Abstract
The discovery of mutations in EGFR significantly changed the treatment paradigm of patients with EGFR-mutant non-small cell lung cancer (NSCLC), a particular group of patients with different clinical characteristics and outcome to EGFR-wild-type patients. In these patients, the treatment of choice as first-line therapy is first- or second-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib, erlotinib, or afatinib. Inevitably, after the initial response, all patients become refractory to these drugs. The most common mechanism of acquired resistance to EGFR-TKIs is the development of a second mutation in exon 20 of EGFR (T790M). Osimertinib is a third-generation EGFR-TKI designed for overcoming T790M-mediated resistance. Based on the results of efficacy and tolerability of Phase II and Phase III studies, osimertinib has been approved for treatment of advanced EGFRT790M+ mutation NSCLC following progression on a prior EGFR-TKI. Occurrence of acquired resistance to osimertinib represents an urgent need for additional strategies including combination with other agents, such as other targeted therapies or checkpoint inhibitors, or development of new and more potent compounds.
Keywords: EGFR-mutant non-small-cell Lung cancer, acquired resistance, T790M mutation, third generation EGFR-TKI, osimertinib
Introduction
Lung cancer is the second most commonly diagnosed cancer and the main cause of cancer-related mortality in both men and women. Non-small cell lung cancer (NSCLC) represents ~85% of lung cancer cases and it presents as metastatic disease in over half of all cases. In the last few years, treatment of NSCLC has radically changed after the discovery that inhibition by target agents of molecular drivers, such as EGFR, could be effective in reducing tumor burden. The prevalence of EGFR mutations in adeno-carcinoma is 10% of Western and up to 50% of Asian patients. It is well known that EGFR mutations are more frequently observed in Asiatic than in Caucasian patients, in female, in never smokers, and mainly in adenocarcinomas, with deletion in exon 19 or point mutation in exon 21 (L858R) as the most common (>90%) types. Nine randomized Phase III clinical trials (OPTIMAL, First Signal, IPASS, WJTOG 3405, NEJSG 002, EURTAC, ENSURE, LUX-3, LUX-6) demonstrated that, in patients harboring classical EGFR mutations, EGFR-tyrosine kinase inhibitors (EGFR-TKIs) such as erlotinib, gefitinib, or afatinib are superior to the standard platinum-based chemotherapy in terms of response rate, progression-free survival (PFS), toxicity profile, and quality of life (Table 1). In up to 60%–80% of patients treated with an EGFR-TKI, there is a meaningful tumor regression, but inevitably, after a median time of 9–12 months, all patients develop acquired resistance and become refractory.1–5 Among the different mechanisms of acquired resistance, a secondary mutation, T790M, in the exon 20 of the EGFR gene is the most frequent event, occurring in ~50%–60% of cases. At the present time, only one agent has been US Food and Drug Administration (FDA) approved for treatment of EGFRT790M+ patients. Phase II studies and more recently a large Phase III trial demonstrated that osimertinib (Tagrisso, AstraZeneca, London, UK) is active in EGFR-TKI-pretreated, EGFRT790M+ patients, representing today the best option in the acquired resistance setting.5–8
Table 1.
Studies of EGFR-TKIs versus chemotherapy as first-line therapy in EGFRmut+ NSCLC
| Study | EGFR-TKI | n | Median PFS in TKI arm (months) | P-value | HR |
|---|---|---|---|---|---|
| OPTIMAL51 | Erlotinib | 154 | 13.7 | <0.0001 | 0.16 |
| First signal52 | Gefitinib | 42 | 8.4 | 0.084 | 0.48 |
| IPASS53 | Gefitinib | 261 | 9.5 | <0.0001 | 0.36 |
| WJTOG 340554 | Gefitinib | 177 | 9.2 | <0.001 | 0.42 |
| NEJSG 00255 | Gefitinib | 200 | 10.8 | <0.001 | 0.36 |
| EURTAC56 | Erlotinib | 174 | 10.4 | <0.0001 | 0.42 |
| ENSURE57 | Erlotinib | 217 | 11.0 | 0.0001 | 0.34 |
| LUX-358 | Afatinib | 308 | 11.1 | 0.001 | 0.58 |
| LUX-659 | Afatinib | 364 | 11.0 | <0.0001 | 0.28 |
Abbreviations: EGFR-TKI, epidermal growth factor-tyrosine kinase inhibitor; HR, hazard ratio; PFS, progression-free survival.
Resistance to EGFR-TKIs
According to Jackman’s criteria, resistant patients should have the following features:
Previously received treatment with a single-agent EGFR-TKI;
Either or both the following elements: a tumor harboring an EGFR mutation known to be associated with drug sensitivity (ie, G719X, exon 19 deletion, L858R, L861Q) or objective clinical benefit from treatment with an EGFR-TKI (documented partial or complete response [CR] according to RECIST or WHO criteria) or significant and durable (≥6 months) clinical benefits (stable disease [SD] as defined by RECIST or WHO) after initiation of an EGFR-TKI.
The following criteria are additional: systemic progression while on continuous treatment with an EGFR-TKI within the last 30 days and no intervening systemic therapy between cessation of EGFR-TKIs and new therapy.9 Acquired resistance to EGFR-TKIs can be target dependent, if it is characterized by the development of a second mutation in EGFR sequence, or target independent, if it is a consequence of the activation of alternative pathways.4 The most frequent mechanism of acquired resistance (up to 60% of cases) is target dependent and consists of the emergence of the T790M mutation, a characteristic point mutation in exon 20 of the EGFR gene. Target-independent mechanisms include MET amplification (4%), human EGFR type 2 (HER2) amplification (8%–13%), PIK3CA mutation (2%), BRAF mutation (1%), histological transformation from NSCLC to SCLC (6%), or epithelial–mesenchymal transition (1%-2%).2,4 In 18% of the cases, the mechanism of acquired resistance is unknown (Figure 1). Histological and biological review of tissue samples, taken after the development of acquired resistance, demonstrated that, in some cases, these mechanisms overlap and are not mutually exclusive.10
Figure 1.
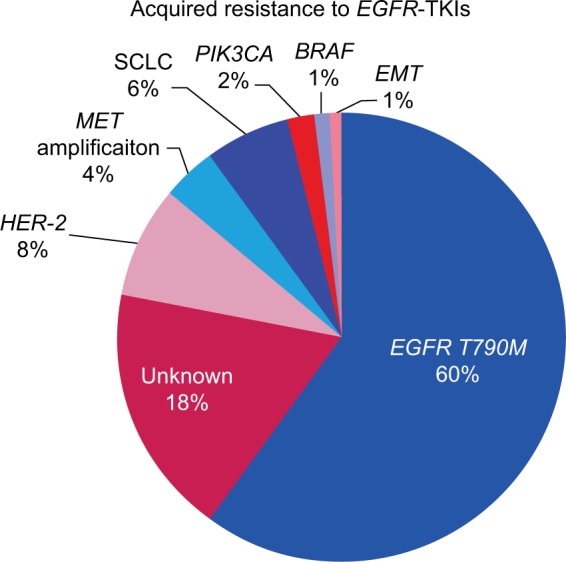
Mechanisms responsible for acquired resistance to EGFR-TKIs.
Abbreviations: EGFR-TKI, epidermal growth factor-tyrosine kinase inhibitor; EMT, epithelial to mesenchymal transition; SCLC, histological transformation to small cell lung cancer.
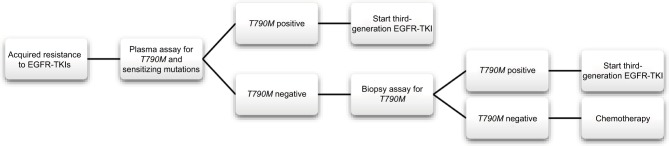
The complexity of resistance mechanisms highlights the importance of repeating a tumor biopsy at the time of disease progression. Moreover, availability of new agents specifically effective only in the presence of EGFRT790M mutation explains why tumor re-biopsy is now entering into clinical practice. Unfortunately, in lung cancer patients, repeating tumor biopsy is not feasible in the majority of cases mainly because of the risk related to a new biopsy in a difficult-to-access disease or patient refusal. Therefore, during the last few years, much interest is growing around the possibility to assess the mutational status on circulating tumor DNA (ctDNA). The so-called “liquid biopsy,” which involves isolating ctDNA in plasma or other biologic fluids, including urine, presents several advantages including the fact that it is easy to perform, rapid, and repeatable, overcoming the problem of tumor heterogeneity.11,12 The only relevant limitation is represented by the relatively low sensitivity (60%–80%). Sensitivity is also influenced by the type of mutation and tumor burden, with patients with low tumor burden at a high risk of a false-negative result. Therefore, in clinical practice, liquid biopsy is now recommended as the first test to offer to the patient, with tumor biopsy recommended only in the case of a negative result (Figure 2).13
Figure 2.
Flowchart for plasma and tumor genotyping for T790M testing.
Abbreviations: EGFR-TKI, epidermal growth factor-tyrosine kinase inhibitor.
Pharmacodynamic
The T790M mutation consists of the substitution of threonine at the “gatekeeper” amino acid 790 by methionine. This mutation makes the receptor refractory to the inhibition by reversible EGFR-TKIs through both steric hindrance and increased ATP affinity (its natural substrate). Osimertinib is an oral, irreversible, third-generation TKI targeting T790M and EGFR-TKI-sensitizing mutation sparing the activity of wild-type EGFR. First-generation reversible TKIs (erlotinib and gefitinib) are ineffective at targeting T790M, while they strongly inhibit wild-type EGFR cell lines with similar potency to sensitizing mutant EGFR. Second-generation irreversible TKIs (afatinib and dacomitinib) show activity against T790M in vitro, but concentrations required to overcome T790M activity preclinically are not achievable in humans due to non-selective inhibition of wild-type EGFR which is associated with significant toxicity.14 The good safety profile and the tolerability of osimertinib are related to the selective inhibition of T790M and EGFR-sensitizing mutation. 1) Osimertinib is less potent at inhibiting phosphorylation of EGFR in wild-type cell lines. It is associated with lower skin and gastrointestinal toxicity.
Clinical trials
AURA5 was a Phase 1 study assessing the safety, tolerability, and efficacy of osimertinib. Eligible patients had a locally advanced or metastatic NSCLC, had a known EGFR-TKI-sensitizing mutation or a prior clinical benefit from treatment with EGFR-TKI, and had a radiologically documented disease progression while receiving such treatment. This study included dose-escalation and dose-expansion cohorts. In the dose-escalation cohorts, patients received a single dose of osimertinib. The first dose tested was 20 mg daily. Each subsequent dose represented a 100% increase from the previous dose, with the exception of the final dose escalation, which was from 160 mg once daily to 240 mg once daily. In the dose-escalation cohorts, pretreatment EGFRT790M testing was optional, while in the dose-expansion cohorts, a new tumor biopsy was required after disease progression on the most recent regimen. Testing for EGFR T790M was performed in a central laboratory or in a local laboratory followed by confirmation in a central laboratory. The objective response rate (ORR) for the entire population was 51% and the disease control rate (DCR) (CR plus partial response plus SD) was 84%. In patients harboring the T790M mutation, the ORR was 61% and the DCR was 95%. There was activity in the T790M-negative patients with an ORR of 21% and DCR of 61%. In EGFRT790M+ patients, the median PFS was 9.6 months and in EGFRT790M– patients, it was 2.8 months. The most common adverse events (AEs) were diarrhea (47%), rash (40%), nausea (22%), and anorexia (21%). Grade 3 or higher AEs were observed in 32% of patients, with AEs leading to dose reduction in 7% of patients and AEs leading to drug discontinuation in 6% of patients.5,15 The optimal dose to obtain the best efficacy with the lower risk of toxicity is 80 mg once daily.
AURA ex,6 a Phase II extension cohort of the Phase I trial, evaluated the efficacy, tolerability, and safety of osimertinib at a dose of 80 mg once daily in the EGFRT790M+patients progressing after EGFR-TKI treatment. Similar to the results from the Phase I trial, the ORR was 61% with a DCR of 91%. Osimertinib was well tolerated with drug-related grade ≥3 AEs reported in 12% of the patients and a discontinuation rate of 4%. These promising results were confirmed also in AURA 2 study,7 a Phase II, single-arm trial conducted in EGFRT790M+, which showed an ORR of 71%, with a DCR of 92% and a median PFS of 6.8 months.6,7 In a combined analysis of 411 patients in both Phase II trials (AURA ex and AURA 2), the most commonly reported all-grade AEs were diarrhea (42%), rash (41%), dry skin (31%), nail toxicity (25%), eye disorders (18%), nausea (17%), decreased appetite (16%), and constipation (15%). These events were primary grade 1/2, with a low rate of grade ≥3 AEs. The most common grade ≥3 AEs were pneumonia (2%) and pulmonary embolism (2%). Across both studies, dose reductions as a result of AEs were needed for 4.4% of patients. The most frequently reported AEs that led to a dose reduction or interruption were QTc prolongation (2%) and neutropenia (2%). Other AEs resulting in treatment discontinuation were interstitial lung disease (ILD) or pneumonitis (2%) and cerebrovascular accident (1%). Fatal AEs occurred in 3.2% of patients and consisted of four cases of pneumonitis, which were attributed to osimertinib.6,7 Since in AURA and AURA 2 trials brain metastases were assessed as non-target lesions, there were no measurements of metastatic brain lesion diameter. Therefore, it was not possible to calculate an ORR or DCR for central nervous system (CNS) disease. In these studies, the proportion of patients with the CNS as the first site of progression was 12%.16 Omuro et al reported that the incidence of the CNS as an initial failure site reached 33% in EGFR-TKI responders with advanced NSCLC.17 Approximately half of patients with EGFR-positive metastatic NSCLC treated with first-line chemotherapy develop CNS disease relapse, and the low rate of primary CNS relapse in AURA and AURA 2 trials may suggest a CNS antitumor activity16 of osimertinib. The mechanism underlying the relationship between clinical benefit from EGFR-TKIs and CNS metastasis may involve several causal factors. Prolonged survival through the use of EGFR-TKIs may coincide with a substantial risk of developing CNS metastasis, as the cranial event occurs in a relatively late phase of the disease. The high frequency of EGFR mutations in brain metastases of lung adenocarcinoma suggests an intrinsic brain tropism of these tumors. Incomplete drug penetration of the brain–blood barrier may account for the increased incidence of CNS metastasis. Metastatic CNS clones may possess an inherited resistance to EGFR-TKIs, or they may acquire earlier drug resistance during EGFR-TKI therapy.17,47 Noteworthy, first-generation EGFR-TKIs hardly penetrate across the blood–brain barrier at the recommended doses.18 Based on the data from Phase II studies (AURA extension6 and AURA 27), osimertinib was approved by FDA, in November 2015, and by European Medicines Agency, in April 2016, for patients with advanced EGFRT790M+ NSCLC following progression on a prior EGFR-TKI.
AURA 3,8 published on December 2016, is a Phase III trial comparing osimertinib with platinum-based doublet chemotherapy in patients with EGFRT790M+ advanced NSCLC after first-line EGFR-TKI therapy. Patients were randomly assigned to received oral osimertinib (80 mg once daily) or intravenous pemetrexed (500 mg/m2) plus either carboplatin area under the curve (AUC) 5 or cisplatin (75 mg/m2). The median PFS was significantly longer with osimertinib than with platinum-based chemotherapy (10.1 months vs 4.4 months). This benefit was observed across all predefined subgroups also among patients with stable, asymptomatic CNS metastases (8.5 months vs 4.2 months), supporting preclinical and clinical data suggesting that osimertinib may be an EGFR-TKI with improved brain exposure.19 The ORR was significantly better with osimertinib than with platinum-based chemotherapy (71% vs 31%). The good clinical profile of osimertinib was confirmed also in AURA 3 trial: the proportion of patients with grade 3 AEs was 23% in the osimertinib group and 47% in the chemotherapy group. Osimertinib was associated with a lower rate of AEs leading to permanent discontinuation.8
Leptomeningeal metastasis is another detrimental complication of advanced EGFR mutation-positive NSCLC. A Phase I study (BLOOM study NCT02228369) is ongoing to test osimertinib monotherapy at 160 mg once daily against brain and leptomeningeal metastasis. Preliminary data demonstrated encouraging results in terms of safety and efficacy.20
According to the clinical activity and tolerability, osimertinib is being tested in other Phase III studies. In FLAURA Phase III trial (NCT02296125), treatment-naïve patients with locally advanced or metastatic EGFR mutant NSCLC were randomly assigned to receive osimertinib (80 mg qd, orally) or standard of care EGFR-TKI (gefitinib 250 mg qd, orally, or erlotinib 150 mg qd, orally).21
Combination treatment is another strategy to improve the efficacy and antitumor activity. TATTON trial (NCT02143466) is a multi-arm, Phase Ib trial investigating osimertinib 80 mg once daily in combination with durvalumab (anti-PD-L1 monoclonal antibody) or with savolitinib (MET inhibitor) or with selumetinib (MEK 1/2 inhibitor) in patients with advanced EGFR-mutant lung cancer. Primary objectives were safety and tolerability and the secondary objective was clinical activity of the combinations. An increase in ILD events was observed with the combination of osimertinib plus durvalumab. Therefore, enrollment in the osimertinib plus durvalumab combination arm has been suspended.22 Other trials assessing the combination treatment are ongoing: osimertinib plus necitumumab (NCT02496663), plus ramucirumab (NCT02789345), or plus bevacizumab (NCT02803203). In addition to metastatic disease, osimertinib is being tested in the adjuvant setting (ADAURA study, NCT02511106).23
Currently, osimertinib is the only EGFR-TKI approved for patients with metastatic EGFRT790M+ NSCLC. Rociletinib (Clovis) is another third-generation EGFR-TKI designed to inhibit both T790M and EGFR-activating mutations while sparing wild-type EGFR. Rociletinib has been investigated in EGFR-mutant patients who progressed after at least one line of EGFR-TKI treatment (TIGER X Phase I/II trial), in first-line setting versus erlotinib in EGFR-mutated patients (TIGER 1 Phase II/III trial), and in second-line post-standard EGFR-TKI treatment (TIGER 2 Phase II trial) versus chemotherapy in patients who have progressed after standard EGFR-TKI and after platinum-based doublet chemotherapy.2 Despite initial promising results, Clovis has stopped the clinical development of rociletinib because of updated data revealing lower response rates than initially reported, a negative vote from the FDA’s Oncologic Drugs Advisory Committee (ODAC), and FDA approval of osimertinib, rociletinib’s main competitor in the setting.24,25 Further third-generation EGFR-TKIs in clinical development are HM61713, ASP8273, EGF816, and PF-06747775.26
Immune checkpoint inhibitors and EGFR-TKIs
Recent data showed that nivolumab (Checkmate 05727 and Checkmate 01728), pembrolizumab (Keyote 01029), and atezolizumab (POPLAR30 and recently OAK31 study) are superior to docetaxel in second-line setting and pembrolizumab (Keynote-02432) also improve survival versus platinum-based chemotherapy in PD-L1-positive untreated NSCLC. As shown in these studies, among EGFR-mutant patients, the efficacy of checkpoint inhibitors was lower than that in wild-type population probably because of the low level of mutational load in EGFR-mutant tumors. EGFR-mutant NSCLC expresses higher PD-L1 levels than wild-type, while gefitinib can reduce the PD-L1 expression, suggesting that combined strategies of EGFR-TKI and immunotherapy may be an interesting approach.33 While promising results come from a combination of nivolumab plus erlotinib in EGFR-mutant advanced NSCLC with acquired resistance to EGFR-TKI34 and from pembrolizumab plus gefitinib in heavily pretreated (up to four prior therapies) EGFR-mutant NSCLC,35 association treatment with osimertinib showed significant toxicity. In the Phase I TATTON trial (NCT02143466) and in Phase III CAURAL trial (NCT02143466), the combination of osimertinib and durvalumab (anti-PD-L1 monoclonal antibody) in patients with EGFR-mutant NSCLC with acquired resistance to EGFR-TKI and T790M positivity showed a high incidence of ILD.22,23 However further Phase I/II combination trials of checkpoint inhibitors are ongoing in EGFR-TKI-naïve and pretreated patients (NCT02013219: erlotinib plus atezolizumab, NCT02364609: afatinib plus pembrolizumab).36 Several clinical studies are underway to assess new immunotherapy strategies in different settings.37 A combination of ipilimumab and nivolumb has been tested as first-line treatment in advanced NSCLC with interesting ORR.48 Durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA4) showed clinical activity in relapsed NSCLC.49 Many studies are ongoing to test new targets for immunotherapy such as inhibitory molecules (indoleamine-dioxygenase, adenosine, TIM-3, LAG-3) or stimulatory molecules (OX40, CD40, CD27), new peptide vaccines targeting novel antitumor antigens, alternative checkpoint inhibitors, chimeric antigen receptor T cells (CAR-T), histone deacetylase (HDAC) inhibitors, and DNA hypomethylating agents target epigenetics for tumor growth suppression.50 Immune checkpoint inhibitors have been approved for therapy of a variety of advanced cancers, and they also can be considered for combination therapy to overcome the acquired resistance to EGFR-TKIs.37
Clinical strategies beyond EGFR-TKI progression
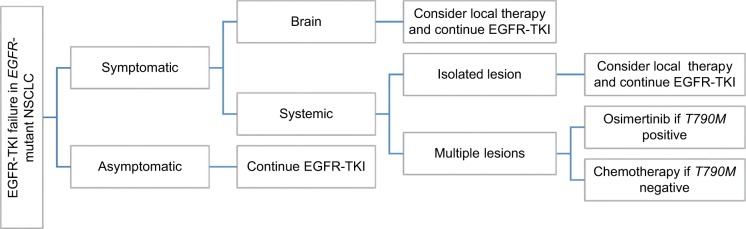
The standard of care for patients with acquired resistance to EGFR-TKIs is rapidly changing after the development of third-generation EGFR-TKIs targeting both T790M and EGFR-TKI-sensitizing mutation. Osimertinib is the new standard of care in patients with metastatic EGFRT790M+ NSCLC after progression on erlotininb, gefitinib, or afatinib. A second-line platinum-based doublet chemotherapy remains the standard of care in patients without T790M mutation or other targetable resistance mechanism especially in the case of dramatic progression.3,36 EGFR-TKIs should be interrupted during chemotherapy, although some clones of EGFR-mutant NSCLC maintain dependence on EGFR signaling after the development of acquired resistance. IMPRESS study is a randomized Phase III trial comparing maintenance gefitinib combined with pemetrexed and cisplatin versus chemotherapy alone in patients with acquired resistance to gefitinib. No difference in PFS was reported in both the arms (median PFS 5.4 months in both the groups). Combination treatment showed a detrimental effect on the overall survival compared with chemotherapy alone. Patients without T790M mutation had a non-significant benefit with combination treatment (PFS 6.7 vs 5.4 months).38 For patients with oligometastatic progression, local therapies such as radiotherapy, surgery, and stereotactic ablative radiotherapy in conjunction with continued EGFR-TKI can extend disease control by over 6 months.39 Finally, patients with indolent, asymptomatic progression, and good performance status may continue to be treated with EGFR-TKI beyond RECIST progression if there is no evidence of deterioration or intolerable toxicity (Figure 3). These strategies are supported by the risk of disease flare after EGFR-TKI cessation, considering that some clones remain sensitive to EGFR inhibition after the acquired resistance.3,36 Second-generation irreversible TKIs failed to overcome T790M-mediated resistance because concentrations at which they overcome T790M activity preclinically are not achievable in humans due to dose-limiting toxicity related to non-selective inhibition of wild-type EGFR.40 Vertical inhibition, the simultaneous inhibition of both extracellular and intracellular receptor domains with the combination of cetuximab and Afatinib, in a Phase Ib clinical trial demonstrated promising results, but the high rate of toxicity limited its use in clinical practice.41
Figure 3.
Algorithm for treatment after acquired resistance in EGFR-mutant NSCLC.
Abbreviations: EGFR-TKI, epidermal growth factor-tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer.
Acquired resistance to a T790M-specific EGFR inhibitor
The main mechanism of resistance to osimertinib and to all third-generation irreversible EGFR inhibitors42 is the acquisition of missense mutation EGFR C797S in exon 20 which consist of the substitution of cysteine with serine at the amino acid position 797 within the kinase-binding site. Osimertinib loses the ability to form covalent bond with EGFR at the position of cysteine residue.12 EGFR C797S arise in approximately one-third of patients treated with osimertinib11 over a period of 9–13 months.42 In preclinical models, the configuration of T790M and C797S mutation affects how cells respond to therapy. If the two mutations are in trans (on different alleles), cells are resistant to third-generation EGFR-TKIs, but a combination of first-generation TKIs can restore EGFR inhibition. If the two mutations are in cis (on the same allele), cells are refractory to any EGFR-TKIs.12,23 In a case report of a patient with an EGFR-mutant lung cancer, next-generation sequencing (NGS) techniques were performed on three biopsy specimens obtained before treatment with erlotinib, after acquired resistance to erlotinib, and after acquired resistance to AZD9291. The original sensitizing EGFR mutation was present in all tumor samples. Under the selective pressure of EGFR-TKIs, the tumor developed secondary (T790M) and tertiary (C797S) mutations to maintain EGFR signaling.43 A subsequent study collected plasma samples from 15 patients who received osimertinib therapy and had preexisting plasma EGFRT790M. A total of 40% of patients had EGFRdel19/T790M/C797S, 33% of patients had EGFRT790M alone, and EGFR T790M was no longer detectable in 27% of patients.44 Interestingly, in patients with chronic lymphocytic leukemia treated with ibrutinib, (Bruton tyrosine kinase [BTK] inhibitor), mutations have been detected in C481 (C481S), the analogous cysteine residue to C797 in EGFR, suggesting that mutations in this conserved residue may be a common mechanism of acquired resistance to covalent kinase inhibitors.45 Additional mechanisms of resistance to osimertinib in patients negative for C797S include HER-2 or MET amplification, loss of T790M mutation,46 EGFR L718Q, EGFR L798I, KRAS G12S.42 In addition to acquired mutations and gene amplifications, phenotype transformation represents a distinct mechanism of resistance. Adenocarcinoma turns into small cell lung cancer with RB1 inactivation as the defining features.42 The genomic heterogeneity associated with resistance to EGFR-TKIs in NSCLC requires the development of target therapy to overcome C797S resistance and combination therapies that can inhibit the emergence of multiple resistance mechanisms.44 EAI045 is the first allosteric TKI purposefully designed to overcome T790M and EGFR C797S mutations. In a genetically engineered mouse model of L858R/T790M mutant-driven lung cancer, EAI045 was tested alone and in combination with cetuximab. While the allosteric inhibitor was ineffective alone due to receptor dimerization, the combination of EAI045 and cetuximab showed significant tumor regression. Clinical trials are required to assess these results in patients with advanced NSCLC.42
Conclusion
Acquired resistance is one of the most significant limitations in lung cancer treatment. Despite an initial benefit to target therapies, all patients become refractory. Identification of mechanisms involved in drug resistance is essential to tailor the best treatment strategy for each patient. This is the reason why identification of biomarkers should be encouraged and appropriate tissue sample or plasma assay is essential for biological characterization. Failure of currently available targeted therapies suggests that a single agent may be not sufficient to overcome drug resistance. New strategies, including combination treatment, are currently under investigation to identify new opportunities of treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Landi L, Cappuzzo F. Irreversible EGFR-TKIs: dreaming perfection. Transl Lung Cancer Res. 2013;2(1):40–49. doi: 10.3978/j.issn.2218-6751.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ou Romanidou, Landi L, Cappuzzo F, Califano R. Overcoming resistance to first/second generation epidermal growth factor receptor tyrosine kinase inhibitors and ALK inhibitors in oncogene-addicted advanced non-small cell lung cancer. Ther Adv Med Oncol. 2016;8(3):176–187. doi: 10.1177/1758834016631531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Guidelines. Non-small cell lung cancer (Version 4) 2016. [Accessed July 12, 2017]. Available from 1) https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 4.Cappuzzo F. Guide to Targeted Therapies: Treatment Resistance in Lung Cancer. Adis-Springer; Switzerland: 2015. [Google Scholar]
- 5.Jänne PA, Yang James Chih-Hsin, Kim Dong-Wan. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study Phase II extension component. J Clin Oncol. 2017;35(12):1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 7.Mitsudomi T, Tsai C, Shepherd F, et al. AZD9291 in pre-treated T790M positive advanced NSCLC: AURA2 Phase II study. J Thorac Oncol. 2015;10(9 Suppl 2):S320. n.d. [Google Scholar]
- 8.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackman D, Pao W, Riely G, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol. 2010;28(2):357–360. doi: 10.1200/JCO.2009.24.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha Yu, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancer. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabon JJ, Simmons AD, Lovejoy AF, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanism in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell R, Karachaliou N. Implications of blood-based T790M genotyping and beyond in epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3361–3362. doi: 10.1200/JCO.2016.68.3458. [DOI] [PubMed] [Google Scholar]
- 13.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3375–3382. doi: 10.1200/JCO.2016.66.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 16.Khozin S, Weinstock C, Blumenthal GM, et al. Osimertinib for the treatment of metastatic epidermal growth factor T790M positive non-small cell lung cancer. Clin Cancer Res. 2017;23(9) doi: 10.1158/1078-0432.CCR-16-1773. [DOI] [PubMed] [Google Scholar]
- 17.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 18.Jamal-Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer metastatic to the brain. Clin Cancer Res. 2012;18(4):938–944. doi: 10.1158/1078-0432.CCR-11-2529. [DOI] [PubMed] [Google Scholar]
- 19.Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res. 2016;22(20):5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 20.Yang JC-H, Kim D-W, Kim S-W, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from nonsmall cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol. 2016;34(Suppl):9002. [Google Scholar]
- 21.Ramalingam S, Rukazenkov Y, Thomaset K, et al. A randomized, phase III study (FLAURA) of AZD9291, a novel EGFR-TKI, versus gefitinib or erlotinib in treatment-naïve patients with advanced non-small cell lung cancer and an EGFR-TKI-sensitizing mutation. Abstract. Meetinglibrary. asco.org; ASCO Annual Meeting; 2015. Abstract number TPS8102. [Google Scholar]
- 22.Oxnard GR, Ramalingam SS, Ahn M-J, et al. Preliminary results of TATTON, a multi-arm phase Ib trial of AZD9291 combined with MEDI4736, AZD6094 or selumetinib in EGFR-mutant lung cancer. ASCO Meeting Abstracts. 2015;33:2509. [Google Scholar]
- 23.Liao BC, Lin CC, Lee JH, Yang Chih-Hsin. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptors tyrosine kinase inhibitors. J Biomed Sci. 2016;23:86. doi: 10.1186/s12929-016-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broderick J M. Clovis ends development of rociletinib in lung cancer. [Accessed July 18, 2017]. OncLive.com. Published Online: Friday, May 6, 2016. Available from http://www.onclive.com/web-exclusives/clovis-ends-development-of-rociletinib-in-lung-cancer.
- 25.Clovis Oncology announces regulatory update for rociletinib NDA filing. Business Wire. Nov 16, 2015. [Accessed July 18, 2017]. Available from http://www.business-wire.com/news/home/20151116005513/en/Clovis-Oncology-Announces-Regulatory-Update-Rociletinib-NDA.
- 26.Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst RS, Baas P, Kim DW, et al. Pembrolizumabversus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomized controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 30.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 31.Barlesi F, Park K, Ciardello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol. 2016;27(Suppl. 6):vi552–vi587. [Google Scholar]
- 32.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 33.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kB. Biochem Biophys Res Commun. 2015;463:95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 34.Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-D1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. Vol. 32; ASCO Meeting Abstracts; 2014. p. 8022. [Google Scholar]
- 35.Creelan BC, Chow LQ, Kim DW, et al. Safety and tolerability results from a phase I study of MEDI4736, a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib in patients (pts) with non-small-cell lung cancer (NSCLC). Vol. 33; ASCO Meeting Abstracts; 2015. p. 3047. [Google Scholar]
- 36.Remon J, Besse B. Unravelling signal escape through maintained EGFR activation in advanced non-small cell lung cancer (NSCLC): new treatment options. ESMO Open. 2016;1(4):e000081. doi: 10.1136/esmoopen-2016-000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9:47. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonia JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 39.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eskens FA, Mom CH, Planting AS, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008;98:80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janjigian YY, Smith EF, Groen HJ, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov. 2014;4:1036–1045. doi: 10.1158/2159-8290.CD-14-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Song Y, Liu D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017;385:51–54. doi: 10.1016/j.canlet.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Yu HA, Tian SK, Drilon AE, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1(7):982–984. doi: 10.1001/jamaoncol.2015.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol. 2016;9:59. doi: 10.1186/s13045-016-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patient with nonsmall-cell lung cancer. Cancer. 2010;116:1336–1343. doi: 10.1002/cncr.24877. [DOI] [PubMed] [Google Scholar]
- 48.Hellman MD, Gettinger SN, Goldman JW, et al. Checkmate 012: safety and efficacy of first-line nivolumab and ipilimumab in advanced NSCLC. ASCO Meeting Abstracts. 2016;34(15_suppl):3001. [Google Scholar]
- 49.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dholaria B, Hammond W, Shreders A, Lou Y. Emerging therapeutic agents for lung cancer. J Hematol Oncol. 2016;9:138. doi: 10.1186/s13045-016-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mok TS, Wu YI, Thongprasert S, et al. Gefitinib or Carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 52.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 53.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 54.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 55.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first line treatment for patients with advanced EGFR mutation-positive non-small cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 56.Rosell R, Carceremy E, Gervais R, et al. Erlotinib versus standard chemotherapy as first line treatment for European patients with advanced EGFR-mutation positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 57.Wu YL, Liam CK, Zhou C, et al. First-line erlotinib versus cisplatin/gemcitabine (GP) in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC): interim analyses from the phase 3, open-label, ENSURE study. J Thorac Oncol. 2013;8:s603. doi: 10.1093/annonc/mdv270. Suppl.2. [DOI] [PubMed] [Google Scholar]
- 58.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 59.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutation (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]