Abstract
Purpose
Prior research has documented associations between mental health and alcohol use, mental health and insomnia, and insomnia and alcohol use. This study examined insomnia severity as a mediator of the association between mental health and alcohol-related outcomes in young adult veterans.
Procedures
Veterans aged 18 to 34 years (N = 622, 83% male) who reported drinking in the past year completed assessments at baseline and one-month follow-up as part of a larger intervention trial. Participants reported symptoms of depression and posttraumatic stress disorder (PTSD) at baseline, insomnia severity at one month, and alcohol use and related consequences at baseline and one month. Mediation analyses using bootstrapped confidence intervals were used to examine the indirect effects of baseline mental health symptoms on alcohol-related outcomes at one month via insomnia severity.
Main Findings
Insomnia severity was associated with both drinking quantity and alcohol-related consequences. Greater depressive (but not PTSD) symptoms were associated directly with more alcohol-related consequences. Neither depressive nor PTSD symptoms had direct effects on drinking quantity when controlling for the other mental health symptoms (e.g., depressive symptoms did not predict drinking quantity when controlling for symptoms of PTSD). However, symptoms of depression and PTSD predicted drinks per week and alcohol-related consequences indirectly through insomnia severity.
Conclusions
Symptoms of depression and PTSD increase risk for alcohol use and related consequences in part by increasing symptoms of insomnia. Findings suggest that insomnia may be an appropriate target for prevention and intervention efforts among heavy-drinking Veterans reporting symptoms of depression or PTSD.
Keywords: sleep, depression, posttraumatic stress, alcohol consequences
1. Introduction
Excessive alcohol consumption is one of largest barriers to physical and mental health in the nation, costing the United States (US) approximately $223 billion per year (CDC, 2016). It is especially concerning among military personnel, 20% of whom report consumption of five or more drinks in one occasion every week in the last month (Bray, 2013; Brown et al., 2010). Heavy-drinking service members (defined as 7/14+ drinks per week in the past year for women/men) report higher levels of general stress (63.4%), anxiety (32.9%), depression (20.8%), posttraumatic stress (PTSD; 13.2%), and suicidal ideation (10.5%) than their lower-drinking counterparts; yet few (<1%) report interest in alcohol treatment in the next six months (Barlas et al., 2013). Such high rates of mental health disorders in comparison to civilian samples (Rudd et al., 2011; Widome et al., 2011), combined with the perceived stigma of mental health treatment (Britt et al., 2008; Vogt et al., 2014), place heavy-drinking service members and Veterans at increased risk for negative health outcomes.
A range of co-occurring mental health symptoms contribute to the burden of alcohol use on the US healthcare system. Symptoms of depression and PTSD, for example, have been associated with relapse to problematic drinking among service members and Veterans who had remitted naturally over time (Williams et al., 2015). They have also been associated with a greater number of drinking-related symptoms among Veterans with alcohol use disorders (Fuehrlein et al., 2014). Moreover, drinking to cope with negative affect has been associated with an increase in alcohol-related consequences among service members and Veterans, both in college (Whiteman and Barry, 2011) and in hospital settings (McDevitt-Murphy et al., 2015). Studies at the daily level are also consistent with self-medication models (Khantzian, 2003), in that elevations in PTSD symptoms during the day are associated with increases in alcohol use and related problems that evening (Gaher et al., 2014; Langdon et al., 2016). Collectively, these findings suggest that symptoms of depression and PTSD significantly increase risk for heavy drinking and alcohol-related consequences among military service members and Veterans.
One symptom that is shared across multiple mental health disorders – and, therefore, may play a transdiagnostic role in alcohol use outcomes – is difficulty falling or staying asleep. Depression and PTSD have both been linked to symptoms of insomnia in military and Veteran populations (Jenkins et al., 2015; Seelig et al., 2010). In turn, symptoms of insomnia have been linked to heavy drinking and alcohol-related consequences among military samples. For example, poor sleep quality is a concurrent predictor of heavy alcohol consumption (Swinkels et al., 2013), and short sleep duration (<6 hours) predicts heavy and unintentional drinking in returning Veterans (Luxton et al., 2011). Insomnia severity has also been associated cross-sectionally with alcohol-related consequences among those exposed to combat (Wright et al., 2011). In longitudinal studies, decreased pre-deployment sleep has been associated with higher risk of post-deployment depression and PTSD (Seelig et al., 2010), and difficulty falling/staying asleep has been found to predict relapse to problem drinking up to three years later among military personnel and Veterans who had naturally remitted (Williams et al., 2015).
The longitudinal associations between insomnia and mental health – and their respective and combined effects on alcohol use among military samples – are complex and not well understood. This is likely due in part to the fact that these symptoms are often difficult to tease apart. For example, there is some evidence that insomnia moderates the impact of combat exposure on PTSD and alcohol use, such that combat exposure is associated with PTSD symptoms and hazardous drinking only among those reporting high levels of insomnia (Wright et al., 2011). This suggests that insomnia compounds the negative impact of certain military experiences (i.e., combat) on health-related outcomes. However, it is also possible that insomnia helps explain the association between health-related outcomes (e.g., PTSD, depression) and subsequent alcohol use. Specifically, the racing and/or intrusive thoughts associated with depression and PTSD may compound problems falling asleep at night, both at bedtime and during nighttime awakenings. Such disturbed sleep may increase use of alcohol as a sedative as well as risk for alcohol-related problems (Swinkels et al., 2013; Williams et al., 2015). Indeed, there is cross-sectional evidence that mental health symptoms (defined as a combination of depression, anxiety, and stress) may impact alcohol use and related consequences indirectly through poor sleep quality in civilian samples (Kenney et al., 2013). Thus, symptoms of PTSD and depression may lead to sleep problems that then exacerbate heavy drinking and alcohol-related consequences.
The current study tested three hypotheses. First, given previous research linking mental health to alcohol use outcomes (Fuehrlein et al., 2014; Williams et al., 2015), we hypothesized that baseline symptoms of depression and PTSD would predict alcohol use and related consequences one month later. Second, we expected symptoms of depression and PTSD to predict greater insomnia severity at one month (Jenkins et al., 2015; Seelig et al., 2010). Finally, we hypothesized that insomnia severity at one month would mediate the association between baseline symptoms of both depression and PTSD and alcohol use and consequences reported one month later.
2. Method
2.1 Participants and Procedure
Veterans aged 18 to 34 years were recruited using targeted Facebook ads as part of a larger randomized controlled trial examining the efficacy of a personalized normative feedback alcohol intervention for Veterans at one-month follow-up (Pedersen et al., in press; National Institutes of Health Clinical Trial NCT02187887). Participants were eligible for the study if they (a) were a U.S. Veteran currently separated from active duty, (b) were between the ages of 18 and 34 years, and (c) scored 3/4+ (for women/men) on the Alcohol Use Disorders Identification Test (Saunders et al., 1993). A total of 1177 individuals responded to the ads and provided informed consent. Of those, 784 participants with reliable demographic information completed the baseline assessment and were randomized to receive either the intervention (n=388) or an attention control (n=396). One month later, 622 participants (79%) completed the online follow-up survey. All surveys were completed online from remote locations, and all procedures were approved by the institutional review board.
2.2 Measures
2.2.1 Demographic information
Participants provided information regarding age, gender, race, ethnicity, and branch of service. Combat exposure and severity were assessed using an 11-item scale used in prior work with service members and veterans (Hoge et al., 2004; Schell and Marshall, 2008). After indicating (yes/no) if they had been exposed to combat during deployment, participants indicated (yes/no) if they had been exposed each of 11 potential combat experiences (e.g., “being responsible for the death of a civilian”). Affirmative responses were summed to calculate combat severity. This measure has been used in previous studies documenting the association between combat severity and mental health problems among returning service members (Schell and Marshall, 2008) and had adequate reliability in this sample (α=.78).
2.2.2 Alcohol use
Participants completed the Daily Drinking Questionnaire, a reliable measure of drinking quantity and frequency that has demonstrated criterion-related validity in young adult samples (Collins et al., 1985; Sobell and Sobell, 2003), at baseline and one-month follow-up. Using a seven-day grid, participants indicated the number of drinks they had consumed on each day of a typical week in the past month. Values were summed to create the drinks per week variable.
2.2.3 Alcohol-related consequences
Participants completed the 24-item Brief Young Adult Alcohol Consequences Questionnaire (Kahler et al., 2008; Kahler et al., 2005) at baseline and one-month follow-up. Each participant indicated (yes/no) if they had experienced each consequence (e.g., “felt sick to my stomach or thrown up”) after drinking in the past month. Affirmative responses were summed to create the alcohol-related consequences variable. This instrument has demonstrated strong psychometric properties in young adult samples (Kahler et al., 2005), and reliability in this sample was high (α=.94).
2.2.4 Symptoms of depression
Symptoms of depression were assessed at baseline using the Patient Health Questionnaire-8 (PHQ-8; Kroenke et al., 2009). Participants indicated the frequency with which they had been bothered by eight depressive symptoms (e.g., “little interest or pleasure in doing things”) in the past two weeks. Response options ranged from 0 (not at all) to 3 (nearly every day) and were summed to create the total symptom severity score used in statistical analyses. Internal consistency was high in this sample (α=.93). For descriptive purposes, a cut-off score ≥10 (Kroenke et al., 2009) was used to classify participants as meeting screening criteria for depression.
2.2.5 Symptoms of PTSD
PTSD symptoms were assessed using the PTSD Checklist for DSM-5 (PCL-5; Weathers et al., 2013). Participants indicated how much in the past month they had been bothered by 20 potential responses (e.g., “repeated, disturbing, and unwanted memories”) to a stressful experience (e.g., serious accidents, combat, assault). Response options ranged from 0 (not at all) to 4 (extremely) and were summed to create the total symptom score used in analyses. The PCL-5 has demonstrated good reliability and convergent and divergent validity in samples of Veterans (Bovin et al., 2016), and internal consistency was high in this sample (α=.98). For descriptive purposes, a cut-off score ≥33 (Bovin et al., 2016) was used to classify participants as meeting screening criteria for PTSD.
2.2.6 Insomnia severity
Symptoms of insomnia were assessed at one-month follow-up using the 7-item Insomnia Severity Index (Morin et al., 2011). Participants indicated the severity of their problems falling or staying asleep in the past two weeks, their satisfaction with their current sleep pattern, the extent to which their sleep problems interfered with daily functioning, how noticeable the impact of their sleep problem may be to others, and the extent to which their sleep caused worry/distress. Response options ranged from 0 (e.g., none) to 4 (very severe) and were summed to create the continuous variable used in analyses. Reliability in this sample was high (α=.93). For descriptive purposes, a cut-off score ≥10 was used to characterize participants as meeting screening criteria for insomnia (Morin et al., 2011).
2.3 Data Screening and Analysis
Data were screened for missing values, normality, multicollinearity, and baseline group differences prior to analysis. No imputation procedures were used for missing values, as only two participants were missing data on either the drinks per week or insomnia severity variables. For predictor and outcome variables, skewness and kurtosis estimates fell within the acceptable range (Tabachnick and Fidell, 2007). The one sleep item in the depression measure (PHQ-8) and the two sleep items in the PTSD measure (PCL-5) were removed for primary statistical analyses to avoid confounds between predictor and proposed mediator variables; however, they were retained in descriptive statistics characterizing our sample.1 We used linear regression to test for multicollinearity between symptoms of insomnia, depression, and PTSD. Diagnostic statistics (O’Brien, 2007) indicated moderate levels of tolerance (0.32) and low variance inflation factor (3.17) among the predictors in the current sample, indicating that the majority of variance in each mental health predictor was unaccounted for by the others.
Zero-order correlations, means, and standard deviations of study variables are presented in Table 1. Age, gender, drinks per week, frequency of binge drinking, and alcohol-related consequences did not differ by treatment condition at baseline. However, there was a significant time by condition interaction in the prediction of drinking and alcohol-related consequences at one month follow-up (Pedersen et al., in press); therefore, intervention condition was included as a covariate in all analyses. Analyses also controlled for gender and age, given evidence that men (Scott et al., 2013) and younger adult Veterans (Fuehrlein et al., 2014) report higher rates of hazardous drinking than women and older adult Veterans, respectively. We controlled for combat severity because combat experience has been linked to more harmful alcohol use among military personnel (Bray et al., 2013).
Table 1.
Descriptive statistics and zero-order correlations between variables (N = 622).
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Intervention condition | – | |||||||||
| 2. | Male gender | −0.07 | – | ||||||||
| 3. | Age | −0.03 | 0.02 | – | |||||||
| 4. | Combat severity | 0.004 | 0.23*** | 0.15*** | – | ||||||
| 5. | Drinks per week (BL) | 0.02 | 0.21*** | −0.002 | 0.20*** | – | |||||
| 6. | Depressive symptoms (BL) | 0.05 | −0.07 | −0.001 | 0.23*** | 0.23*** | – | ||||
| 7. | PTSD symptoms (BL) | 0.04 | −0.02 | 0.04 | 0.32*** | 0.25*** | 0.83*** | – | |||
| 8. | Insomnia severity (1mo) | 0.07 | −0.07 | −0.04 | 0.22** | 0.22*** | 0.58*** | 0.58*** | – | ||
| 9. | Drinks per week (1mo) | 0.13** | 0.17*** | 0.03 | 0.11** | 0.65*** | 0.11** | 0.13** | 0.16*** | – | |
| 10. | Alcohol consequences (1mo) | 0.13** | 0.05 | −0.04 | 0.12** | 0.41*** | 0.31*** | 0.27*** | 0.33*** | 0.51*** | |
|
|
|||||||||||
| N | N | M | M | M | M | M | M | M | M | ||
|
|
|||||||||||
| 309 | 514 | 28.95 | 3.99 | 17.92 | 8.02 | 25.52 | 12.31 | 11.49 | 4.52 | ||
|
|
|||||||||||
| % | % | SD | SD | SD | SD | SD | SD | SD | SD | ||
|
|
|||||||||||
| 49.7 | 82.6 | 3.35 | 3.08 | 17.81 | 6.35 | 20.67 | 7.58 | 13.53 | 6.00 | ||
Note. Combat severity scores ranged 0–11. Depressive symptom scores ranged 0–21 (sleep items excluded). PTSD symptom scores ranged 0–72 (sleep items excluded). Insomnia severity scores ranged 0–28. Alcohol consequence scores ranged 0–24. BL = baseline. 1mo = 1-month follow-up.
p < .05.
p < .01.
p < .001.
Mediational process models were examined using the PROCESS macro for SAS, model 4 (Hayes, 2013). The original design of the study did not allow us to account for the temporal precedence between the mediator and outcome variables. However, given previous studies establishing symptoms of insomnia as prospective predictors of alcohol use and problems (Haario et al., 2013; Williams et al., 2015), insomnia severity (measured at one month) was modeled as a potential mediator of the effects of two mental health predictors (depressive and PTSD symptoms at baseline) on two separate alcohol use outcomes (drinks per week and alcohol-related consequences at one-month follow-up). Mediation was evaluated by computing the indirect path following the ab product term approach (MacKinnon et al., 2002). We used bootstrapped (nboot=1,000) 95% asymmetric confidence intervals around the indirect effect (Hayes, 2013; MacKinnon et al., 2002). The indirect path was considered statistically significant if the confidence interval did not include zero.
3.0 Results
3.1 Descriptive Statistics
Participants who completed the one-month follow-up (N=622) were included in the current study (see Table 1). The majority were members of the Army (58.4%) followed by the Marines (21.5%), Air Force (10.8%), and Navy (9.3%). Represented racial groups included White (84.6%), bi/multiracial (6.1%), African American (4.2%), American Indian or Alaskan Native (2.1%), Asian (1.3%), Native Hawaiian/Pacific Islander (0.5%), and other (1.1%). Most participants (82.3%) reported some exposure to combat during their military service. Approximately half (48.9%) scored above the optimal screening cut-off for insomnia in community samples (≥10; Morin et al., 2011). Similarly, approximately half (46.6%) met the screening cut-off for depression on the PHQ-8 (≥10; Kroenke et al., 2009), and a little over a third (39.3%) met screening criteria for PTSD on the PCL-5 (≥33; Bovin et al., 2015).
3.2 Models Predicting Drinks per Week
First, we examined insomnia severity as a mediator of the association between depressive symptoms and drinks per week (see Figure 1A). There were significant positive associations between depressive symptoms and insomnia severity (a-path) and between insomnia severity and drinks per week (b-path). There was no direct association between depressive symptoms and drinks per week (c-path), and this association remained non-significant when accounting for insomnia severity (c′-path). However, depressive symptoms did impact drinks per week indirectly through insomnia severity, ab = 0.08 (SE = 0.04), CI95 (0.01, 0.19).
Figure 1.
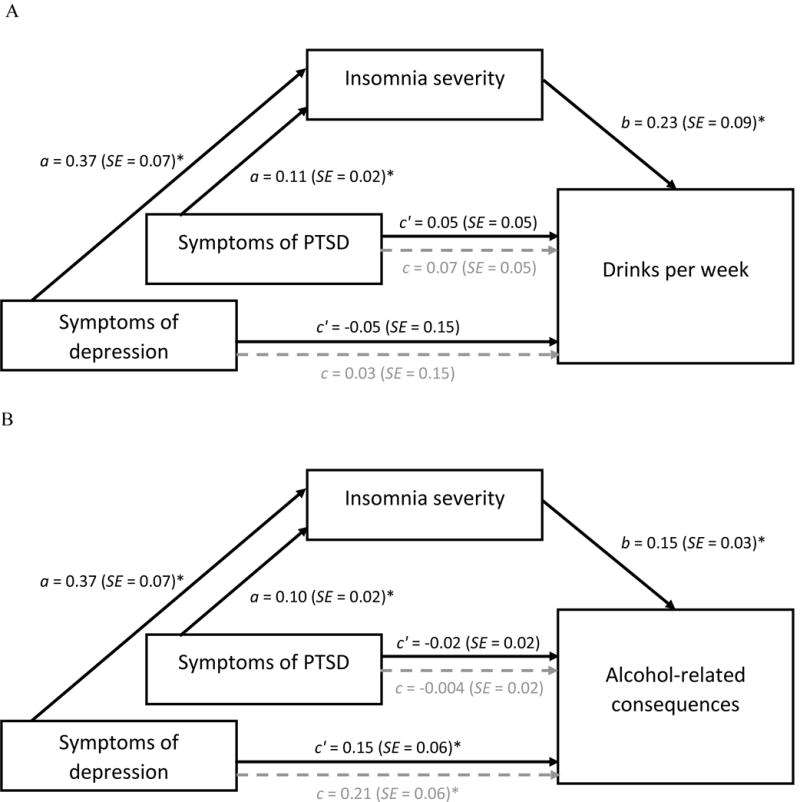
a. Indirect effects of depression/PTSD symptoms on drinks per week.
b. Indirect effects of depression/PTSD symptoms on alcohol-related consequences.
Path coefficients for simple mediation analysis examining insomnia symptoms as a mediator of the association between depression/PTSD symptoms and alcohol-related outcomes. Lighter, dotted lines represent the effect of depression/PTSD symptoms on alcohol outcomes when mediators are not included in the model. All models include intervention condition, gender, age, combat severity, and the opposite mental health diagnosis as covariates (e.g., depressive symptom models include PTSD symptoms as a covariate). The model predicting alcohol-related consequences also controlled for drinks per week at baseline. *p < .05.
We then examined insomnia severity as a mediator of the association between PTSD symptoms and drinks per week. PTSD symptoms were significantly associated with insomnia severity (a-path), and insomnia severity remained a significant predictor of drinks per week (b-path). There was no direct association between symptoms of PTSD and drinks per week (c-path), even when accounting for the mediator (c′-path). However, PTSD symptoms impacted drinks per week indirectly through insomnia severity, ab = 0.03 (SE = 0.01), CI95 (0.01, 0.06).
3.3 Models Predicting Alcohol-Related Consequences
We conducted parallel models to examine insomnia severity as a mediator of the association between depressive symptoms and alcohol-related consequences (see Figure 1B). Again, there were significant positive associations between depressive symptoms and insomnia severity (a-path) and insomnia severity and alcohol-related consequences (b-path). The direct effect of depressive symptoms on alcohol-related consequences (c-path) was positive and significant, indicating that, as symptoms of depression increased, participants reported more alcohol-related consequences. This association weakened but remained significant when accounting for the mediator (c′-path). There was a significant indirect effect of depressive symptoms on alcohol-related consequences through insomnia severity, ab = 0.06 (SE = 0.02), CI95 (0.03, 0.10).
Finally, we examined insomnia severity as a mediator of the association between PTSD symptoms and alcohol-related consequences. Again, PTSD symptoms were significantly associated with insomnia severity (a-path), and insomnia severity was significantly associated with alcohol-related consequences (b-path). No direct association between PTSD symptoms and alcohol-related consequences was observed (c-path), even when insomnia severity was included in the model (c′-path). However, there was a significant indirect effect of PTSD symptoms on alcohol-related consequences through insomnia severity, ab = 0.02 (SE = 0.01), CI95 (0.01, 0.03).
4.0 Discussion
This is the first study of which we are aware to document that insomnia severity accounts in part for the impact of symptoms of depression and PTSD on alcohol use and related consequences in Veterans. Approximately half of the participants in this study reported clinically significant symptoms of insomnia and depression, and more than a third met screening criteria on a self-report measure of PTSD. The prevalence of these mental health issues and their respective influences on alcohol-related outcomes indicate a need to prevent and treat these problems in young adults who have been discharged from military service.
We were surprised to find that symptoms of neither depression nor PTSD had direct effects on drinking quantity, and only symptoms of depression had a direct effect on alcohol-related consequences. This seems to suggest that, in community samples of Veterans, symptoms of depression and PTSD may not be associated with increases in drinking quantity unless they lead to symptoms of insomnia. This interpretation of our findings is consistent with data indicating that sleep disturbance is a core feature, rather than a secondary symptom, of PTSD (Spoormaker and Montgomery, 2008). Yet it contradicts findings that self-reported depression, PTSD, and trouble sleeping are independent predictors of relapse to problem drinking among military personnel (Williams et al., 2015). However, all models in the current study controlled for symptoms of the other included mental health problem; therefore, ‘symptoms of the depression’ in the current study represent depressive symptoms that do not overlap with PTSD, and ‘symptoms of PTSD’ represent those that do not overlap with depression. Few studies have examined the unique effects of these predictors on alcohol use outcomes over time. Thus, it is possible that depression, PTSD, and insomnia operate through one another in such a way that independent effects are attenuated when all symptoms are included in the model. Alternatively, it is possible that rates of clinically significant PTSD were too low in this sample to establish direct associations between PTSD symptoms and alcohol-related outcomes, as a number of studies linking PTSD symptoms to alcohol outcomes included clinician-administered assessments of PTSD and/or larger proportions of participants who met criteria for PTSD (Fuehrlein et al., 2014; McDevitt-Murphy et al., 2015; Simons et al., 2017).
Despite lack of direct mental health effects on alcohol-related outcomes, symptoms of both depression and PTSD predicted symptoms of insomnia one month later, and insomnia severity was a concurrent predictor of alcohol use and consequences. The longitudinal association between mental health symptoms and insomnia severity is consistent with previous findings among military and Veteran populations (Jenkins et al., 2015; Seelig et al., 2010). Similarly, the association between insomnia and alcohol-related outcomes is consistent with cross-sectional and longitudinal findings in both military (Luxton et al., 2011; Swinkels et al., 2013; Williams et al., 2015) and civilian samples (Breslau et al., 1996; Haario et al., 2013). The current study extends this literature by documenting that insomnia severity statistically mediates this longitudinal association between mental health symptoms and alcohol-related outcomes. While we were unable to examine the specific mechanism by which symptoms of depression and PTSD contribute to insomnia severity, we speculate that the racing and/or intrusive thoughts associated with depression and PTSD make it difficult for individuals to fall and stay asleep at night. This hypothesis is supported by research indicating that perseverative thinking (e.g., “my thoughts repeat themselves”) and sleep-specific worry are associated with sleep impairment (Lancee et al., 2017). These issues may be particularly relevant for veterans, who may be trained to be hypervigilant during service in conflict environments (e.g., standing guard at night, using caffeine, perceiving need for little sleep as a sign of ‘toughness’) and may not receive training in alternative behaviors following deployment (Troxel et al., 2015).
The mechanism by which insomnia severity then influences alcohol use and related consequences is also unclear. Research has demonstrated bidirectional associations between mental health disorders and sleep disturbance, such that major depressive disorder and PTSD are associated with disturbed sleep, and in turn, disturbed sleep is associated with increased risk of mental health disorders (Krystal, 2012). Given this bidirectional association, it is possible that insomnia exacerbates symptoms of existing (or perhaps premorbid) mental health disorders. From a self-medication perspective (Khantzian, 2003), individuals experiencing mental health symptoms (especially symptoms of insomnia, which cut across multiple disorders) may use alcohol to cope with negative affect or to help with sleep. Indeed, up to 40% of heavy-drinking veterans engaged in VA care and 50% of individuals with alcohol use disorders report use of alcohol as a sleep aid (Cucciare et al., 2011; Kolla et al., 2015). Unfortunately, alcohol use before bedtime may also disrupt sleep physiology (Roehrs and Roth, 2001) and weekly sleep patterns (Van Reen et al., 2016), leading to a vicious cycle of daytime dysfunction, insomnia, and increased alcohol use, which may result in increased alcohol problems. Alternatively, it is possible that compounded deficits in executive functioning (Alhola and Polo-Kantola, 2007; Benitez and Gunstad, 2012) lead to poor decision-making (e.g., increased alcohol use) and increased risk of alcohol-related consequences.
Given the prevalence and significance of insomnia symptoms in Veterans, increased efforts to prevent and intervene in sleep problems within this population are warranted. Cognitive behavioral therapy (CBT-I) is the gold standard treatment for insomnia (Siebern and Manber, 2011), and a number of randomized controlled trials have documented its efficacy in reducing symptoms of insomnia in military and Veteran populations (Edinger et al., 2009; Germain et al., 2014). It yields comparable effects for younger and older Veterans (Karlin et al., 2015), and effects are maintained in the presence of medical and mental health disorders (Smith et al., 2005). Yet CBT-I is underutilized in VA settings (Karlin et al., 2013). While replication is needed, findings of this study suggest that insomnia may be an appropriate target for prevention and intervention efforts for heavy-drinking Veterans reporting symptoms of depression or PTSD.
The results of this study should be interpreted with limitations in mind. First, data were collected as part of a larger research trial, which assessed insomnia severity and alcohol-related outcomes at the same time; therefore, finding should be replicated accounting for the temporal precedence of mediator and outcome variables. Findings are also confounded by the bidirectional association between sleep and alcohol use. Specifically, while insomnia seems to predict subsequent alcohol use and consequences (Seelig et al., 2010; Williams et al., 2015), alcohol use before bedtime may also impact sleep architecture (Ebrahim et al., 2013; Roehrs and Roth, 2001) and weekly sleep patterns in adults (Van Reen et al., 2016). Thus, insomnia cannot be isolated as a cause or consequence of alcohol use in this sample. Third, we used self-report rather than clinician-administered or objective measures of mental health, insomnia, and alcohol use. However, the measures of depression, PTSD, and insomnia used in this study are the standard measures used in military research and clinical settings and have been validated against clinical criterion standards (Bovin et al., 2016; Kroenke et al., 2009; Morin et al., 2011). Similarly, actual drinking quantities reported using quantity-frequency measures may be underestimated (Sobell and Sobell, 2003); however, self-report of substance use has been shown to be reliable and accurate in comparison to biomarkers of alcohol use when confidentiality is assured (Leffingwell et al., 2013; Simons et al., 2015). Finally, the sample consisted of veteran drinkers only, as the trial focused on reducing alcohol use and consequences among young adult veterans; and participants were primarily male young adults who were not necessarily seeking treatment for alcohol or other mental health disorders. Therefore, findings need to be replicated in older adult, female, non-veteran, and clinical samples.
Heavy drinking and mental health symptoms co-occur among young adult Veterans and result in significant public health cost (Barlas et al., 2013). Yet research documenting the mechanisms by which mental health symptoms influence alcohol-related outcomes in this population is limited. The results of this study indicate that insomnia severity accounts in part for the impact of symptoms of depression and PTSD on alcohol use and related consequences in young adult Veterans. Given the prevalence and consequences of these symptoms, insomnia may be an appropriate target for prevention and intervention efforts among heavy-drinking Veterans. Healthcare providers are encouraged to screen for sleep disorders within this population and recommend treatment as appropriate.
Highlights.
Insomnia is associated with greater drinking quantity and more alcohol problems.
Symptoms of depression predict more alcohol problems.
Insomnia accounts in part for the impact of mental health symptoms on alcohol use.
Insomnia may be an appropriate target for intervention.
Acknowledgments
Role of Funding Source
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (grant numbers R34-AA022400, Pedersen; T32-AA007459, Monti). NIH had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health, or the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Author ERP designed the study and wrote the protocol. Author MBM conducted literature searches and provided summaries of previous research studies. Authors MBM and AMD conducted statistical analyses. All authors (including KBC and BB) contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to report.
Sensitivity analyses were conducted including and excluding the sleep-related items within the depression and PTSD measures. We found no differences between analyses in terms of statistical significance, with slight differences in correlations/coefficients.
References
- Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsych Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health Related Behaviors Survey of Active Duty Military Personnel. ICF International, Fairfax; 2013. [Google Scholar]
- Benitez A, Gunstad J. Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. Clin Neuropsychol. 2012;26:214–223. doi: 10.1080/13854046.2012.658439. [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in Veterans. Psychol Assess. 2016;28:1379–1391. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- Bray RM. Substance use in the US active duty military. In: Moore BA, Barnett JE, Moore BA, Barnett JE, editors. Military psychologists’ desk reference. Oxford University Press; New York: 2013. pp. 221–226. [Google Scholar]
- Bray RM, Brown JM, Williams J. Trends in binge and heavy drinking, alcohol-related problems, and combat exposure in the U.S. military. Subst Use Misuse. 2013;48:799–810. doi: 10.3109/10826084.2013.796990. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychol. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Britt TW, Greene-Shortridge TM, Brink S, Nguyen QB, Rath J, Cox AL, Hoge CW, Castro CA. Perceived stigma and barriers to care for psychological treatment: Implications for reactions to stressors in different contexts. J Soc Clin Psychol Psychology. 2008;27:317–335. [Google Scholar]
- Brown JM, Bray RM, Hartzell MC. A comparison of alcohol use and related problems among women and men in the military. Mil Med. 2010;175:101–107. doi: 10.7205/milmed-d-09-00080. [DOI] [PubMed] [Google Scholar]
- CDC. Alcohol and Public Health. 2016 Retrieved March 13, 2016 from http://www.cdc.gov/alcohol/
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. J Consult Clin Psych. 1985;53:189–200. doi: 10.1037//0022-006x.53.2.189. [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Darrow M, Weingardt KR. Characterizing binge drinking among U.S. military veterans receiving a brief alcohol intervention. Addict Behav. 2011;36:362–367. doi: 10.1016/j.addbeh.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: Effects on normal sleep. Alcohol Clin Exp Res. 2013;37:539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Olsen MK, Stechuchak KM, Means MK, Lineberger MD, Kirby A, Carney CE. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: A randomized clinical trial. J Sleep Sleep Disorders Res. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehrlein B, Ralevski E, O’Brien E, Jane JS, Arias AJ, Petrakis IL. Characteristics and drinking patterns of veterans with alcohol dependence with and without post-traumatic stress disorder. Addict Behav. 2014;39:374–378. doi: 10.1016/j.addbeh.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Gaher RM, Simons JS, Hahn AM, Hofman NL, Hansen J, Buchkoski J. An experience sampling study of PTSD and alcohol-related problems. Psychol Addict Behav. 2014;28:1013–1025. doi: 10.1037/a0037257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Richardson R, Stocker R, Mammen O, Hall M, Bramoweth AD, Begley A, Rode N, Frank E, Haas G, Buysse DJ. Treatment for insomnia in combat-exposed OEF/OIF/OND military Veterans: Preliminary randomized controlled trial. Behav Res Ther. 2014;61:78–88. doi: 10.1016/j.brat.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haario P, Rahkonen O, Laaksonen M, Lahelma E, Lallukka T. Bidirectional associations between insomnia symptoms and unhealthy behaviors. J Sleep Res. 2013;22:89–95. doi: 10.1111/j.1365-2869.2012.01043.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; New York: 2013. [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Jenkins MM, Colvonen PJ, Norman SB, Afari N, Allard CB, Drummond SPA. Prevalence and mental health correlates of insomnia in first-encounter veterans with and without military sexual trauma. Sleep. 2015;38:1547–1554. doi: 10.5665/sleep.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Hustad J, Barnett NP, Strong DR, Borsari B. Validation of the 30-day version of the Brief Young Adult Alcohol Consequences Questionnaire for use in longitudinal studies. J Stud Alcohol Drugs. 2008;69:611–615. doi: 10.15288/jsad.2008.69.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Read JP. Toward efficient and comprehensive measurement of the alcohol problems continuum in college students: The Brief Young Adult Alcohol Consequences Questionnaire. Alcohol Clin Exp Res. 2005;29:1180–1189. doi: 10.1097/01.alc.0000171940.95813.a5. [DOI] [PubMed] [Google Scholar]
- Karlin BE, Trockel M, Spira AP, Taylor CB, Manber R. National evaluation of the effectiveness of cognitive behavioral therapy for insomnia among older versus younger veterans. In J Geriatr Psych. 2015;30:308–315. doi: 10.1002/gps.4143. [DOI] [PubMed] [Google Scholar]
- Karlin BE, Trockel M, Taylor CB, Gimeno J, Manber R. National dissemination of cognitive behavioral therapy for insomnia in veterans: Therapist- and patient-level outcomes. J Consult Clin Psych. 2013;81:912–917. doi: 10.1037/a0032554. [DOI] [PubMed] [Google Scholar]
- Kenney SR, Lac A, LaBrie JW, Hummer JF, Pham A. Mental health, sleep quality, drinking motives, and alcohol-related consequences: A path-analytic model. J Stud Alcohol Drugs. 2013;74:841–851. doi: 10.15288/jsad.2013.74.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis revisited: The dually diagnosed patient. Prim Psychiat. 2003;10:47–48. [Google Scholar]
- Kolla BP, Schneekloth T, Mansukhani MP, Biernacka JM, Hall-Flavin D, Karpyak V, Geske J, Frye MA. The association between sleep disturbances and alcohol relapse: A 12-month observational cohort study. Am J Addiction. 2015;24:362–367. doi: 10.1111/ajad.12199. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disorders. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Krystal AD. Psychiatric disorders and sleep. Neurologic Clinics. 2012;30:1389–1413. doi: 10.1016/j.ncl.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancee J, Eisma MC, van Zanten KB, Topper M. When thinking impairs sleep: Trait, daytime, and nighttime repetitive thinking in insomnia. Behav Sleep Med. 2017;15:53–69. doi: 10.1080/15402002.2015.1083022. [DOI] [PubMed] [Google Scholar]
- Langdon KJ, Fox AB, King LA, King DW, Eisen S, Vogt D. Examination of the dynamic interplay between posttraumatic stress symptoms and alcohol misuse among combat-exposed Operation Enduring Freedom (OEF) / Operation Iraqi Freedom (OIF) Veterans. J Affect Disorder. 2016;196:234–242. doi: 10.1016/j.jad.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, Barnett NP. Continuous objective monitoring of alcohol use: twenty-first century measurement using transdermal sensors. Alcohol Clin Exp Res. 2013;37:16–22. doi: 10.1111/j.1530-0277.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton DD, Greenburg D, Ryan J, Niven A, Wheeler G, Mysliwiec V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. J Sleep Sleep Dis Res. 2011;34:1189–1195. doi: 10.5665/SLEEP.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt-Murphy ME, Fields JA, Monahan CJ, Bracken KL. Drinking motives among heavy-drinking veterans with and without posttraumatic stress disorder. Addict Res Theory. 2015;23:148–155. doi: 10.3109/16066359.2014.949696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. J Sleep Sleep Dis Res. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- Pedersen ER, Parast L, Marshall GN, Schell TL, Neighbors C. A randomized controlled trial of a web-based personalized normative feedback alcohol intervention for young adult veterans. J Consult Clin Psych. doi: 10.1037/ccp0000187. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25:101–109. [PMC free article] [PubMed] [Google Scholar]
- Rudd MD, Goulding J, Bryan CJ. Student veterans: A national survey exploring psychological symptoms and suicide risk. Prof Psychol-Res Pr. 2011;42:354–360. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schell TL, Marshall GN. Survey of individuals previously deployed for OEF/OIF. RAND Corporation; Santa Monica: 2008. [Google Scholar]
- Scott JC, Pietrzak RH, Mattocks K, Southwick SM, Brandt C, Haskell S. Gender differences in the correlates of hazardous drinking among Iraq and Afghanistan veterans. Drug Alcohol Depend. 2013;127:15–22. doi: 10.1016/j.drugalcdep.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Seelig AD, Jacobson IG, Smith B, Hooper TI, Boyko EJ, Gackstetter GD, Gehrman P, Macera CA, Smith TC, Team M.C.S Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33:1615–1622. doi: 10.1093/sleep/33.12.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebern AT, Manber R. New developments in cognitive behavioral therapy as the first-line treatment of insomnia. J Psychol Res Behav Manag. 2011;4:21–28. doi: 10.2147/PRBM.S10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Simons RM, O’Brien CP, Stoltenberg SF, Keith JA, Hudson JA. PTSD, alcohol dependence, and conduct problems: Distinct pathways via lability and disinhibition. Addict Behav. 2017;64:185–193. doi: 10.1016/j.addbeh.2016.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Wills TA, Emery NN, Marks RM. Quantifying alcohol consumption: Self-report, transdermal assessment, and prediction of dependence symptoms. Addict Behav. 2015;50:205–212. doi: 10.1016/j.addbeh.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: J.A.a M.C, editor. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. National Institute on Alcohol Abuse and Alcoholism; Bethesda: 2003. pp. 75–101. (NIAAA Treatment Handbook Series 4, NIH Publication No 95-3745). [Google Scholar]
- Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Med Rev. 2008;12:169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Swinkels CM, Ulmer CS, Beckham JC, Buse N, Calhoun PS. The association of sleep duration, mental health, and health risk behaviors among U.S. Afghanistan/Iraq Era veterans. J Sleep Sleep Dis Res. 2013;36:1019–1025. doi: 10.5665/sleep.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th. Harper and Row; New York: 2007. [Google Scholar]
- Troxel WM, Shih RA, Pedersen ER, Geyer L, Fisher MP, Griffin BA, Haas AC, Kurz J, Steinberg PS. Sleep in the military: Promoting healthy sleep among US service members. RAND Corporation; Santa Monica: 2015. [PMC free article] [PubMed] [Google Scholar]
- Van Reen E, Roane BM, Barker DH, McGeary JE, Borsari B, Carskadon MA. Current alcohol use is associated with sleep patterns in first-year college students. Sleep. 2016;39:1321–1326. doi: 10.5665/sleep.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Fox AB, Di Leone BA. Mental health beliefs and their relationship with treatment seeking among U.S. OEF/OIF veterans. J Trauma Stress. 2014;27:307–313. doi: 10.1002/jts.21919. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL-5) National Center for PTSD; Boston: 2013. [Google Scholar]
- Whiteman SD, Barry AE. A Comparative Analysis of Student Service Member/Veteran and Civilian Student Drinking Motives. J Stu Aff Res Pract. 2011;48:297–313. doi: 10.2202/1949-6605.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widome R, Laska MN, Gulden A, Fu SS, Lust K. Health risk behaviors of Afghanistan and Iraq War veterans attending college. Am J Health. 2011;26:101–108. doi: 10.4278/ajhp.090826-QUAN-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, Frasco MA, Jacobson IG, Maynard C, Littman AJ, Seelig AD, Crum-Cianflone NF, Nagel A, Boyko EJ. Risk factors for relapse to problem drinking among current and former US military personnel: A prospective study of the Millennium Cohort. Drug Alcohol Depend. 2015;148:93–101. doi: 10.1016/j.drugalcdep.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wright KM, Britt TW, Bliese PD, Adler AB. Insomnia severity, combat exposure, and mental health outcomes. Stress Health. 2011;27:325–333. [Google Scholar]


