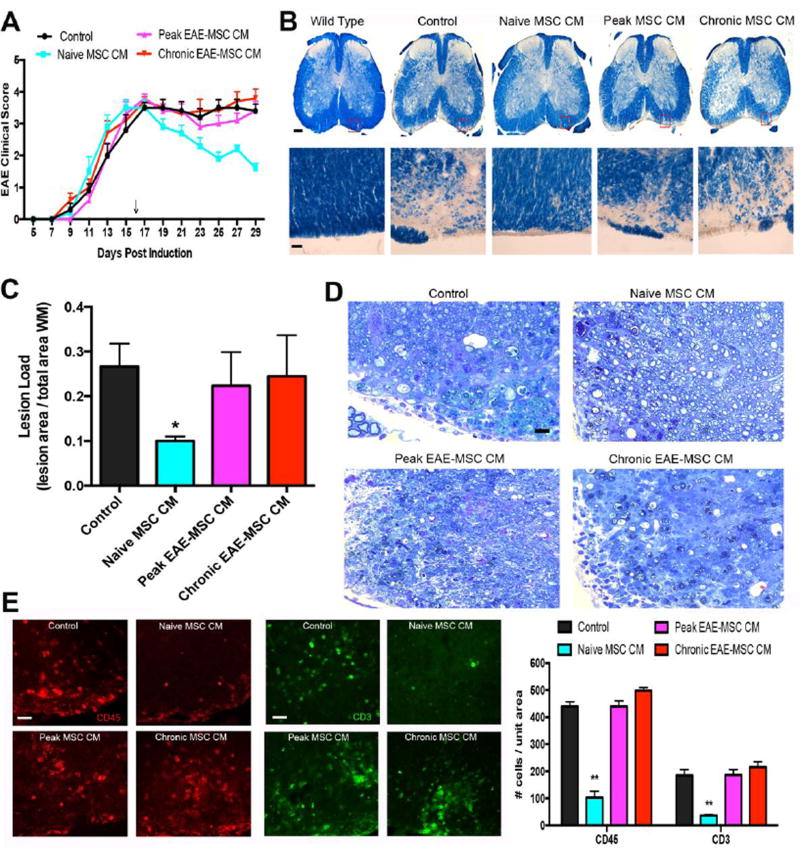
Figure 2. Conditioned medium (CM) from EAE-MSCs fails to improve functional recovery or CNS pathology in EAE mice.
(A) Intravenous infusion of conditioned medium (CM) from naïve MSCs leads to improved functional recovery in MOG35–55 EAE-mice, while infusion of CM from either peak or chronic EAE-MSCs does not improve functional recovery relative to controls (EAE mice that received unconditioned medium). 0.5 mg conditioned medium was given 16 days after EAE induction (arrow), data shown represents mean + SEM, n=11–12 mice per group, from 3 independent experiments. (B) Representative images of solochrome cyanine stained spinal cord sections showing myelin loss in EAE mice 14 days after they received CM from naïve MSCs versus peak or chronic EAE-MSCs. (C) EAE mice treated with naïve MSC CM have less myelin loss and lower lesion load, while mice treated with EAE-MSC CM have a higher lesion load comparable to controls. Data shown = mean + SEM, with lesion load quantified from 6 solochrome cyanine stained sections per mouse, 3 mice per group; *P<0.05, One-way ANOVA. (D) Toluidine blue staining of spinal cord sections in EAE mice 14 days after treatment shows more myelinated axons in mice that received naïve MSC CM, while mice receiving EAE-MSC CM have less myelinated axons and more inflammatory infiltrates similar to controls. (D) EAE-mice treated with naïve MSC-CM have significantly lower numbers of CD45 positive inflammatory cells (red) and CD3 positive T-cells (green) in their spinal cords 14 days after treatment, whereas mice treated with EAE-MSC CM show no significant difference from controls. Data shown in graph = mean + SEM, quantified from 5 sections per animal, 3 animals per group. **P<0.01, One-way ANOVA. Scale bars in (B) (Top)= 500um, (Bottom) = 25um (D) = 25 um, (E) = 20um.
The lack of improvement in functional recovery in mice treated with EAE-MSC CM was paralleled by a lack of improvement in CNS histopathology. Solochrome cyanine staining of spinal cord sections from mice treated with naïve MSC CM had less white matter loss and a significantly lower lesion load compared to controls at 14 days post treatment, whereas mice treated with either peak or chronic EAE-MSC CM had no significant difference in demyelination or lesion load compared to controls (Figure 2B and 2C). Likewise the number of CD45-positive immune cells and CD3-positive T-cells was significantly decreased in spinal cord sections from mice treated with naïve MSC CM compared to controls, but there was no such reduction in the number of inflammatory cells or T cells in mice treated with peak or chronic EAE-MSC CM (Figure 2E).
EAE-MSCs secrete higher levels of pro-inflammatory cytokines
These results suggest the lack of therapeutic functionality observed in EAE-MSCs relative to naïve MSCs is due to differences in their secretion of paracrine factors. To identify factors that are differentially secreted by EAE-MSCs, an antibody array was used to detect and compare expression levels of 40 different candidate inflammatory proteins in the CM of EAE-MSCs versus the CM of naïve MSCs. This array profiles both pro-inflammatory and anti-inflammatory factors important in immune response and EAE pathogenesis, and many of these factors, including TGFB, IL-4, IL6, IL-10, and others, have previously described roles in MSC immunomodulation in EAE or other diseases (Kyurkchiev et al., 2014).
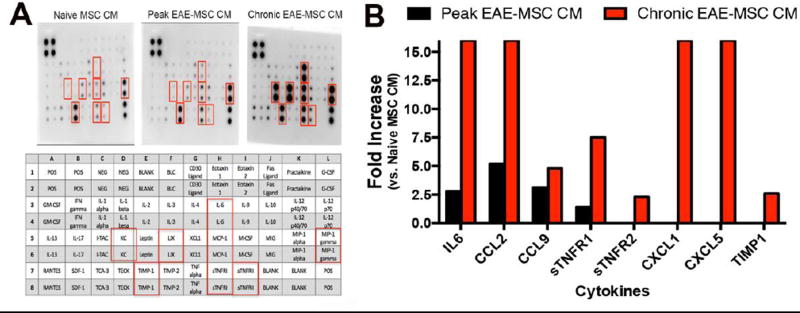
Conditioned medium from EAE-MSCs had considerably higher levels of many pro-inflammatory cytokines compared to conditioned medium from naïve MSCs. Comparison of spot intensity values on respective arrays showed that peak EAE-MSCs secreted 2 to 4 fold higher levels of the pro-inflammatory cytokines IL-6, CCL2, and CCL9 compared to naïve MSCs (Figure 3A and 3B). Chronic EAE-MSCs also secreted higher levels of these three cytokines, but at an even greater magnitude. Conditioned medium from chronic EAE-MSCs had 16 fold higher levels of IL-6 and CCL2, and nearly 5 hold higher levels of CCL9. Also, chronic EAE-MSC CM had a 16-fold increase in the pro-inflammatory cytokines CXCL1 and CXCL5 (Figure 3A and 3B). In total, the antibody array detected 21 out of 40 proteins profiled in the MSC conditioned medium samples; 4 of these proteins (IL-6, CCL2, CCL9, and sTNFR1) were higher in peak EAE-MSC CM compared to naïve MSC CM and 8 of these proteins (IL-6, CCL2, CCL9, sTNFR1, sTNFR2, CXCL1, CXCL5, and TIMP1) were higher in chronic EAE-MSC CM compared to naïve MSC CM. The array found no proteins that were down-regulated in EAE-MSC CM relative to naïve MSC CM.
Figure 3. EAE-MSCs secrete higher levels of pro-inflammatory cytokines.
(A) Representative images of antibody arrays treated with conditioned medium (CM) from naïve MSCs, peak EAE-MSCs, or chronic EAE-MSCs. Cytokines found to be up-regulated in the arrays are indicated by red boxes and identified in the array diagram depicted below; CCL2 = MCP-1, CCL9 = MIP-1 gamma, CXCL1 = KC, and CXCL5 = LIX. (B) Fold changes of proteins increased in conditioned medium from peak or chronic EAE-MSCs relative to naïve MSCs; note conditioned medium from EAE-MSCs contains higher levels of pro-inflammatory cytokines, including IL6, CCL2, and CCL9. Fold changes were calculated by densitometric quantification of spot intensity values from 3 separate antibody arrays per group, n = 3 experiments. Fold changes were capped at 16, with no fold decreases of any cytokines tested observed in the arrays.
In a previous study, hepatocyte growth factor (HGF) was found to be an important mediator of MSC-induced recovery in EAE (Bai et al., 2012). Because HGF was not profiled in the antibody arrays, quantitative ELISA was used to compare HGF levels in naive MSC CM versus EAE-MSC CM. Unexpectedly, peak EAE-MSC CM had significantly higher levels of HGF (approximately a 2 fold increase) compared to naïve MSC CM (Supplemental Figure 2). In contrast, CM from chronic EAE-MSCs had significantly lower levels of HGF (approximately 50%) compared to CM from naïve MSCs (Supplemental Figure 2).
Conditioned medium from chronic EAE-MSCs lacks immunosuppressive functionality in-vitro
The elevated levels of pro-inflammatory cytokines in EAE-MSC CM suggests that these cells may no longer have the immunosuppressive, anti-inflammatory functionality commonly associated with naïve MSCs (Zappia et al., 2005). To test this hypothesis, a splenocyte restimulation assay was employed, in which spleenic lymphocytes were cultured from MOG35–55 induced EAE mice and subsequently restimulated by 20uM MOG35–55 antigen in-vitro driving lymphocyte activation and increased proliferation.
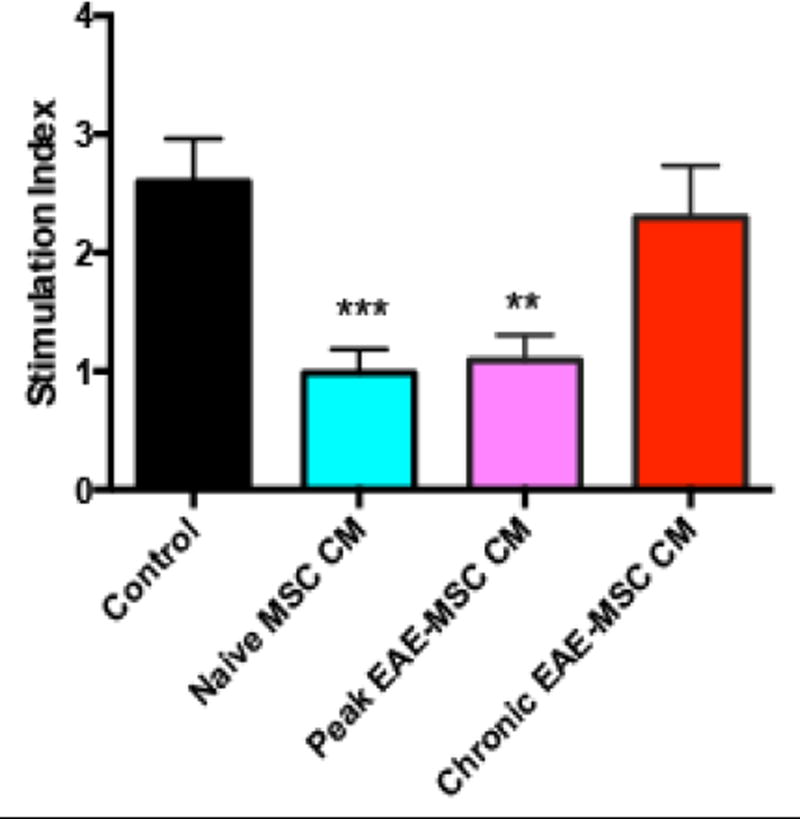
Consistent with previous studies (Gerdoni et al., 2007; Zappia et al., 2005), conditioned medium from naïve MSCs effectively suppressed MOG35–55 induced restimulation of splenocytes in-vitro. As measured by BrdU incorporation, proliferation was significantly decreased in MOG stimulated splenocytes treated with naïve MSC CM compared to control cultures that were treated with unconditioned medium (Figure 4). Surprisingly, conditioned medium from peak EAE-MSCs significantly reduced proliferation of MOG restimulated splenocytes (Figure 4). By contrast, conditioned medium from chronic EAE-MSCs failed to suppress splenocyte response to MOG, as MOG stimulated cultures treated with chronic EAE-MSC CM showed no significant difference in proliferation compared to controls (Figure 4).
Figure 4. Effects of EAE versus naïve MSC CM on MOG-stimulated splenocytes.
Conditioned medium from naïve MSCs or peak EAE-MSCs suppresses MOG induced restimulation of splenocytes in-vitro, whereas conditioned medium from chronic EAE-MSCs fails to significantly inhibit splenocyte proliferation. Proliferation was assessed 48 hours after MOG stimulation (20uM) via BrdU Elisa. Data shown = mean + SEM, n = 3 experiments, **P<0.01, ***P<0.005, One-way ANOVA.
EAE-MSCs differ from naïve MSCs in differentiation and proliferation
EAE-MSCs had similar cellular characteristics that classically define naïve MSCs. For example, EAE-MSCs had a similar morphology to naïve MSCs (Figure 5A), and expressed common mesenchyme and stem cell markers used to identify MSCs in-vitro (Figure 5B, Supplemental Figure 3). EAE-MSCs could also be differentiated along common mesenchymal lineages, into adipocytes and osteoblasts under appropriate culture conditions. However, EAE-MSCs showed significant differences in differentiation potential compared to naïve MSCs (Figure 5C). For instance, when cultured under identical conditions, fewer peak EAE-MSCs differentiated into Oil-Red O positive adipocytes compared to naïve MSCs. Also, significantly more chronic EAE-MSCs differentiated into Alizarin Red positive osteoblasts/osteocytes compared to naïve MSCs, even though all cells were cultured under identical conditions (Figure 5C).
EAE-MSCs proliferated at a significantly higher rate than naïve MSCs in-vitro. As measured by BrdU pulse (10 uM for 16 hours) and subsequent immunolabeling, the proportion of both peak and chronic EAE-MSCs that were BrdU positive was significantly higher versus the percentage of naïve MSCs (Figure 5D). Collectively, this data suggests that while EAE-MSCs have the same functional characteristics used to define naïve MSCs in-vitro, they also possess intrinsic differences in properties like growth rate and differentiation potential, in addition to important differences in cytokine expression and therapeutic functionality.
MSCs derived from MS patients lack therapeutic efficacy in modulating EAE
We next asked whether the diminished therapeutic functionality observed in EAE-MSCs was in fact paralleled by human MSCs derived from MS patients (MS-MSCs). To address this question, BM-MSCs were obtained from 3 different donors diagnosed with relapsing-remitting MS and expanded in-vitro. These MS-MSCs were phenotypically similar to naïve MSCs in their expression of common MSC markers (Supplemental Figure 4). MS-MSCs were then intravenously infused (1×10^6 cells/animal) into MOG35–55 induced EAE mice 14 days after EAE induction to compare them against naïve MSCs (from age and sex matched healthy donors) in their therapeutic potential.
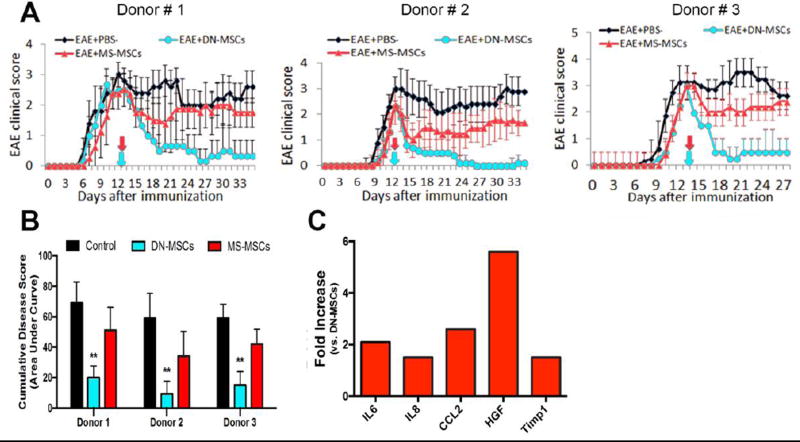
For each of the respective donors, MS-MSCs were less effective at improving functional recovery in MOG - induced EAE mice compared to naïve MSCs. Although differences in therapeutic potential between MS-MSCs derived from different donors were observed. For instance, EAE mice treated with MS-MSCs derived from Donor 1 showed a limited improvement (about 1 point) in functional recovery compared to saline-treated controls for about 7 days after infusion, but this improvement rapidly reversed (Figure 6A). In contrast, MS-MSCs derived from Donor 2 promoted a more sustained improvement in functional recovery compared to the MS-MSCs derived from Donor 1, although these cells were still less effective than naïve MSCs (Figure 6A). Overall, only EAE mice treated with naïve MSCs showed a significant reduction in cumulative disease score (Figure 6B).
Figure 6. MS-MSCs lack therapeutic efficacy compared to naïve MSCs in modulating EAE.
(A) MSCs derived from MS patients (MS-MSCs) are less effective at improving functional recovery when transplanted into EAE mice compared to naïve MSCs (DN-MSCs) derived from healthy donors. MOG35–55 induced EAE mice were infused with 1×10^6 MSCs at 14 days post EAE induction (arrows), with MS-MSCs derived from 3 separate donors diagnosed with relapsing-remitting MS and DN-MSCs derived from corresponding sex and age-matched healthy donors. Data shown = mean ± SEM, n = 11–13 mice per group. (B) EAE mice treated with DN-MSCs from each of the respective donors show a significant reduction in cumulative disease scores (area under the curve), whereas EAE mice treated with MS-MSCs show no significant difference versus saline treated controls. Data shown = mean + SEM, **P<0.01, One-way ANOVA. (C) Fold changes of proteins increased in conditioned medium from MS-MSCs relative to naïve MSCs; note conditioned medium from MS-MSCs contains higher levels of pro-inflammatory cytokines, including IL-6, IL-8, and CCL2. Fold changes were calculated by comparing spot intensity values from 2 separate antibody arrays per group, with each array treated with an independent sample. No fold decreases of any cytokine tested were observed in the arrays.
Since MS-MSCs and EAE-MSCs both lacked therapeutic efficacy in treating EAE, we next asked if MS-MSCs secreted higher levels of pro-inflammatory factors like EAE-MSCs. An antibody array was used to profile and compare CM from MS-MSCs to CM from naïve MSCs. While these arrays detected a relatively small number of proteins in human MSC CM (12 factors detected out of 80 targets profiled), this approach nevertheless identified several pro-inflammatory cytokines that were elevated in MS-MSC CM. Conditioned medium from MS-MSCs contained higher levels of both IL-6 and CCL2 compared to CM from naïve MSCs (Figure 6C), paralleling the increase observed in CM from EAE-MSCs (Figure 3). Additionally, MS-MSC CM had higher levels of IL-8 and Timp1 compared to naïve MSC CM. MS-MSC CM also had much higher levels of HGF compared to naïve MSC CM (Figure 6C), although this increase parallels the increase in HGF levels observed in CM from peak EAE-MSCs.
Discussion
Here, we demonstrate for the first time that bone marrow MSCs isolated from EAE mice and MS patients have reduced therapeutic efficacy compared to naïve MSCs in modulating EAE. These results have important clinical implications, as most clinical trials evaluating BM-MSCs to treat MS utilize autologous MSCs (Meamar et al., 2016). Indeed, results from completed trials report good safety but little or no therapeutic efficacy in systemically transplanting autologous BM-MSCs into MS patients (Connick et al., 2012; Karussis et al., 2010; Mohyeddin Bonab et al., 2007; Yamout et al., 2010). Our results show that autologous EAE-MSCs and MS-MSCs likewise have little therapeutic effect when transplanted into MOG EAE mice, and are comparatively worse than naïve MSCs in modulating EAE.
Despite the fact that most clinical trials utilize autologous BM-MSCs to treat MS, few previous studies have compared autologous diseased MSCs to naïve cells. Two studies reported cultured MS-MSCs were similar to naïve MSCs in proliferation, differentiation, and expression of common MSC cell surface markers, although one of these studies did report MS-MSCs secreted higher levels of immunomodulatory cytokines including CXCL10 (Mallam et al., 2010; Mazzanti et al., 2008). A more recent study that compared gene expression profiles of bone marrow MSCs derived from MS patients compared to those from healthy controls found that MS-MSCs down-regulated anti-inflammatory genes like IL-10 and up-regulated pro-inflammatory genes like IL-6 (de Oliveira et al., 2015).
Studies comparing bone marrow EAE-MSCs to naïve MSCs report conflicting results. One study found BM-MSCs isolated from MOG induced EAE mice are no different than naïve MSCs in terms of growth rate or differentiation, and are just as effective as naïve MSCs in ameliorating disease when transplanted systemically into EAE mice (Kassis et al., 2013). This study isolated MSCs from EAE mice early on in the disease, when the mice showed clinical symptoms such as tail paralysis or hind limb weakness. In contrast, a more recent study compared BM-MSCs isolated from MOG EAE mice later in disease, when symptoms are more severe, and found that these EAE-MSCs were different from naïve MSCs in terms of growth rate, differentiation potential, and mRNA expression levels of important histone-modifying genes (Zacharaki et al., 2013). This later study did not compare EAE-MSCs to naïve MSCs in terms of therapeutic efficacy. Our results show that EAE-MSCs isolated from mice later in disease when symptoms are more severe (full hind limb paralysis) lack therapeutic efficacy compared to naïve MSCs and have distinct differences in growth rate and differentiation potential.
While a number of factors, such as differences in MSC isolation protocol or culture conditions, might explain these conflicting results, one intriguing possibility for these different findings is that changes in the functionality to BM-MSCs in EAE is dependent on the severity and stage of the disease. Our data lends support to this hypothesis, as we found that the progression of disease in EAE seems to correlate to loss of functionality in BM-MSCs. While both peak EAE-MSCs and chronic EAE-MSCs lacked therapeutic efficacy in modulating EAE compared to naïve MSCs, mice that received peak EAE-MSCs did show small, albeit temporary, improvements in functional recovery. Furthermore, while conditioned medium from chronic EAE-MSCs failed to inhibit proliferation of MOG-stimulated splenocytes in-vitro, conditioned medium from peak EAE-MSCs effectively suppressed proliferation in a manner similar to naïve MSC CM. Thus, while lacking therapeutic efficacy compared to naïve MSCs, peak EAE-MSCs still seem to have some limited therapeutic effect in-vivo and immunosuppressive functionality in-vitro.
One of the striking differences observed between peak and chronic EAE-MSCs was in their secretion of immunomodulatory cytokines and growth factors. Analysis of conditioned medium from peak EAE-MSCs showed they secrete higher levels of IL-6, CCL2, and CCL9 compared to naïve MSCs. Chronic EAE-MSCs also secrete higher levels of the same proteins relative to naïve MSCs, but at a much greater magnitude. For example, peak EAE-MSC CM had an approximately 2.5-fold increase in IL-6 versus naïve MSC CM, while chronic EAE-MSC CM had 16-fold higher IL-6 levels. In addition, chronic EAE-MSCs also secreted much higher levels of two other cytokines: CXCL1 and CXCL5. There were also differences between peak and chronic EAE MSCs in their secretion of HGF, which has anti-inflammatory and pro-regenerative properties and has been implicated as an important mediator of MSC-induced recovery in EAE (Bai et al., 2012). Peak EAE-MSC CM actually had higher levels of HGF compared to naïve MSC CM, whereas chronic EAE-MSC CM had significantly lower levels of HGF. The findings that peak EAE-MSC CM contains higher levels of HGF may contribute to its ability to inhibit MOG induced lymphocyte proliferation in-vitro, whereas chronic EAE-MSC CM has no effect. Independent of HGF, peak EAE-MSCs secrete higher levels of pro-inflammatory cytokines relative to naïve MSCs, and this might account for their reduced therapeutic efficacy compared to naïve MSCs when transplanted in-vivo.
Results from experiments in which EAE mice were treated with conditioned medium from naïve MSCs or EAE-MSCs suggest the lack of therapeutic efficacy in EAE-MSCs is due to differences in factors they secrete relative to naïve MSCs. Previous studies have shown naïve MSCs mediate recovery in EAE by secreting various anti-inflammatory cytokines, chemokines, and trophic factors (Bai et al., 2012; Jumah and Abumaree, 2012; Matysiak et al., 2011). These in turn act to suppress auto-reactive immune cells and inhibit inflammation while also promoting remyelination and neural repair (Morando et al., 2012). In agreement with these studies, we found that conditioned medium (CM) from naïve MSCs improves both functional recovery and neuropathology in EAE mice, reducing lesion load and the number of immune and T-cells in the CNS. While infusion of CM from peak EAE-MSCs into EAE mice results in a small improvement in functional recovery, this effect was very limited and only temporary, and so overall peak EAE-MSC CM failed to significantly improve clinical recovery or neuropathology. Similarly, infusion of CM from chronic EAE-MSCs did not improve functional or histological recovery.
While a number of different cytokines are up regulated in the CM of both peak and chronic EAE-MSCs, it is unclear which, if any, of these factors might account for the loss of therapeutic efficacy observed in EAE-MSCs. All of the cytokines identified have pro-inflammatory functionality and are known mediators of inflammation in EAE. IL-6 in particular is thought to be an important driver of T-cell activity in diseases like MS and EAE (Hunter and Jones, 2015), as it biases the development of immature or regulatory T-cells into pro-inflammatory Th17 cells (Korn et al., 2008). Both CCL2 and CCL9 are known chemokines for monocytes and inflammatory macrophages (Conductier et al., 2010), with CCL2 recruiting inflammatory immune cells into the CNS of EAE mice (Mahad and Ransohoff, 2003). Both CXCL1 and CXCL5 are chemokines important in the recruitment of neutrophils to sites of inflammation (De Filippo et al., 2013; Nouailles et al., 2014), and both are systemically up regulated in MS patients during active lesion formation (Rumble et al., 2015).
Conditioned medium from MS-MSCs had higher levels of some of the same pro-inflammatory cytokines, including IL-6 and CCL2. MS-MSCs were also similar to peak EAE-MSCs in that they secreted higher levels of HGF compared to naïve MSCs. One question raised by these findings is whether secretion of HGF or other immunomodulatory factors by MS-MSCs is affected by severity or duration of disease. For instance, are BM-MSCs from MS patients different during relapse versus remittance, or are MSCs from relapsing-remitting MS patients different from MSCs from progressive MS patients? While MSCs can be isolated from EAE mice at different phases of disease, it is more challenging to isolate and compare MSCs from MS patients during specific periods of disease or control for variables like disease duration, severity of disease, or differences in medications donors were receiving at the time their bone marrow was collected. Future work including both prospective and retrospective studies comparing MS-MSCs to naïve MSCs are required to better document the effect of such factors as well as genetic and environmental variables that could also influence the functionality of bone marrow MSCs. The benefits of autologous MSC therapy in MS will likely be unrealized until there is a better understanding of how disease status and severity correlate to loss of therapeutic functionality in these cells.
The demonstration of differences in the functionality of bone marrow derived MSCs following CNS inflammation and demyelination is consistent with the observation of changes in bone physiology in patients with MS. Osteoporosis and osteopenia are commonly associated with MS, with MS patients having a higher risk of both conditions, but the mechanisms underlying bone degradation in MS are currently unclear (Dobson et al., 2012; Marrie et al., 2009). It has been proposed that various factors contribute to reduced bone density in patients with MS, including paralysis and musculoskeletal atrophy, vitamin D deficiency, and inflammation that directly damages bone structure (Dionyssiotis, 2011). Our data shows that diseases like EAE and MS can dramatically alter the functionality of bone marrow MSCs, including aspects of their proliferation and differentiation, raising the possibility that changes to bone marrow MSCs in MS could contribute to changes in bone density observed in MS patients.
Conclusions
The pronounced lack of therapeutic efficacy of both EAE and MS-MSCs in an experimental model of MS suggests that autologous MSCs may be a poor candidate for cell-based therapies to treat MS. The present study thus supports the advancement of allogenic MSC trerapy over autologous MSC therapy for the treatment of MS, and raises important concerns over the efficacy of using autologous BM-MSCs in clinical trials.
Supplementary Material
Highlights.
Mesenchymal stem cells (MSCs) derived from EAE mice fail to ameliorate EAE
EAE-MSCs secrete higher levels of pro-inflammatory cytokines
Similar functional changes are seen in MSCs from patients with multiple sclerosis
Acknowledgments
This work was supported by NS30800 (NIH) and the Myelin Repair Foundation. The authors thank the Southwest Hospital of the Third Military Medical University for providing human bone marrow samples, and the CWRU stem cell core and GWU imaging core for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
AS planned and conducted experiments, analyzed data, and co-wrote manuscript. LB conducted human MSC functional experiments. GS and LR provided technical assistance and scored EAE animals. MK and EG assisted in tissue processing and Toluidine blue staining. SMP and JC derived and provided human MSC samples and helped with manuscript preparation. RHM planned experiments, analyzed data, and co-wrote manuscript.
References
- Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98(5):1076–84. doi: 10.1002/jcb20886. [DOI] [PubMed] [Google Scholar]
- Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol. Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. Journal of the neurological sciences. 2013;330(0):43–49. doi: 10.1016/j.jns.2012.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon J-L, Rovère C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan S-L, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- de Oliveira GLV, de Lima KWA, Colombini AM, Pinheiro DG, Panepucci RA, Palma PVB, Brum DG, Covas DT, Simões BP, de Oliveira MC, Donadi EA, Malmegrim KCR. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplant. 2015;24:151–165. doi: 10.3727/096368913X675142. [DOI] [PubMed] [Google Scholar]
- DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, Busch SA, Silver J. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep. 2015;5:16795. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionyssiotis Y. Bone loss and fractures in multiple sclerosis: focus on epidemiologic and physiopathological features. Int J Gen Med. 2011;4:505–509. doi: 10.2147/IJGM.S22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R, Ramagopalan S, Giovannoni G. Bone health and multiple sclerosis. Mult. Scler. 2012;18:1522–1528. doi: 10.1177/1352458512453362. [DOI] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nature Immunology. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Jumah, Al MA, Abumaree MH. The Immunomodulatory and Neuroprotective Effects of Mesenchymal Stem Cells (MSCs) in Experimental Autoimmune Encephalomyelitis (EAE): A Model of Multiple Sclerosis (MS) Int J Mol Sci. 2012;13:9298–9331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis I, Petrou P, Halimi M, Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol. Lett. 2013;154:70–76. doi: 10.1016/j.imlet.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Zhang JF, Sun B, Peng HS, Kong Q-F, Bai S-S, Liu Y-M, Wang G-Y, Wang J-H, Li H-L. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clin. Exp. Immunol. 2009;158:37–44. doi: 10.1111/j.1365-2249.2009.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin. Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Mallam E, Kemp K, Wilkins A, Rice C, Scolding N. Characterization of in vitro expanded bone marrow-derived mesenchymal stem cells from patients with multiple sclerosis. Mult. Scler. 2010;16:909–918. doi: 10.1177/1352458510371959. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Cutter G, Tyry T, Vollmer T. A cross-sectional study of bone health in multiple sclerosis. Neurology. 2009;73:1394–1398. doi: 10.1212/WNL.0b013e3181beece8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J. Neuroimmunol. 2011;233:106–111. doi: 10.1016/j.jneuroim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Mazzanti B, Aldinucci A, Biagioli T, Barilaro A, Urbani S, Dal Pozzo S, Amato MP, Siracusa G, Crescioli C, Manuelli C, Bosi A, Saccardi R, Massacesi L, Ballerini C. Differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors: implication for assessment of disease activity and treatment. J. Neuroimmunol. 2008;199:142–150. doi: 10.1016/j.jneuroim.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Meamar R, Nematollahi S, Dehghani L, Mirmosayyeb O, Shayegannejad V, Basiri K, Tanhaei AP. The role of stem cell therapy in multiple sclerosis: An overview of the current status of the clinical studies. Adv Biomed Res. 2016;5:46. doi: 10.4103/2277-9175.178791. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miller RH, Bai L, Lennon DP, Caplan AI. The potential of mesenchymal stem cells for neural repair. Discov Med. 2010;9:236–242. [PubMed] [Google Scholar]
- Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, Ghavamzadeh A, Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50–57. [PubMed] [Google Scholar]
- Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, Furlan R, Uccelli A. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J, Faé KC, Arrey F, Kuhlmann S, Bandermann S, Loewe D, Mollenkopf H-J, Vogelzang A, Meyer-Schwesinger C, Mittrücker H-W, McEwen G, Kaufmann SHE. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J. Clin. Invest. 2014;124:1268–1282. doi: 10.1172/JCI72030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu-Mourez R, François M, Boivin M-N, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J. Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, Wang L, Segal BM. Neutrophil-related factors as biomarkers in EAE and MS. J. Exp. Med. 2015;212:23–35. doi: 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent A, Miller RH. MSC Therapeutics in Chronic Inflammation. Current Stem Cell Reports. 2016:1–6. doi: 10.1007/s40778-016-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NMA, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Zacharaki D, Lagoudaki R, Touloumi O, Kotta K, Voultsiadou A, Poulatsidou K-N, Lourbopoulos A, Hadjigeorgiou G, Dardiotis E, Karacostas D, Grigoriadis N. Characterization of in vitro expanded bone marrow-derived mesenchymal stem cells isolated from experimental autoimmune encephalomyelitis mice. J. Mol. Neurosci. 2013;51:282–297. doi: 10.1007/s12031-013-9992-9. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.