Abstract
One Health is defined as the intersection and integration of knowledge regarding humans, animals, and the environment, yet as the One Health scientific literature expands, there is considerable heterogeneity of approach and quality of reporting in One Health studies. In addition, many researchers who publish such studies do not include or integrate data from all three domains of human, animal, and environmental health. This points to a critical need to unify guidelines for One Health studies. This report details the Checklist for One Health Epidemiological Reporting of Evidence (COHERE) to guide the design and publication format of future One Health studies. COHERE was developed by a core writing team and international expert review group that represents multiple disciplines, including human medicine, veterinary medicine, public health, allied professionals, clinical laboratory science, epidemiology, the social sciences, ecohealth and environmental health. The twin aims of the COHERE standards are to 1) improve the quality of reporting of observational or interventional epidemiological studies that collect and integrate data from humans, animals and/or vectors, and their environments; and 2) promote the concept that One Health studies should integrate knowledge from these three domains. The 19 standards in the COHERE checklist address descriptions of human populations, animal populations, environmental assessment, spatial and temporal relationships of data from the three domains, integration of analyses and interpretation, and inclusion of expertise in the research team from disciplines related to human health, animal health, and environmental health.
Keywords: One Health, Reporting guidelines, Observational studies, Epidemiology, Environmental health
Highlights
-
•
A Checklist for One Health Epidemiological Reporting of Evidence (COHERE) is proposed.
-
•
COHERE was developed by a core writing team and international expert review group.
-
•
The aim is to improve studies that report on data from humans, animals and the environment.
1. Introduction
With the increased recognition that diseases often emerge out of interactions of human, animal, and environmental factors, a new approach to address these issues has arisen, known as One Health. The roots of this paradigm lie in the fertile grounds of comparative pathology, driven by the remarkable efforts, perspectives, and writings of William Osler, Calvin Schwabe, Rudolf Virchow, and many others [1]. This early foundation, focused mainly on the “one medicine” intersection of human and animal health, has grown into an effort that also incorporates preventative medicine and public health approaches, particularly environmental health and ecohealth [2]. In recent years, One Health has been described as “the collaborative effort of multiple disciplines — working locally, nationally, and globally — to attain optimal health for people, animals and our environment” [3]. The One Health approach, therefore, involves combined assessment of health risks across the three domains of humans, animals, and the environment, and it involves design and implementation of intervention and prevention strategies that address all three sectors with a goal to produce integrated knowledge [4].
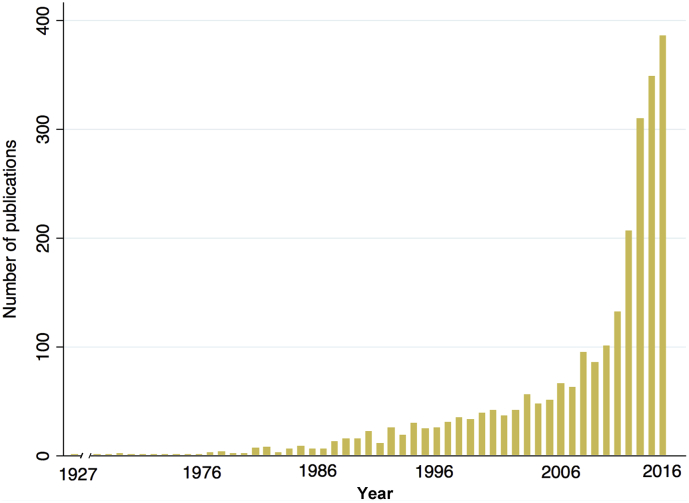
A One Health approach, by definition, encompasses many fields, and these include, but are not limited to, infectious diseases, chronic diseases, toxicology, ecology, agriculture and sustainability, conservation medicine, economics, anthropology, ethnography, and the social sciences. The approach can inform efforts to develop and implement studies or programs related to human and animal wellness, mental health and wellbeing, and the human-animal bond. However, the fields in question often are segregated by methodology, funding, and publication [5]. Requirements from funding sources and publication silos may contribute to the fracture of One Health studies into multiple, discipline-specific studies and/or publications. At the same time, the term “One Health” has become increasingly common in the biomedical literature (Fig. 1). As the literature expands, authors of this document and contributing experts who have conducted systematic reviews have noted considerable heterogeneity of approach and quality of reporting in One Health studies [4], [6], [7]. Such lack of consensus on criteria that constitute a well-designed and clearly-presented One Health study jeopardizes the impact of this growing field and limits the ability of the reader to judge the strengths and limitations of this literature.
Fig. 1.
Number of papers published per year identified with the search terms “One Health” or “One Medicine” in Pubmed (1927–2016).
To build on the foundation of scholarship and provide scope and guidance for future work, we propose the following Checklist for One Health Epidemiological Reporting of Evidence (COHERE) for research publications classified as One Health studies. We intend this work to apply primarily to the approach and reporting of observational and interventional One Health studies that employ epidemiological methods (see Box 1), although these guidelines may also benefit other One Health study designs. Given that interdisciplinary work can serve as an incubator for innovation, we further intend this checklist to be a living document informed by iterative feedback from authors, editors, and readers of the One Health literature.
Box 1. Glossary of key terms used in the standards and text.
Ecohealth: an integrated systemic approach to health incorporating the sustainability of ecosystem health services and social stability to maintain peaceful coexistence of humans, animals and their environments [27].
Captive exotic animal: An animal of a non-domesticated species that is living under human control.
Domestic animal: Companion and food-producing species that have lived for many generations with humans and whose characteristics and traits are generally considered to be under human control.
Epidemiological studies: Studies that determine the distribution of diseases in populations and the factors that may drive this distribution.
Free-ranging wild animal: An animal of a non-domesticated species that is living largely outside of human control.
Generalizability: applicability of research study findings from a sample population to the larger, target population.
Inter-professional education: training approach that brings together and fosters collaboration among students of various disciplines in order to enhance collaboration and promote acquisition of interdisciplinary knowledge.
Qualitative data: data that are non-numerical.
Quantitative data: numerical data.
Semi-qualitative data: data that have a numerical hierarchy but are presented in terms of categories or scales.
Signalment: An animal's age, sex, species and breed.
Alt-text: Box 1
2. Aims and use of the COHERE standards
The Checklist for One Health Epidemiological Reporting of Evidence (COHERE) provides a set of standards that should be included in articles reporting on the results of One Health studies that use epidemiological methods. Box 1 provides a glossary of terms that may be useful to those who will use these standards.
The twin aims of COHERE are to 1) improve the quality of reporting of observational or interventional epidemiological studies that collect and integrate data from humans, animals and/or vectors, and their environments (the One Health domains); and 2) promote the concept that One Health studies should integrate knowledge from all three of these domains. Fig. 2 offers an illustration of this concept, which is further explained in Box 2 (Definition of the Three Domains) and Box 3 (Incorporation of the Three Domains). We intend this guidance to aid, not only to report data at the point of manuscript preparation, but to design and plan studies, since certain criteria not obtained or observed during the execution of the study cannot be corrected at the reporting stage. While the scope of this document and these standards is limited to epidemiological studies (aim 1), the concept that all One Health studies should strive to integrate knowledge from human, animal, and environmental domains (aim 2) can be applied regardless of study methodology.
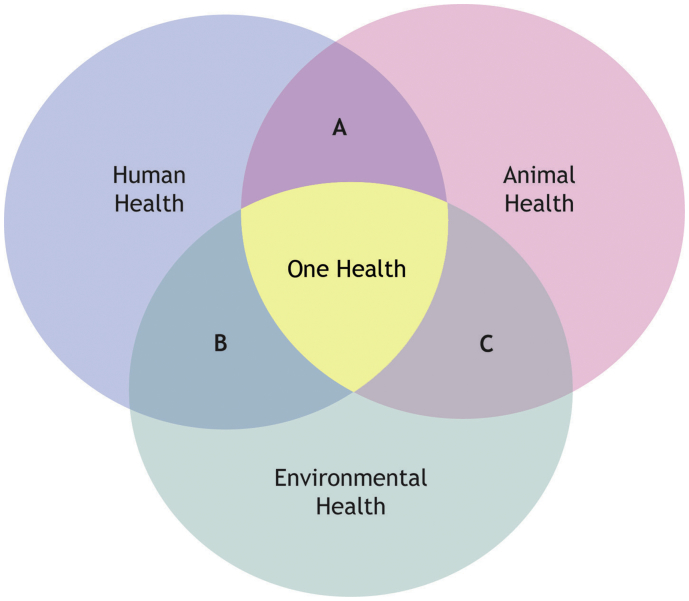
Fig. 2.
Venn diagram illustrating the three domains of One Health. (A) Epidemiological studies relating factors between animal and human health; (B) Epidemiological studies relating factors between environmental and human health; (C) Epidemiological studies relating factors between animal and environmental health.
Box 2. Definition of the Three Domains.
Although the three domains of humans, animals and the environment are quite broad, their application in a One Health context requires specific focus on health-related aspects of these sectors (see Fig. 2). That said, since health, as defined by the World Health Organization, is “a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity,” this allows for evaluation of not just disease factors, but well-being, societal resilience, and other beneficial aspects [28]. For example, the human-animal bond may reduce human and animal stress; safe and walkable neighborhoods may promote greater activity and reduced obesity in human and companion animal inhabitants; and preservation of ecosystem services may improve resilience of human and animal populations threatened by changes in climate. COHERE standards allow for authors whose work may not fall into traditional definitions of human health, animal health, and environmental health to describe and defend the definitions used for the study being reported. It is then incumbent upon editors, reviewers, and readers to determine if any non-traditional definitions are reasonable and justified.
The COHERE standards require that studies report data in all three domains, but sometimes, the boundary between domains may not be sharply delineated. Vectors are particularly problematic to classify. The term itself has multiple definitions that encompass the fields of mathematics, computer science, and biology. This can challenge accurate communication in a scientific arena as multidisciplinary as One Health. For the purposes of these standards, we will use two different definitions of vector: 1) mechanical vector, and 2) biological vector. In both these cases, invertebrate arthropods (i.e., insects and arachnids) operate in the context of transmission of infectious agents from one host to another. However, some definitions of mechanical vector also include inanimate fomites that move, such as vehicles. The diversity of research that surrounds insect vectors and vector-borne diseases can cloud consensus on whether vectors should be considered in the animal domain or environmental domain.
Mechanical vectors, which more appropriately fall in the environmental domain, transfer infectious agents, chemicals, or other substances from one point in the environment to another point in the environment, much the same way a car could transfer or disperse a substance it contacts. When a research group considers vectors in this way, we argue that these data should be grouped with other environmental variables or treated as findings from a fomite. Biological vectors, in contrast, are part of an ecosystem or are part of the lifecycle of an infectious agent. They are living members of communities, and when researchers evaluate them as such and target their biology and not just their carriage of a pathogen, may belong in the sector with animals. Consider the example of malaria, a well-studied vector-borne disease. Research Group A studies how changes in the geographic dispersal of mosquitoes that correlate with changes in temperature and humidity result in changes to the geographic dispersal of malaria in human populations. In this case, COHERE would group data on the geographic distribution of mosquito populations with the environmental sector. Research Group B instead studies how temperature and humidity impact mosquito fecundity and the extrinsic incubation period of the malaria parasite within the mosquitoes. COHERE would group these data on the mosquitoes with the animal sector.
Alt-text: Box 2
Box 3. Incorporation of the Three Domains.
The COHERE standards advocate that studies which purport to be “One Health” in nature should present data from human, animal, and environmental domains of the problem, as illustrated in Fig. 2. However, a recent systematic review of integrated parasite surveillance identified more publications that integrated two One Health sectors than those that addressed all three [7]. Studies integrating human and animal data (illustrated as zone A in Fig. 2) appear to be most common, and report useful information that includes, but is not limited to, differences in prevalence, clinical features, and local reservoirs for infection. However, these studies (illustrated as zones A, B, and C in Fig. 2) represent a missed opportunity to present a complete picture of disease transmission, and may adversely affect pathogen control interventions due to unexpected pathogen dynamics in the uncharacterized One Health domain, whichever one that is.
By following the COHERE standards, investigators will be encouraged to include data about the human, animal, and environmental health domains of a particular disease or health issue. Authors who have completed data collection in only two of the three domains, or who do not have access to one of the three domains for a variety of reasons may still benefit from consideration of as many of the COHERE standards that apply to their work. Indeed, such studies may be necessary precursors to the successful launch of a One Health study, and the data derived from these efforts often are critical to inform interpretation of the results of subsequent One Health research. Therefore, this group makes a number of suggestions for how to use and incorporate the COHERE standards when only two of the three domains are well represented, as described in Table 2. As long as the research group incorporates expertise from all three domains and applies this multidisciplinary expertise diligently to the study design, conduct of the research, and interpretation of the findings, then use of partial data or surrogate assessment of one of the domains would not disqualify the research from being considered a One Health study. (N.B.: In considering expertise, individual researchers may have the background needed to represent more than one domain.) While some studies may be difficult to categorize, a necessary component to reporting a One Health study is explicit incorporation of all three domains in the introduction, methods, results, and discussion of the manuscript. Studies where authors reference the literature on an otherwise neglected third domain in the introduction and discussion alone do not fulfill the standards. This group recommends that studies that 1) cannot fulfill the majority of the standards and 2) do not have the necessary component of data reporting on all three domains as described above should not formally claim to be One Health studies, e.g., in the title or keywords. Nonetheless, these research efforts are important and can be foundational to later One Health research efforts, and authors are encouraged to discuss One Health implications and position their findings within the larger One Health literature.
Alt-text: Box 3
3. Development of the COHERE standards
The COHERE standards were developed by a multidisciplinary core team of experts who determined the process for standard development, drafted the initial checklist, and chose the membership of the COHERE expert review group. This review group was an extended panel of experts who individually and jointly reviewed the standards and whose feedback was incorporated by the core team. The COHERE expert review group was chosen to represent both professional and global diversity in the field. Experts in the conduct of One Health epidemiological studies were included from human medicine, veterinary medicine, public health, allied professionals, clinical laboratory science, ecohealth, and environmental health. By design, multiple institutions and countries were represented in the core team and expert review group.
The structure of the COHERE checklist was designed to closely follow the model of STROBE and its extensions, with the intention that authors of One Health manuscripts should additionally follow these and related standards where appropriate [8], [9]. The COHERE checklist is intended to cover content description felt to be critical to report and interpret studies conducted at the intersection of human, animal, and environmental health.
4. The COHERE standards
Table 1 provides the checklist of standards that should be considered when undertaking, reporting, and reviewing “One Health” epidemiological studies with the goal to produce integrated knowledge. The 19 standards encompass the approach and reporting of findings related to the following manuscript sections: Introduction [1-2], Methods [3-10], Results [11-15], Discussion [16-17], One Health contribution [18], and acknowledgements [19]. As indicated in the standards, authors should additionally follow other guidance documents, e.g., STROBE and its extensions (STROBE-VET, STROME-ID, etc.), CONSORT, PRISMA, in accord with the study design [10], [11], [12], [13], [14], [15]. Authors should use these standards as guidance for the types of information to include in a manuscript, but not as a constraint to the narrative flow of the text. For example, authors are not required to report human, animal, and environmental data separately if an integrated presentation will be more clear for the reader, nor are they required to combine all findings into a single construct if the data are better presented using separate table or figures.
Table 2.
Suggested categorization of One Health and related epidemiological studies involving at least two of the three domains.
| Data reported |
Researcher expertise |
Description | Hypothetical example | Example studies | |||||
|---|---|---|---|---|---|---|---|---|---|
| H | A | E | H | A | E | ||||
| One Health | X | X | X | X | X | X | Data and researcher expertise are well represented among all three domains | Concurrent assessment of a zoonotic pathogen in humans, animals, and a shared environmental reservoir | [29], [30], [31], [32], [33], [34], [35], [36] |
| One Health | [X] | X | X | X | X | X | Researcher expertise covers all domains, but data are limited or surrogate assessments are used for one of the domains | Concurrent investigation of a worksite where animals and the environment are sampled, but access to workers is limited; breathing zone and ambient assessments are made and although humans are not sampled, overall study inference is to worker health | [37], [38], [39] |
| One Health | X | [X] | X | X | X | X | Researcher expertise covers all domains, but data are limited or surrogate assessments are used for one of the domains | Concurrent investigation of the association between an environmental exposure and human disease where access to animals is restricted but detailed data are collected instead on human-animal contacts or proximity as a primary risk factor or effect modifier of interest | [40], [41] |
| One Health | X | X | [X] | X | X | X | Researcher expertise covers all domains, but data are limited or surrogate assessments are used for one of the domains | Concurrent investigation of the overlapping spatial distribution of clinical disease in humans and animals where data from the literature are used to model or infer the environmental exposure | [42] |
| One Medicine | X | X | X | X | Data and expertise capture human and animal domains | Concurrent investigation of zoonotic disease transmission between animal and human populations, where animal disease is known to emerge from an environmental reservoir, but contributions from environmental factors are neither assessed nor modeled | [43], [44], [45] | ||
| Environmental Health | X | X or [X] | X | X | Data and expertise capture human and environmental domains | Concurrent investigation of an environmental contaminant that may derive from animals and the effects of that contaminant on human health, where animals are not assessed | [46], [47], [48], [49] | ||
| Veterinary Preventative Medicine | X | X or [X] | X | X | Data and expertise capture animal and environmental domains | Concurrent investigation of a pathogen reservoir in animal housing and the effects of that reservoir on animal carriage of the pathogen, where humans may interact with the animals and/or housing reservoir but human carriage is neither assessed directly nor are human interactions with animals and environment quantified using survey or other surrogate measures | [50], [51], [52] | ||
H: human domain; A: animal domain; E: environmental domain.
X: fully represented; [X] surrogate used or partially represented.
Example studies were not assessed for research team composition and are included primarily as illustrations of data reporting in the context of the three domains.
Table 1.
The COHERE standards.
| Item | Standard number | Recommendation |
|---|---|---|
| Introduction | ||
| Background | 1 | Review the human, animal, and environmental context of the problem and justify why a One Health study is appropriate to address the scientific question |
| Rationale | 2 | Clearly state the research aims and/or hypotheses in the context of the relationship among the three domains (human, animal and environment), or state and defend the nature of the study if it is not hypothesis-driven |
| Methods | ||
| Study design | 3 |
|
| Human participants | 4 |
|
| Animal participantsb | 5 |
|
| Environmentb | 6 |
|
| Measurement | 7 |
|
| Analysis | 8 |
|
| Study team | 9 |
|
| Ethics | 10 |
|
| Results | ||
| Human participants | 11 |
|
| Animal participants | 12 |
|
| Environment | 13 |
|
| Measurement | 14 |
|
| Analysis | 15 |
|
| Discussion | ||
| Overall | 16 |
|
| Limitations | 17 |
|
| One Health Contribution | 18 |
|
| Acknowledgment | 19 | Indicate funding source(s) and potential conflicts of interest |
Please adhere closely to STROBE or extension (e.g. STROBE-VET, STROME-ID, etc.) guidelines for reporting of observational epidemiology studies, which may impact placement of these COHERE checklist data. Where indicated, data should be placed in methods or results sections per STROBE guidance.
Please see additional discussion of definitions of biological vectors and when and how to report them as part of the animal participants or part of the environment.
The authors and working group strongly encourage collection of and consideration of additional data on human subjects as appropriate, particularly occupation/work-related exposures, socioeconomic parameters, and other community parameters.
5. Assessment of the COHERE standards
Given the anticipated evolution of the field, driven by interdisciplinary collaboration and innovation, and given expected feedback from diverse users, the COHERE checklist is intended to be a living document. Authors, editors and readers may provide suggestions to the corresponding authors. The online checklist of standards will be updated as needed at intervals driven by input from users or advances in the field [16].
6. Discussion
Adoption of One Health approaches is growing, driven in part by the inherent integration of diverse disciplines and in part through the emergence of financial and resource support for policy and program initiatives, educational programs, and research [17], [18], [19], [20]. However, such growth may be constrained by the preferences of journal editors, boundaries on resources, lack of sustainable government commitment, and a paucity of international educational opportunities [5], [17], [18], [20], [21], [22]. As One Health re-emerges as a top priority for those involved in the mitigation of diseases and promotion of wellness, it is important to recognize the challenges faced by those who work collaboratively to integrate the knowledge of multiple disciplines. Examples of such challenges may include budget, competing timelines, long-term sustainability, data sharing, media contact, language and/or culture differences, and synching research interests. Policy makers and academic leaders can alleviate some pressures of multi-disciplinary collaboration through the introduction of data-sharing agreements between governmental agencies, by the promotion of opportunities for inter-professional education [23], and by the prioritization of funding for research teams composed of members from different fields. Institutional animal and human research ethics committees—such as the Institutional Care and Use Committee (IACUC) and Institutional Review Board (IRB)—can collaborate to improve oversight of One Health studies [24]. Studies that monitor the effectiveness of One Health approaches are underrepresented in the literature to date [4], [6]. To overcome this, authors of One Health studies can contribute to the evidence for the cost-effectiveness and efficacy of integrated approaches to investigation and intervention among the three domains by including such outputs in the report of their findings.
If a One Health approach is to continue to thrive, it also will require a shift within academia to recognize the efforts and rewards of interdisciplinary collaborations that may take more time to complete, require more initial funding or resource investment, and require shared credit by multiple investigators. This includes a shift away from “publication splitting,” a temptation for academics who rely on a productive publication record to compete for tenure and promotion [7]. Shared lead and senior authorship may be one solution to overcome such challenges when findings are presented in an integrated fashion rather than piecemeal in journals specific to the discipline of the expertise groups within the research team. In addition, authors may contend with preferences of journal editors who give priority to manuscripts with more discipline-specific focus. The emergence of journals focused on One Health studies provides one avenue for publication where integrated manuscripts have priority [25].
The COHERE standards are designed to strengthen the quality of reporting of One Health observational or interventional epidemiological studies that integrate knowledge and expertise from all three domains. This process challenges authors, editors, and readers to support efforts to break down publication silos, improve collaborative reporting, and foster innovation. (See Box 3 for additional discussion of how to undertake and report studies focused on only two of the three domains.) The types of studies that will employ COHERE standards are likely to be quite diverse. Data may be quantitative, semi-quantitative, qualitative, or a combination, and study designs may be retrospective or prospective. The study findings may be pertinent to local, national, or international scales, and the work may be conducted in both well-served and underserved areas. Diseases tackled using a One Health approach are not limited to infection outcomes in humans caused by zoonotic pathogens. Studies may include a wide clinical spectrum of both acute and chronic diseases in animals as well as humans, or they may consider ecosystem and planetary health. Hence, authors of One Health studies will need to be careful to avoid jargon and to emphasize clarity in communication that can bridge the various disciplines involved in the field. Some One Health expert groups have endorsed involvement of communication specialists in this effort [26].
These standards are not intended to constrain the evolution of the field nor to limit One Health research funding. Given the intrinsic multidisciplinary nature of the field, it is perhaps inevitable that the number of disciplines represented by researchers in this arena will increase and include some areas of expertise less well represented in the health disciplines, e.g., the earth sciences and engineering. As a result, the standards may have to be adapted to new perspectives. Our author group invites feedback via communication with the corresponding author, and we will iteratively update the checklist of standards. We also encourage development of extensions to COHERE for other study designs, such as basic research, mathematical modeling, systematic and narrative literature reviews, economic analyses, program evaluation, the social sciences, policy and law. As the acceptance and adoption of a One Health approach continues to grow, the criteria used to conduct and report research studies should grow with it.
Acknowledgments
Acknowledgments and funding
This work was not funded by any specific source. MFD was supported by the U.S. NIH Office of Research Infrastructure Programs (K01OD019918). JS was supported by the Integrated Training Program in Infectious Diseases, Food Safety and Public Policy (ITraP) funded by the Natural Sciences and Engineering Research Council (NSERC) (414153-2012). This work was presented at the 4th International One Health Congress & 6th Biennial Congress of the International Association for Ecology and Health.
Conflicts of interest
None.
Contributor Information
Meghan F. Davis, Email: mdavis65@jhu.edu.
Peter Rabinowitz, Email: peterr7@uw.edu.
COHERE Expert Review Group:
Gregory Gray, Laura Kahn, Catharine Machalaba, Jonna Mazet, Marguerite Pappaioanou, Jan Sargeant, Andrew Thompson, Scott Weese, and Jakob Zinnstag
References
- 1.Monath T.P., Kahn L.H., Kaplan B. One health perspective. ILAR J. 2010;51(3):193–198. doi: 10.1093/ilar.51.3.193. [DOI] [PubMed] [Google Scholar]
- 2.Zinsstag J., Schelling E., Waltner-Toews D., Tanner M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev. Vet. Med. 2011;101(3–4):148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AVMA . American Veterinary Medical Association: One Health Initiative Task Force Final Report. 2008. One health: a new professional imperative; pp. 1–76. [Google Scholar]
- 4.Rabinowitz P.M., Kock R., Kachani M., Kunkel R., Thomas J., Gilbert J. Toward proof of concept of a one health approach to disease prediction and control. Emerg. Infect. Dis. 2013;19(12) doi: 10.3201/eid1912.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manlove K.R., Walker J.G., Craft M.E., Huyvaert K.P., Joseph M.B., Miller R.S. “One health” or three? Publication silos among the one health disciplines. PLoS Biol. 2016;14(4):e1002448–14. doi: 10.1371/journal.pbio.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum S.E., Machalaba C., Daszak P., Salerno R.H. Evaluating one health: are we demonstrating effectiveness? One Health. 2016 doi: 10.1016/j.onehlt.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schurer J.M., Mosites E., Li C., Meschke S., Rabinowitz P. Community-based surveillance of zoonotic parasites in a ‘One Health’ world: a systematic review. One Health. 2016;2:166–174. doi: 10.1016/j.onehlt.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The STROBE Statement. 2007. http://strobe-statement.org/index.php?id=strobe-home Available at:
- 9.The CONSORT Statement. 2010. http://www.consort-statement.org/ Available at:
- 10.Begg C., Cho M., Eastwood S., Horton R., Moher D., Olkin I. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276(8):637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann. Intern. Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 12.Field N., Cohen T., Struelens M.J., Palm D., Cookson B., Glynn J.R. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): an extension of the STROBE statement. Lancet Infect. Dis. 2014;14(4):341–352. doi: 10.1016/S1473-3099(13)70324-4. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Hopewell S., Schulz K.F., Montori V. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010;63:e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Sargeant J.M., O'Connor A.M., Dohoo I.R., Erb H.N., Cevallos M., Egger M. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology—veterinary (STROBE-Vet) statement. Prev. Vet. Med. 2016;134:188–196. doi: 10.1016/j.prevetmed.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 16.The COHERE Standards. 2017. http://deohs.washington.edu/cohr/cohere Available at:
- 17.McKenzie J.S., Dahal R., Kakkar M., Debnath N., Rahman M., Dorjee S. One Health research and training and government support for One Health in South Asia. Infect. Ecol. Epidemiol. 2016;6(0):16–19. doi: 10.3402/iee.v6.33842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rwego I.B., Olajide Babalobi O., Musotsi P., Nzietchueng S., Keambo Tiambo C., David Kabasa J. One Health capacity building in sub-Saharan Africa. Infect. Ecol. Epidemiol. 2016;6(0):75–79. doi: 10.3402/iee.v6.34032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroud C., Kaplan B., Logan J.E., Gray G.C. One Health training, research, and outreach in North America. Infect. Ecol. Epidemiol. 2016;6(0):1–16. doi: 10.3402/iee.v6.33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikkema R., Koopmans M. One Health training and research activities in Western Europe. Infect. Ecol. Epidemiol. 2016;6(0):2142–2149. doi: 10.3402/iee.v6.33703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid S.A., McKenzie J., Woldeyohannes S.M. One Health research and training in Australia and New Zealand. Infect. Ecol. Epidemiol. 2016;6(0):48–49. doi: 10.3402/iee.v6.33799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Liu L., Wang G., Lu J. One Health in China. Infect. Ecol. Epidemiol. 2016;6(0):1–8. doi: 10.3402/iee.v6.33843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabinowitz P.M., Natterson-Horowitz B., Kahn L.H., Kock R., Pappaioanou M. Incorporating one health into medical education. BMC Med. Educ. 2017;17(1):45. doi: 10.1186/s12909-017-0883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dell R.B. Animal studies and one health: IACUC considerations. ILAR J. 2010;51(3):288–290. doi: 10.1093/ilar.51.3.288. [DOI] [PubMed] [Google Scholar]
- 25.Osterhaus A., MacKenzie J. The ‘One Health’ journal: filling a niche. One Health. 2016;2:18. doi: 10.1016/j.onehlt.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willingham A.L., Cruz-Martinez L., Scorpio D.G., Gallagher C.A. Global solutions to regional challenges: bridging the one health divide in the Caribbean. One Health. 2016;2:8–10. doi: 10.1016/j.onehlt.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Association for Ecology and Health 2017. https://ecohealth.net/en/ Available at:
- 28.WHO Constitution of the World Health Organization. 1948. http://www.who.int/about/mission/en/ Available at:
- 29.Mosites E., Sammons M., Otiang E., Eng A., Noecker C., Manor O. Microbiome sharing between children, livestock and household surfaces in western Kenya. PLoS One. 2017;12(2):e0171017–15. doi: 10.1371/journal.pone.0171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purohit M., Chandran S., Shah H., Diwan V., Tamhankar A., Stalsby Lundborg C. Antibiotic resistance in an Indian rural community: a ‘One-Health’ observational study on commensal coliform from humans, animals, and water. Int. J. Environ. Res. Public Health. 2017;14(4):386–413. doi: 10.3390/ijerph14040386. (04/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohmen W., Schmitt H., Bonten M., Heederik D. Air exposure as a possible route for ESBL in pig farmers. Environ. Res. 2017;155:359–364. doi: 10.1016/j.envres.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Garcia M.N., O'Day S., Fisher-Hoch S., Gorchakov R., Patino R., Feria Arroyo T.P. One Health interactions of Chagas disease vectors, canid hosts, and human residents along the Texas-Mexico border. PLoS Negl. Trop. Dis. 2016;10(11):e0005074–10. doi: 10.1371/journal.pntd.0005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons M.B., Travis D., Lonsdorf E.V., Lipende I., Roellig D.M., Collins A. Epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl. Trop. Dis. Feb 20, 2015;9(2):e0003529. doi: 10.1371/journal.pntd.0003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima M.M., Sarquis O., de Oliveira T.G., Gomes T.F., Coutinho C., Daflon-Teixeira N.F. Investigation of Chagas disease in four periurban areas in northeastern Brazil: epidemiologic survey in man, vectors, non-human hosts and reservoirs. Trans. R. Soc. Trop. Med. Hyg. Mar 2012;106(3):143–149. doi: 10.1016/j.trstmh.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Johnson J.L., Ginsberg H.S., Zhioua E., Whitworth U.G., Markowski D., Hyland K.E. Passive tick surveillance, dog seropositivity, and incidence of human lyme disease. Vector Borne Zoonotic Dis. 2004;4(2):137–142. doi: 10.1089/1530366041210710. [DOI] [PubMed] [Google Scholar]
- 36.Davis M.F., Misic A.M., Morris D.O., Moss J.T., Tolomeo P., Beiting D.P. Genome sequencing reveals strain dynamics of methicillin-resistant Staphylococcus aureus in the same household in the context of clinical disease in a person and a dog. Vet. Microbiol. 2015;180(3–4):304–307. doi: 10.1016/j.vetmic.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shender L., Niemela M., Conrad P., Goldstein T., Mazet J. Habitat management to reduce human exposure to Trypanosoma cruzi and western conenose bugs (Triatoma protracta) EcoHealth. 2016;13(3):525–534. doi: 10.1007/s10393-016-1153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sotomayor-Bonilla J., Chaves A., Rico-Chavez O., Rostal M.K., Ojeda-Flores R., Salas-Rojas M. Dengue virus in bats from southeastern Mexico. Am. J. Trop. Med. Hyg. 2014;91(1):129–131. doi: 10.4269/ajtmh.13-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schurer J.M., Hill J.E., Fernando C., Jenkins E.J. Sentinel surveillance for zoonotic parasites in companion animals in indigenous communities of Saskatchewan. Am. J. Trop. Med. Hyg. 2012;87(3):495–498. doi: 10.4269/ajtmh.2012.12-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang C.F., Ma M.J., Zhan B.D., Lai S.M., Hu Y., Yang X.X. Nosocomial transmission of avian influenza a (H7N9) virus in China: epidemiological investigation. BMJ. 2015;351:h5765. doi: 10.1136/bmj.h5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey J.A., Curriero F.C., Cosgrove S.E., Nachman K.E., Schwartz B.S. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern. Med. 2013;173(21):1980–1990. doi: 10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Suarez N., Rial C., Boada L.D., Henriquez-Hernandez L., Valeron P.F., Camacho M. Are pet dogs good sentinels of human exposure to environmental polycyclic aromatic hydrocarbons, organochlorine pesticides and polychlorinated biphenyls? J. Appl. Anim. Res. 2016;44(1):135–145. [Google Scholar]
- 43.Daley P., Bajgai J., Penney C., Williams K., Whitney H., Golding G.R. A cross sectional study of animal and human colonization with Methicillin-resistant Staphylococcus aureus (MRSA) in an Aboriginal community. BMC Public Health. 2016;16(1):595. doi: 10.1186/s12889-016-3220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conlan J.V., Vongxay K., Khamlome B., Gomez-Morales M., Pozio E., Blacksell S.D. Patterns and risks of Trichinella infection in humans and pigs in northern Laos. PLoS Negl. Trop. Dis. 2014;8(7):e3034–10. doi: 10.1371/journal.pntd.0003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stull J.W., Peregrine A.S., Sargeant J.M., Weese J.S. Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada. BMC Public Health. 2013;13(1):520. doi: 10.1186/1471-2458-13-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui E.C., Perzanowski M., Peng R.D., Wise R.A., Balcer-Whaley S., Newman M. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma. JAMA. 2017;317(10):1027–1110. doi: 10.1001/jama.2016.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Exum N.G., Olortegui M.P., Yori P.P., Davis M.F., Heaney C.D., Kosek M. Floors and toilets: association of floors and sanitation practices with fecal contamination in Peruvian Amazon peri-urban households. Environ. Sci. Technol. 2016 doi: 10.1021/acs.est.6b01283. (acs.est.6b01283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carnes M.U., Hoppin J.A., Metwali N., Wyss A.B., Hankinson J.L., O'Connell E.L. House dust endotoxin levels are associated with adult asthma in a U.S. farming population. Ann. Am. Thorac. Soc. 2017;14(3):324–331. doi: 10.1513/AnnalsATS.201611-861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knox J., Sullivan S.B., Urena J., Miller M., Vavagiakis P., Shi Q. Association of environmental contamination in the home with the risk for recurrent community-associated, methicillin-resistant Staphylococcus aureus infection. JAMA Intern. Med. 2016;176(6):807–815. doi: 10.1001/jamainternmed.2016.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Rivera L., Cummings K.J., Loneragan G.H., Rankin S.C., Hanson D.L., Leone W.M. Salmonella prevalence and antimicrobial susceptibility among dairy farm environmental samples collected in Texas. Foodborne Pathog. Dis. 2015:2037. doi: 10.1089/fpd.2015.2037. [DOI] [PubMed] [Google Scholar]
- 51.Chow K., Hearn L.K., Zuber M., Beatty J.A., Mueller J.F., Barrs V.R. Evaluation of polybrominated diphenyl ethers (PBDEs) in matched cat sera and house dust samples_ investigation of a potential link between PBDEs and spontaneous feline hyperthyroidism. Environ. Res. 2015;136:173–179. doi: 10.1016/j.envres.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 52.Stoddard R.A., Atwill E.R., Gulland F.M.D., Miller M.A., Dabritz H.A., Paradies D.M. Risk factors for infection with pathogenic and antimicrobial-resistant fecal bacteria in northern elephant seals in California. Public Health Rep. 2008;123(3):360–370. doi: 10.1177/003335490812300316. [DOI] [PMC free article] [PubMed] [Google Scholar]