ABSTRACT
Upon exposure to seawater, euryhaline teleosts need to imbibe and desalinate seawater to allow for intestinal ion and water absorption, as this is essential for maintaining osmotic homeostasis. Despite the potential benefits of increased mixing and transport of imbibed water for increasing the efficiency of absorptive processes, the effect of water salinity on intestinal motility in teleosts remains unexplored. By qualitatively and quantitatively describing in vivo intestinal motility of euryhaline rainbow trout (Oncorhynchus mykiss), this study demonstrates that, in freshwater, the most common motility pattern consisted of clusters of rhythmic, posteriorly propagating contractions that lasted ∼1–2 min followed by a period of quiescence lasting ∼4–5 min. This pattern closely resembles mammalian migrating motor complexes (MMCs). Following a transition to seawater, imbibed seawater resulted in a significant distension of the intestine and the frequency of MMCs increased twofold to threefold with a concomitant reduction in the periods of quiescence. The increased frequency of MMCs was also accompanied by ripple-type contractions occurring every 12–60 s. These findings demonstrate that intestinal contractile activity of euryhaline teleosts is dramatically increased upon exposure to seawater, which is likely part of the overall response for maintaining osmotic homeostasis as increased drinking and mechanical perturbation of fluids is necessary to optimise intestinal ion and water absorption. Finally, the temporal response of intestinal motility in rainbow trout transitioning from freshwater to seawater coincides with previously documented physiological modifications associated with osmoregulation and may provide further insight into the underlying reasons shaping the migration patterns of salmonids.
KEY WORDS: Fish, Osmoregulation, Enteric electrical activity, Spatio-temporal maps, Video recordings, Salinity
Summary: Intestinal contractile activity of euryhaline teleosts dramatically increases when in seawater to optimise intestinal ion and water absorption, which is most likely necessary to maintain osmotic homeostasis.
INTRODUCTION
The capacity of euryhaline teleosts, such as rainbow trout (Oncorhynchus mykiss), to maintain osmotic homeostasis whilst inhabiting freshwater (FW) or seawater (SW) depends on various behavioural, morphological and physiological mechanisms (Evans, 2008; McCormick and Saunders, 1987). In FW, a dilute ionic and osmotic medium, teleosts face a diffusive osmotic gain of water and loss of ions across their permeable body surfaces (Edwards and Marshall, 2013). To counter this, FW teleosts minimise drinking rates, increase glomerular filtration rates and produce copious amounts of dilute urine (Perry et al., 2003). Furthermore, ions are actively absorbed from the surrounding water via mitochondria-rich cells in the gills (Evans, 2008). With respect to osmoregulation, the gastrointestinal system of FW teleosts is relatively inactive during the fasted state but, following the ingestion of a meal, it becomes increasingly more important as the quantities of most major electrolytes absorbed from the meal far exceed those transported from the water by the gills (Wood and Bucking, 2011).
In contrast, teleosts in SW are constantly faced with dehydration due to the diffusive loss of water to their relatively concentrated environment (Grosell, 2011). To counter this, teleosts continuously imbibe SW at rates typically ranging between 1 and 5 ml kg−1 h−1 (Grosell, 2011; Marshall and Grosell, 2005; Smith, 1930). The imbibed SW is then substantially desalinated in the oesophagus and stomach prior to entering the intestine to allow for solute-coupled water absorption, which is essential for the survival of teleosts in marine environments (Ando et al., 2003; Grosell, 2006; Hirano, 1974). The excess ions are subsequently excreted via branchial and renal pathways to maintain ionic balance (Evans, 2008; Marshall and Grosell, 2005). Regardless of feeding state, it is clear that the gastrointestinal system plays a prominent role in osmoregulation for teleosts in SW (Shehadeh and Gordon, 1969); however, the effects of water salinity on gastrointestinal motility remains unexplored despite representing the only mechanism for transporting and mixing imbibed SW.
For the effective mixing and propulsion of gastrointestinal contents, the contractions and relaxations of gastrointestinal smooth muscle, including the sphincters separating the different sections of the tract, are coordinated and tightly regulated via multiple layers of regulatory mechanisms [i.e. interstitial cells of Cajal, enteric neurons, hormones and paracrine substances (Gräns and Olsson, 2011; Olsson and Holmgren, 2001; Sanders et al., 2012)]. For example, mechano-receptors (responding to distension of the stomach and intestine by imbibed SW) as well as intestinal iono-receptors have been demonstrated to regulate the entry of SW into the oesophagus by stimulating the contraction of the cholinergically innervated upper oesophageal sphincter (Ando and Nagashima, 1996; Ando et al., 2003). The passage and processing of imbibed SW could also be expected to be associated with an increase in gastrointestinal motility, as the optimal transportation and mixing of imbibed SW would increase the efficiency of ion and water absorption by preventing the depletion of ions in the boundary layers near the epithelial surfaces (Lee, 1983). Furthermore, teleosts in SW continuously produce carbonate precipitates in the intestine (an osmoregulatory by-product of drinking calcium- and magnesium-rich SW), which facilitates intestinal water absorption and reduces calcium absorption (Wilson and Grosell, 2003; Wilson et al., 2009, 2002). Because these carbonate precipitates are produced at relatively high rates [e.g. 18–40 μmol C kg−1 h−1 in European flounder (Platichthys flesus) and Gulf toadfish (Opsanus beta) (Wilson et al., 2009, and references therein)], an increase in intestinal motility is most likely necessary to prevent the mucosal accumulation of these precipitates by propelling them along the tract for excretion.
List of abbreviations.
- EEA
enteric electrical activity
- FW
freshwater
- MMCs
migrating motor complexes
- SW
seawater
Intestinal motility patterns can be qualitatively and quantitatively described using spatio-temporal maps generated from video recordings of the intestine (Brijs et al., 2014; Hennig et al., 1999). Spatio-temporal maps allow the identification of a range of different contraction types, as well as their respective spatial (i.e. regional distribution) and temporal (i.e. frequency, duration, velocity and amplitude) characteristics. This method has successfully been used in teleosts to describe numerous motility patterns (Brijs et al., 2014, 2017a; Holmberg et al., 2007; Rich et al., 2013; Rönnestad et al., 2000), as well as to demonstrate differences in motility with respect to feeding state (Brijs et al., 2014). Furthermore, these studies highlight the similarities and differences between in vivo motility patterns as well as their respective control mechanisms in teleosts with those that are well documented in mammals, such as ripples, migrating motor complexes (MMCs), peristaltic contractions and standing contractions (D'Antona et al., 2001; Huizinga and Lammers, 2009; Husebye, 1999). However, a limitation of this method is that it requires direct visualisation of the intestine, necessitating the use of anaesthesia, which prevents recordings of intestinal motility from conscious animals in response to environmental changes such as those experienced by euryhaline species during a transition from FW to SW.
Gastrointestinal motility can also be investigated by surgically implanting electrodes into the wall of the stomach or intestine (Gräns et al., 2009, 2013). These electrodes are then connected to an amplifier and enable the detection of the electrical activity of smooth muscle, similar to electromyography in skeletal muscles. This electrical activity has recently been termed ‘enteric electrical activity’ (EEA) and is defined as the sum of all the electrical activity that occurs in the specific section of the gastrointestinal tract where the electrodes are implanted (Gräns, 2012; Gräns and Olsson, 2011). Recordings of electrical activity or EEA from gastrointestinal smooth muscle have been shown to correlate well with smooth muscle contractions in both mammals and teleosts (Aviv et al., 2008; Gräns, 2012; Gräns and Olsson, 2011; Perkins, 1971; Sanmiguel et al., 2007). An advantage of this methodology is that it allows the investigation of gastrointestinal motility in vivo in conscious animals and real-time responses to various challenges such as temperature changes and food ingestion (Fioramonti and Bueno, 1984; Gräns et al., 2009, 2013; Rodriguez Membrilla et al., 1995; Yin et al., 2004).
The overall aim of the present study was to describe in vivo intestinal motility patterns of rainbow trout in FW, as well as the acute and chronic response to SW exposure, in order to provide further insight into the physiological modifications required for the maintenance of osmotic homeostasis in euryhaline teleosts species transitioning from FW to SW. Specifically, we hypothesised that, upon a transition to SW, rainbow trout would exhibit an increase in intestinal motility to improve the efficiency of intestinal ion and water absorption via the mixing and transport of imbibed SW. Furthermore, this elevated level of intestinal motility would remain in fully SW-acclimated individuals, as the increased contractile activity serves to enhance the osmoregulatory function of the intestine, enabling rainbow trout to maintain osmotic homeostasis at sea.
MATERIALS AND METHODS
Experimental animals and holding conditions
Rainbow trout [Oncorhynchus mykiss (Walbaum 1792)] of mixed sex, acclimated to FW, were obtained from a local hatchery (Antens Laxodling AB, Alingsås, Sweden). They were initially held at 10–11°C on a 12 h:12 h light:dark photoperiod in 1000 l tanks containing recirculating aerated FW (salinity 0.1 ppt; pH 8.0; conductivity 286 µS cm−1; [ions] Na+ 6, K+ 0.4, Ca2+ 0.5 mmol l−1) for 3 weeks to recover from stress invoked during handling, transportation and the new environment. Following this period, one group of fish remained in FW, whereas another group were netted and transferred to similar 1000 l tanks containing artificial SW (salinity 30–33 ppt; source of artificial sea salt: Grotech, GmbH, Ahorn, Germany; pH 7.6–8.0; conductivity 45–65 mS cm−1; [ions] Na+ 425–530, K+ 4–7, Ca2+ 6–8 mmol l−1) at 10–11°C. In accordance with the SW acclimation protocol used in our previous study (Brijs et al., 2016), the animals were acclimated to these conditions for at least 6 weeks prior to experimentation. During this time there was no mortality and all fish were voluntarily feeding on dry commercial trout pellets (9 mm Protec Trout pellets, Skretting, Stavanger, Norway) three times per week. Animal care and all experimental procedures were performed in accordance with the guidelines and regulations set by an ethical permit (165-2015) approved by the Ethical Committee on Animal Research in Gothenburg, Sweden.
Investigating long-term changes in the intestinal motility of FW- and SW-acclimated trout using video recordings and spatio-temporal maps
Surgical procedure for video recordings
Rainbow trout acclimated to either FW or SW were fasted for 1 week prior to surgery. Individual fish were anaesthetised in FW or SW (depending on their acclimation salinity) containing 100 mg l−1 MS222 (ethyl-3-aminobenzoate methanesulphonic acid, Sigma-Aldrich Inc., St Louis, Missouri, USA), which was buffered with 200 mg l−1 NaHCO3. Body mass of the fish was determined prior to placing the individual on an operating table covered with soft, water-soaked foam. To maintain anaesthesia, the gills were continuously irrigated with aerated water of appropriate salinity containing 75 mg l−1 MS222 buffered with 150 mg l−1 NaHCO3 at 10°C.
Throughout the experiment, relative cardiac output was monitored to assess the depth of anaesthesia. This was achieved by making a small skin incision in the opercular cavity to expose the ventral aorta, and a 20 MHz Doppler flow crystal (Iowa Doppler Products, Iowa City, IA, USA) mounted in 2.3 mm cuffs was placed on the vessel (Fig. 1A). The lead from the Doppler flow probe was connected to a directional-pulsed Doppler flow meter (model 545C-4, Iowa Doppler Products), which in turn was connected to a PowerLab 8/30 system (ADInstruments, Castle Hill, Australia). Data was collected on a PC using ADInstruments acquisition software Chart™ 7 Pro v7.2.5, at a sampling rate of 100 Hz. ‘Pre-operative’ cardiac output of anaesthetised fish was measured for 15 min prior to opening the abdominal cavity. Based on observations from previous studies (Brijs et al., 2014, 2017a), if cardiac output had decreased by more than 30% of ‘pre-operative’ values during the experimental period then the fish would have been excluded owing to insufficient perfusion of the gastrointestinal system and overall health of the individual. However, all fish maintained stable values of cardiac output throughout the course of the experiment and therefore no individuals had to be excluded.
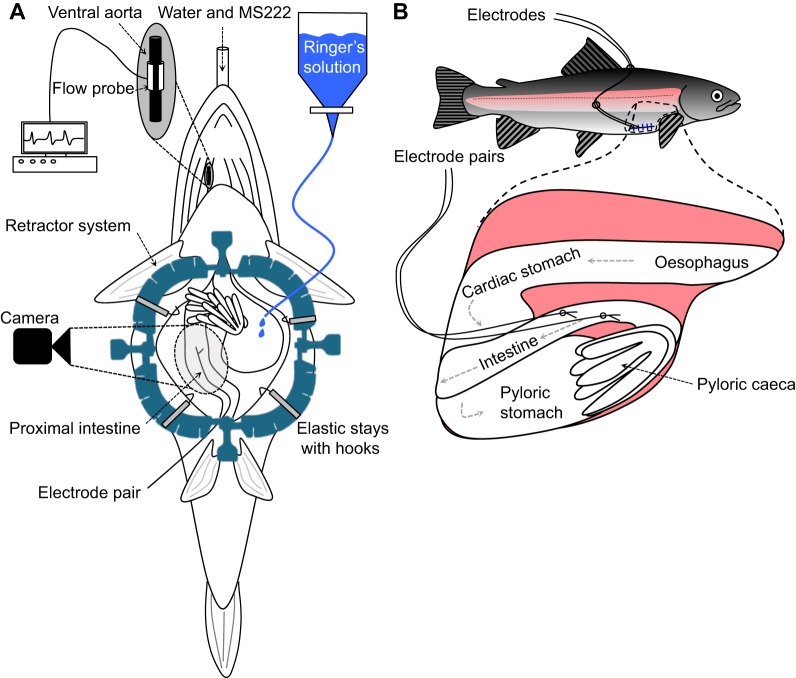
Fig. 1.
Schematic drawing of the surgical instrumentations. (A) Anaesthetised rainbow trout (Oncorhynchus mykiss) ventral side up, as it is surgically prepared for the video recording of the movements of the middle intestine. In some individuals that were surgically prepared for the video recording, pairs of electrodes were also implanted to record enteric electrical activity (EEA). (B) The middle intestine of rainbow trout instrumented with two pairs of electrodes exiting the abdominal cavity via the ventrolateral incision and secured to the fish with silk sutures.
The abdominal cavity of the fish was opened via a mid-ventral incision starting 10 mm anterior of the anus and finishing 10 mm caudal of the pectoral fins (Fig. 1A). The abdominal cavity was then held open using a disposable retractor system with elastic stays (Lone Star®, CooperSurgical, Trumbull, CT, USA). Depending on the size of the fish, approximately 50–80 mm of the intestine beginning just after the pyloric caeca could be visualised. This section of intestine most likely corresponds to a small section of the posterior proximal intestine and the majority of the middle intestine (Olsson, 2011), but for simplicity's sake will hereafter be referred to as the ‘middle intestine’. In some individuals it was necessary to reposition overlaying fat tissue to the side of the intestine in order to provide a clear image. Care was taken not to twist, restrict or damage any blood vessels or nerves during this process. Rainbow trout Ringer's solution (140 mmol l−1 NaCl, 2.5 mmol l−1 KCl, 1.5 mmol l−1 CaCl2, 0.8 mmol l−1 MgSO4, 15 mmol l−1 NaHCO3, 1 mmol l−1 KH2PO4, 5 mmol l−1 HEPES, 10 mmol l−1 glucose, pH 8.1–8.2 at 10–11°C) was contained in a custom-made jacketed organ bath and bubbled with a mixture of 99.7% air and 0.3% CO2. The Ringer's solution was continuously supplied to the abdominal cavity to ensure that the intestine was completely submerged throughout the course of the experiment. Following the surgical procedure, the fish was left undisturbed for 1 h before video recording commenced.
Video recordings
A ruler was placed in the field of view as a spatial reference and fibre-optic lights were strategically placed to achieve optimum contrast between the background and intestine. Images were captured using a DMK31AF03 monochrome FireWire camera (The Imaging Source, Putzbrunn, Germany). The resolution was 1024×768 pixels, corresponding to a field of view at the magnification used of 54×39 mm. Video recordings consisted of 1 h videos (7200 frames at 2 frames s−1). During this time, fish were left undisturbed and the temperature of both the Ringer's solution submerging the intestine and water irrigating the gills was maintained at 10°C. At the end of the experiment, the fish was removed from the surgery table and euthanised with a sharp cranial blow. Post-mortem examinations of the gastrointestinal tract were conducted to ensure that no food content was present.
Spatio-temporal map construction and analyses
Spatio-temporal maps were constructed from the video recordings and allowed us to examine the type, speed, direction and frequency of intestinal contractions. The maps display the changes in diameter of the intestine over time. The first frame of each movie was imported into Volumetry G8d (author: G.W.H.) where a cropping mask was drawn over the intestine. Movies were imported and calibrated for distance and time. Owing to the interspecific differences in the arrangement of blood vessels, adipose tissue and positioning of the intestine between rainbow trout and shorthorn sculpin (Myoxocephalus scorpius), the previously described protocol for the construction of spatio-temporal maps in fish by Brijs et al. (2014) required modification. A mid-point line was manually drawn to follow any bends of the intestine in the region of interest, and the average transmitted/reflected intensity (colour) was calculated perpendicular to the mid-point line extending out ±30% of total diameter. During circular contractions, the intestine tended to whiten, likely due to many factors such as blanching of blood flow, greater thickness of wall material, reduced luminal volume and changes in the angle of light reflecting off the gut surface, which made it possible to determine changes in intestinal diameter. The diameter of the intestine at each point along the section was then colour coded using a 16-bit greyscale with the smallest recorded diameter (i.e. fully contracted) represented by a black pixel and the largest diameter (i.e. dilated or distended) by a white pixel. Thus, each video frame produced a single row of pixels, corresponding to the diameter of each point along the preparation. These video frames were then compiled together to form a spatio-temporal map with the top row representing the beginning of the experiment and the bottom row the end. Although transmitted/reflected intensity could not reliably be correlated to the strength of contraction, the pattern of intestinal circular muscle contractions could be measured in terms of their frequency (intervals between successive contractions, duration of the periods of quiescence, position along the intestine, propagation velocity and propagation coherence). Changes in longitudinal length could be observed between blood vessels that branched off the celiacomesenteric artery and ran perpendicular to the long axis of the intestine. Manual measurements of diameter were made in small regions where there was sufficient edge contrast to resolve the edges of the intestine.
Investigating dynamic changes in the intestinal motility of FW-acclimated trout following an acute transition to SW using EEA recordings
Surgical instrumentation for EEA recordings
Rainbow trout acclimated to FW were fasted for 1 week prior to surgery (n=7). Individual fish were prepared for surgery using the same anaesthesia protocol and surgical table as described above. Body mass of the individuals was also determined prior to beginning the surgical procedure.
To access the intestine, a 30 mm ventro-lateral incision was made beginning 10 mm from the pectoral fins of the animal and heading in a caudal direction (Fig. 1B). The EEA electrodes were prepared by removing ∼2 mm of the insulation from the ends of a twisted pair lead (model AS631-2, Cooner Wire, Chatsworth, LA, USA). The bare ends were inserted into a modified hypodermic needle setup previously described in Gräns et al. (2009), which consists of two 23 gauge, 25 mm-long hypodermic needles glued together so that the tips of the needles were 2 mm apart. This ensures that the electrodes were similarly spaced apart in all of the individuals. The insulated leads were then bent backwards and temporarily secured to a 1 ml syringe that acted as a handle for the two hypodermic needles. Using the hypodermic needle setup, two pairs of electrodes were inserted into the intestinal wall approximately 10 and 30 mm caudal of the pyloric caeca and secured to the intestine with a 6.0 silk suture (this very fine suture material ensures that the leads are securely fastened to the intestine while causing as little damage to the outer layer of the intestine as possible; Vömel, Frankfurt, Germany). The electrodes were exteriorised through the ventro-lateral incision, which was subsequently closed with interrupted stitches using monofilament 3.0 Prolene sutures (this suture material prevents the wicking effect seen in braided material and thus minimises the chance of infection; Ethicon Inc., Somerville, NJ, USA). The electrodes were also secured on the back of the fish with a 3.0 braided silk suture (this suture material ensures that the electrodes are securely fastened to the skin; Fig. 1B).
Following instrumentation, fish were individually placed in opaque experimental chambers (width 150 mm, length 600 mm, height 150 mm) with flow-through, aerated FW at 10.0±0.1°C. The chambers were shielded with black plastic drapes to avoid stressful visual input, and the fish were left undisturbed to recover for 24 h.
Experimental protocol and data acquisition
Following the recovery period, the EEA electrodes and ground leads placed in the water surrounding the fish were connected to Animal Bio Amps (model FE136, ADInstruments). The sensitivity range (±2 mV), low-pass filter (200 Hz), high-pass filter (0.1 Hz) and 50 Hz notch filter were adjusted in the Animal Bio Amp to optimise EEA recordings. The signals were then relayed to a PowerLab 8/30 system (ADInstruments). Data were collected on a PC, via PowerLab 8/30, using ADInstruments acquisition software LabChart™ 7 Pro v7.2.5, at a sampling rate of 100 Hz.
Intestinal EEA recordings of undisturbed rainbow trout in FW were captured continuously for 24 h. The FW was then gradually replaced with SW (salinity: 30–33 ppt) during a transitional period of approximately 2.5 h. Intestinal EEA recordings were captured for another 96 h in SW. Throughout the experiments, water temperature was kept at 10.0±0.1°C. At the end of the experiments, fish were removed from the experimental chambers and killed with a sharp cranial blow. Post-mortem examinations of the gastrointestinal tract were conducted to ensure that no food content was present.
Data analysis
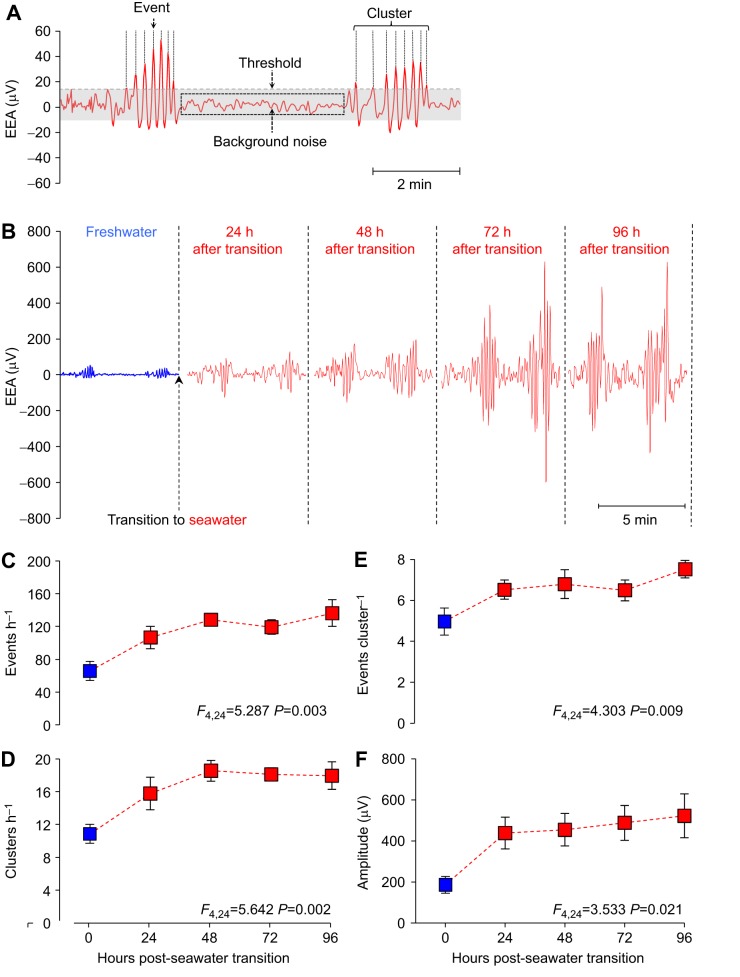
The raw EEA signal was further filtered with a low-pass digital filter (0.25 Hz) in LabChart in order to filter out the majority of the background noise. From the filtered EEA trace a range of variables were calculated (event frequency, cluster frequency, events per cluster and event amplitude) as previously described in Gräns et al. (2009). Briefly, the remaining background noise from an individual's EEA trace was determined by calculating the mean of ‘silent’ sections of the EEA trace (i.e. the periods of quiescence). This value was then used to calculate a threshold (150% of mean noise) from which we could define ‘events’ (a peak in EEA that exceeded the threshold value, see later). The correlation between these EEA events and gastrointestinal contractions has been verified in shorthorn sculpin (Gräns, 2012; Gräns and Olsson, 2011). A time stamp for each event was collected and a frequency histogram was created using the time between consecutive events. As in Gräns et al. (2009), visual analysis of the histograms revealed that ≥1 min between consecutive events was a suitable criterion for identifying ‘clusters’ (a group of events; see Fig. 6A). Events per cluster were calculated by dividing the total number of events within clusters by the total number of clusters. As demonstrated by Gräns (2012), this variable provides information on the mean duration of clusters (i.e. an increasing number of events per cluster equates to a longer cluster duration). Event amplitude was determined by calculating the average cyclic height of each event in LabChart. The abovementioned EEA variables were calculated from a representative 2 h period, within the 24 h FW period, as well as within every 24 h period following the transition to SW (i.e. 24, 48, 72 and 96 h).
Fig. 6.
Enteric electrical activity (EEA) in the middle intestine of rainbow trout transitioning from FW to SW. (A) An example of how the EEA signal is analysed. Electrical spikes that exceed the threshold value (150% signal noise) were defined as ‘events’, which correspond to individual contractions, and groups of events were defined as ‘clusters’, which directly correspond to the clusters of contractions within the MMCs. The clusters were separated by periods of quiescence or low EEA (background noise). (B) Different sections of a raw EEA trace from an individual rainbow trout demonstrating the typical increase that occurs in intestinal contractile activity, as observed by the increase in the number and amplitude of EEA events that occur during the 96 h SW acclimation period. (C) Event frequency, (D) cluster frequency (equal to MMC frequency), (E) events per cluster (corresponding to the duration of the clusters within the MMC) and (F) event amplitude during the transition of rainbow trout (n=7) from FW (blue square) to SW (red squares). Statistical analyses for each EEA variable were generated using a one-way repeated measures ANOVA and are summarised at the bottom of each figure. Significance is defined as P<0.05. The parameter estimates generated by the model are presented as means±s.e.m.
Correlating EEA with video-recorded intestinal movements
The methods used in the abovementioned experiments were also combined to provide further insight on how to interpret EEA recordings. By implanting a pair of electrodes in the middle intestine of an anaesthetised rainbow trout whilst simultaneously video recording the intestinal movements around these electrodes, it was possible to correlate the EEA with specific motility patterns (Fig. 1A).
Statistical analysis
Statistical analyses were performed using SPSS Statistics 21 (IBM Corp., Armonk, NY, USA). An independent samples t-test was used to determine whether significant differences occurred between body mass, intestinal diameter and the mean interval duration between successive MMCs of FW- and SW-acclimated rainbow trout. A one-way repeated measures ANOVA was used to determine whether there were any statistically significant differences between the means of the EEA variables (i.e. events h−1, clusters h−1, events cluster−1 and event amplitude) in rainbow trout during the 96 h period following a transition to SW. All data subjected to statistical analyses were assessed to ensure they met the assumptions required of the specific statistical tests (independent samples t-test: no outliers, normally distributed and homogeneity of variance; repeated measures ANOVA: no outliers, normally distributed and sphericity). F- or t- and P-values obtained from the models are reported throughout the text, with significance defined at P<0.05. Unless otherwise specified, all data are presented as means±s.e.m.
RESULTS
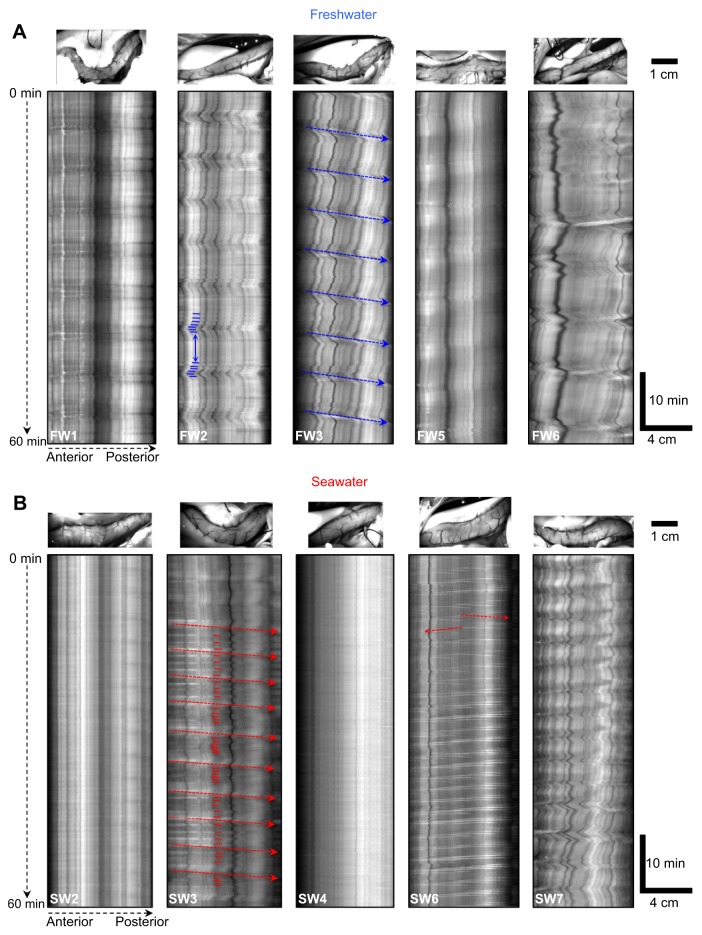
Body mass of FW-acclimated (957±73 g, n=8) and SW-acclimated (992±35 g, n=8) rainbow trout did not differ (t14=−0.435, P=0.670). However, intestinal diameter of trout acclimated to SW (7.4±0.5 mm) was almost twofold greater than those acclimated to FW (4.1±0.2 mm, t14=−6.321, P<0.001, cf. top panels in Fig. 2A with 2B).
Fig. 2.
Spatio-temporal maps displaying the differences in intestinal motor activity of freshwater (FW)- and seawater (SW)-acclimated rainbow trout. The spatio-temporal maps display changes in the diameter (portrayed as a greyscale value with black pixels representing contractions and white pixels representing dilations) of a section of intestine (the horizontal axis of each spatio-temporal map represents the distance along the section of intestine beginning shortly after the pyloric caeca and extending posteriorly) during the 1 h recording period (the vertical axis represents the duration of the experiment) of five individual FW- and SW-acclimated rainbow trout. (A) In FW, trout generally displayed rhythmically occurring clusters of contractions that were separated by periods of quiescence, which resembled migrating motor complexes (MMCs). A clear example of successive clusters of contractions (small blue horizontal lines) separated by a period of quiescence (blue double-headed arrow) can be observed in FW2. When the contractile front was distinct enough to measure, as seen in FW3 (blue dashed arrows), propagation velocity could be determined. (B) In SW, MMCs occurred at frequencies twofold to threefold higher than in FW, which resulted in a concomitant shortening or absence of periods of quiescence between successive contractile fronts of MMCs. A clear example of this can be observed in SW3, in which an increased frequency of contractions (small red horizontal lines) results in a concomitant reduction in, or absence of, the periods of quiescence between successive contractile fronts (red dashed arrows). In addition, ripples propagating either anteriorly (from right to left) or posteriorly (from left to right) were more apparent in SW (see red dashed arrows in SW6 for clear examples). Above each spatio-temporal map is an image of the section of intestine that the map was generated from.
In vivo motility patterns in FW-acclimated rainbow trout
Video recordings and spatio-temporal maps revealed a mixture of anteriorly and posteriorly propagating contractions (Fig. 2). The most commonly observed intestinal motility pattern in FW-acclimated trout consisted of a cluster of contractions lasting ∼1–2 min followed by a period of quiescence (see FW2 in Fig. 2A). This rhythmic pattern showed similarities in its characteristics with mammalian MMCs, and will henceforth be referred to as MMCs. In FW-acclimated trout, MMCs rhythmically occurred every 6.9±0.5 min. In half of the FW-acclimated trout, the contractile front of MMCs was distinct enough to measure and propagated at a velocity of 0.53±0.07 mm s−1 (see FW3 in Fig. 2A).
In vivo motility patterns in SW-acclimated rainbow trout
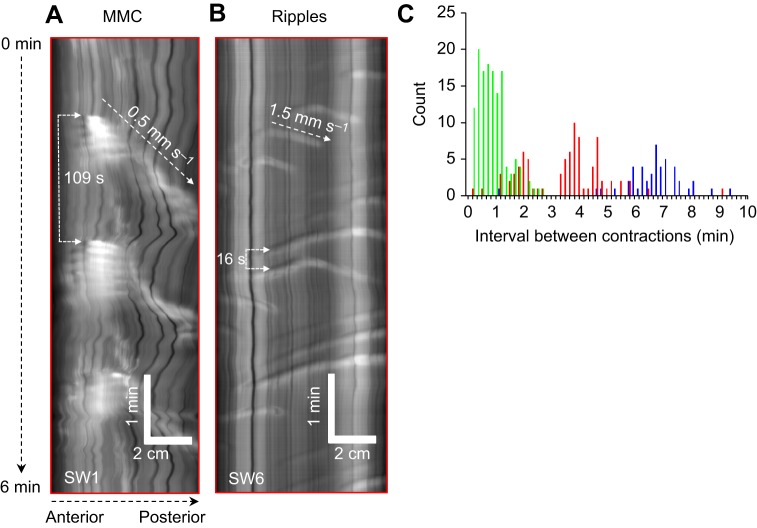
Intestinal motility patterns of SW-acclimated rainbow trout were not only markedly different to FW-acclimated individuals (Fig. 2), but substantial variation also existed between individuals (see ‘Effect of resting diameter on patterns of contractions’ for more details). The differences between FW- and SW-acclimated individuals was most noticeably due to an increase in the frequency of MMCs, which occurred every 3.6±0.6 min (t14=5.672. P<0.001; Fig. 3). This resulted in a concomitant shortening or absence of periods of quiescence between the contractile fronts of successive MMCs (see SW3 in Fig. 2B). In addition, another type of propagating contraction that displayed similarities in its characteristics with mammalian ripples was more apparent during these periods (Fig. 3B, see SW6 in Fig. 2B). Ripples are contractions that can propagate in either direction. The majority of these contractions occurred at a frequency of one contraction every 12 to 60 s (Fig. 3C) and propagated more rapidly along the intestine at a velocity of 1.66±0.18 mm s−1.
Fig. 3.
Frequency and velocity of migrating motor complexes (MMCs) and ripples in freshwater (FW)- and seawater (SW)-acclimated rainbow trout. Magnified view of spatio-temporal maps (horizontal axis: distance along the section of intestine; vertical axis: duration of the experiment) displaying examples of (A) posteriorly propagating MMCs and (B) bi-directionally propagating ripples in the middle intestine of SW-acclimated individuals. (C) Histogram displaying duration of intervals between successive contractions of FW- and SW-acclimated rainbow trout (n=8 for each group). SW-acclimated individuals displayed a high frequency of ripples (green bars), whereas the interval between successive MMCs (red bars) was twofold to threefold shorter than in FW-acclimated individuals (blue bars).
Characteristics of MMCs in rainbow trout
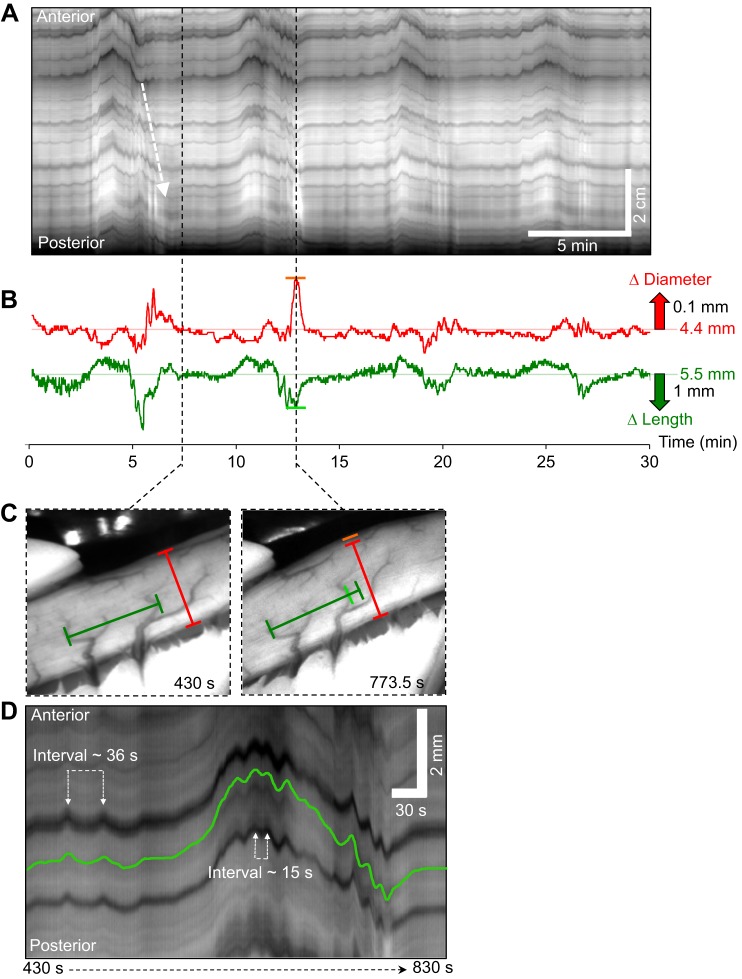
In FW-acclimated rainbow trout, the intervals between the contractile fronts of successive MMCs were longer, and thus it was possible to examine the characteristics of MMCs in more detail. Immediately following the cluster of contractions, intestinal activity was subdued for ∼2 min with only small, mainly longitudinal movements detected, as seen by small oscillations in the position of the black artifacts left in spatio-temporal maps by blood vessels running circumferentially around the intestine (Fig. 4A, dark lines after arrow). As time progressed, the amplitude and regularity of these phasic longitudinal movements increased and were accompanied by an increasing degree of sustained shortening/longitudinal displacement (for examples see FW3 and FW6 in Fig. 2A). This continued for ∼5 min until there was a distinct transition, consisting of a rapid longitudinal shortening accompanied by sustained or high frequency circular contractions (Fig. 4B–D). During the 1–2 min when the cluster of contractions was occurring, the frequency of phasic contractions essentially doubled (Fig. 4D, interval between successive contractions decreased from ∼36 s to ∼15 s). After the initial phase of circular and longitudinal shortening, the intestine often appeared to dilate (Fig. 4B, red diameter trace; orange line in 4C). This could be due to a region of inhibition distal to the contraction front and/or a passive dilation due to the propulsion of fluid from the contractile front into more distal segments.
Fig. 4.
Characteristics of migrating motor complexes (MMCs) in the middle intestine of rainbow trout. (A) Spatio-temporal map displaying four successive MMCs propagating along the middle intestine of rainbow trout in a posterior direction. Note that this map differs from previous maps, as the horizontal axis represents the duration of the experiment (from the left to right), whereas the vertical axis displays the distance along the intestine (the top represents the anterior end of the section). The white dashed arrow represents the contractile front of the MMC. (B) Traces demonstrating the changes occurring in intestinal diameter (red trace) and longitudinal length (green trace) of the middle intestine during four successive MMCs. (C) Images showing the intestinal diameter (red bar) and distance between two blood vessels (green bar), which correspond to the horizontal red and green lines in B, shortly after the contractile front of the MMC had ceased (430 s) and during the period of sustained longitudinal shortening and intestinal dilation (773.5 s; changes indicated by orange and light green bars in B and C). (D) Magnified view of a spatio-temporal map demonstrating the period between 430 and 830 s, which shows one complete MMC cycle, beginning with relatively subdued intestinal activity, followed by a steady increase in strength and regularity of longitudinal contractions, before transitioning into a period of rapid longitudinal shortening which was accompanied by high frequency circular and longitudinal contractions.
Effect of resting diameter on patterns of contractions
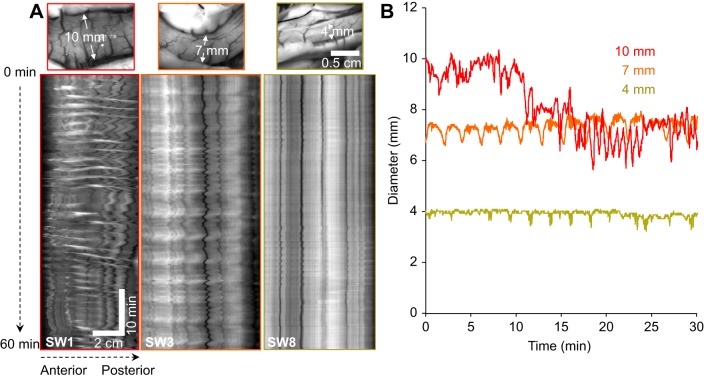
Given the almost twofold increase in resting diameter of most SW-acclimated rainbow trout, the question arose whether the changes in motility patterns corresponded to the degree of distension. As we chose not to manipulate the degree of distension in these in vivo experiments, we relied on the differences in resting diameter between individuals to investigate any potential relationships. Rainbow trout acclimated to FW showed little differences in resting intestinal diameter (range: 3.3–4.8 mm), whereas SW-acclimated individuals displayed intestinal diameters ranging between 4.0 and 10.1 mm.
Using three SW-acclimated individuals with a wide range of resting intestinal diameters, we observed dramatically different patterns of intestinal contractions (Fig. 5). At high levels of distension (SW1 in Fig. 5A and red trace in 5B), MMCs occurred at a high frequency (∼every 2 min) with strong phasic and sustained circular and longitudinal contractions. These MMCs had a well-defined contractile front that propagated along the intestine and were associated with emptying (sustained reduction in diameter; see red trace in Fig. 5B after 20 min). At mid-levels of resting intestinal diameter, observed in most of the SW-acclimated rainbow trout (SW3 in Fig. 5A and orange trace in 5B), the frequency of MMCs was reduced (∼every 4 min) and the amplitude of circular and longitudinal contractions was diminished compared to at high levels of distension. However, contractile activity between the clusters of contractions of successive MMCs remained, with ripples and other types of circular/longitudinal contractions being present (interval between successive contractions ranged from 15 to 30 s). One SW-acclimated individual had a resting intestinal diameter of 4 mm, which was similar to most FW-acclimated rainbow trout. The contractile activity in this fish was greatly diminished as longitudinal and circular contractions were of low amplitude and short duration (SW8 in Fig. 5A and green trace in Fig. 5B).
Fig. 5.
Relationship of intestinal diameter and contractile activity in seawater (SW)-acclimated rainbow trout. A demonstration of the degree and amplitude of intestinal contractile activity related to resting diameter levels is presented in three individual SW-acclimated rainbow trout with varying degrees of intestinal distension (from left to right: high, medium and low distension) and their corresponding (A) intestinal motility patterns and (B) changes in intestinal diameter. The spatio-temporal maps in A display the changes in the diameter (portrayed as a greyscale value with black pixels representing contractions and white pixels representing dilations) of the section of middle intestine from the three individual SW-acclimated rainbow trout (horizontal axis: distance along section of middle intestine; vertical axis: duration of the experiment).
In vivo motility patterns during an acute transition to SW
Visual and statistical analyses of the EEA data revealed clear changes in the intestinal motility patterns of euryhaline rainbow trout transitioning from FW to SW (n=7, mass=1148±54 g). EEA consisted of clusters of activity followed by periods of quiescence (Fig. 6A). Analyses of the simultaneous recordings of EEA and intestinal movements revealed that EEA clusters were coincident with the clusters of contractions within the MMCs (Movie 1). Fig. 6B illustrates the typical increase in the number and amplitude of EEA events that occurred during the transition from FW to SW in an individual rainbow trout.
EEA event frequency, which provides a measure of the overall contractile activity of the intestine, significantly increased from 66±12 events h−1 in FW to reach values twofold higher after 96 h in SW (136±16 events h−1; Fig. 6C). The frequency of rhythmically occurring EEA clusters (corresponding to the clusters of contractions within MMCs) also significantly increased from a cluster every 5–6 min in FW to every 3–4 min after 48 h of SW acclimation (Fig. 6D). The EEA events per cluster, which is associated with the duration of the cluster of contractions within the MMCs, significantly increased during the acclimation period from ∼5 in FW to 7–8 events per cluster after 96 h in SW (Fig. 6E) and event amplitude significantly increased twofold to threefold (Fig. 6F).
DISCUSSION
The present study provides qualitative and quantitative descriptions of in vivo intestinal motility patterns in rainbow trout, and clearly demonstrates the substantial influence that salinity of the surrounding environment has on the intestinal motility of a euryhaline teleost.
The presence and potential osmoregulatory role of MMCs in rainbow trout
The main pattern of intestinal motor activity observed in the rainbow trout was an ongoing, cyclical pattern consisting of a period of quiescence, followed by steadily increasing longitudinal and circular oscillations that culminated in a cluster of contractions. Similar to MMC-like contractions previously described in teleosts (Brijs et al., 2014, 2017a; Holmberg et al., 2007, 2006, 2003, 2004), this propagating complex closely resembles the different phases of mammalian MMCs in rats and mice (Bush et al., 2000; Deloose et al., 2012; Husebye, 1999). However, the relatively long period of quiescence and build-up of contractile activity prior to the cluster of contractions is also reminiscent of neurally mediated peristalsis (Costa and Furness, 1976; Hennig et al., 1999; Waterman et al., 1994).
In FW, the MMCs in rainbow trout most likely serve a housekeeping function to prevent the overgrowth of bacteria and remove waste products such as undigested food and sloughed enterocytes from the gastrointestinal tract (Grzesiuk et al., 2001; Nieuwenhuijs et al., 1998; Vantrappen et al., 1977). However, in SW, the twofold to threefold increase in the frequency and strength of this motility pattern suggests that it plays an important role in the mixing, propulsion and emptying of intestinal fluids. The appearance of ripples, a contraction type well documented to be involved in the mixing of intestinal contents in guinea pigs (D'Antona et al., 2001; Schulze-Delrieu et al., 1996), also suggests that increased mixing of fluids is important in SW environments. The contractile activity of the intestine likely aids in the maintenance of osmotic homeostasis, as the optimal transportation and mixing of imbibed SW increases the efficiency of ion and water absorption by preventing the depletion of ions in the boundary layers near the epithelial surfaces (Lee, 1983). Furthermore, increased intestinal contractile activity would most likely be necessary for the transportation and expulsion of carbonate precipitates that are formed as a result of intestinal bicarbonate secretion (Grosell, 2011; Wilson and Grosell, 2003; Wilson et al., 2009, 2002). An area that warrants further investigation is whether or not euryhaline teleosts acclimated to SW maintain an elevated level of intestinal motility following the ingestion of a meal when compared to individuals acclimated to FW. If so, then this may enhance digestion and nutrient absorption in SW and could potentially, at least in part, underlie the substantial increases in daily growth rate that salmonids can experience when at sea, which allows them to grow larger than non-migratory conspecifics (Gross et al., 1988; McDowall, 1993, 2001; Neilson et al., 1985).
One of the surprising findings of this study was the substantial increase in the diameter of the intestine in trout exposed to SW. Whether the increase in diameter is due to a greater volume of intestinal contents maintained at higher pressures or a greater degree of relaxation by the external muscle layers of the intestine (Smith et al., 2003) could be resolved by measuring intraluminal pressure, but was beyond the scope of the present study. The resting diameter appeared to be correlated to the frequency and strength of contractions, similar to what occurs in peristalsis in a range of rodents (Costa and Furness, 1976; Hennig et al., 1999; Trendelenburg, 2006; Waterman and Costa, 1994; Waterman et al., 1994), and likely involves increased input from mechano-receptors in the intestinal wall mediated by neurogenic and/or myogenic mechanisms (Smith et al., 1990; Tonini et al., 1996; Won et al., 2005). However, it is well documented that euryhaline teleosts acclimated to SW also display elevated levels of ions such as Na+ and Cl− in the tissue and cells when compared to levels displayed in FW (Houston, 1959; Eddy, 1982; Bath and Eddy, 1979a,b). Therefore, the influence of iono-receptors and the direct effects of ions on intestinal smooth muscle and/or interstitial cells of Cajal warrants further investigation.
Temporal changes in intestinal motility during a transition to SW
The correlation between EEA and gastrointestinal smooth muscle contractions has previously been verified in teleosts (Gräns, 2012; Gräns et al., 2009, 2013). However, prior to the present study it could not be conclusively stated whether the rhythmically occurring EEA clusters were propagating. By directly comparing EEA recordings to video recordings of intestinal motility, we demonstrate that EEA clusters correspond to the clusters of contractions within the MMCs (see Movie 1). Using this information, we are now better able to interpret the changes that occur in the intestinal motility of conscious individuals in response to changing environmental conditions, such as those faced by euryhaline teleosts transitioning from FW to SW.
Continuous recordings of EEA in conscious rainbow trout revealed a similar pattern of contractions to that observed in anaesthetised individuals, as FW-acclimated trout displayed rhythmically occurring clusters of contractions separated by periods of quiescence, albeit at a slightly higher frequency (a complex every ∼6 min compared to ∼7 min in anaesthetised individuals). The slight differences in frequency can most likely be attributed to the effects of anaesthesia, which are known to alter the contraction frequency in a range of mammals (Bueno et al., 1978; Fujimiya and Inui, 2000). During the transition from FW to SW, intestinal contractile activity of rainbow trout gradually increased and plateaued at a significantly elevated level after ∼2 days in SW. Specifically, the frequency and duration of the clusters of contractions within the MMCs increased concomitantly with a reduction in the period of quiescence between complexes, which is consistent with the findings from the video recordings of anaesthetised individuals.
Previous research on anadromous salmonids has demonstrated that migration speed significantly decreases when individuals transition from the rivers to saline estuaries (Aarestrup et al., 2014, 2002; Aldvén et al., 2015). The median residence time documented for sea trout (Salmo trutta) in the inner estuary prior to migration into the sea was ∼2–3 days, and was suggested to be due to differences in current velocity or exploration of the environment of the individual for feeding or imprinting purposes (Aldvén et al., 2015; Hansen and Jonsson, 1994). Interestingly, the residence time approximately matches the temporal responses of intestinal motility documented in the present study (∼2 days), as well as essential cardiovascular modifications associated with osmoregulation in trout (e.g. gastrointestinal blood flow and cardiac output increase between ∼2 and 4 days) (Brijs et al., 2015, 2016, 2017,b). Collectively, this may indicate that, even in the face of increased predation pressure and mortality associated with these shallow estuarine habitats (Aldvén et al., 2015; Gregory, 1993), certain physiological modifications in migrating salmonids that are likely triggered by exposure to waters of increasing salinity must occur prior to completing the migration to sea.
Conclusions
The present study shows a dramatic elevation in intestinal contractile activity in vivo in a euryhaline teleost species during acute and chronic exposure to SW that is likely important for the maintenance of osmotic homeostasis by optimising intestinal water and ion absorption. The temporal dynamics in the frequency and patterning of intestinal motility in rainbow trout transitioning from FW to SW coincides with previously documented cardiovascular modifications associated with osmoregulation, and may provide further insight into the underlying reasons behind migration patterns of anadromous salmonids.
Acknowledgements
We gratefully acknowledge Anna-Maria Kellermann for technical assistance in the laboratory.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualisation: J.B.; Methodology: J.B., G.W.H., A.G.; Software: G.W.H., A.G., M.A.; Validation: J.B., G.W.H., A.G.; Formal analysis: J.B., G.W.H., A.G., E.D.; Investigation: J.B., A.G., E.D.; Resources: J.B., G.W.H., M.A., C.O.; Data curation: J.B., G.W.H., C.O.; Writing - original draft: J.B.; Writing - review & editing: J.B., G.W.H., A.G., E.D., M.A., C.O.; Visualisation: J.B., G.W.H.; Supervision: M.A., C.O.; Project administration: J.B., C.O.; Funding acquisition: J.B., G.W.H., A.G., M.A., C.O.
Funding
This research was funded by the Swedish Research Council (Vetenskapsrådet) (to M.A.); Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Svenska Forskningsrådet Formas) (to A.G.); Stiftelserna Wilhelm och Martina Lundgrens (to J.B.); Herbert och Karin Jacobssons Stiftelse (to J.B.); Kungl. Vetenskaps- och Vitterhets-Samhället i Göteborg (to J.B.); Helge Ax:son Johnsons Stiftelse (to J.B.); National Institute of General Medical Sciences [P20GM103513] (to G.W.H.] and the National Center for Research Resources from the National Institute of Health [P20RR018751] (to G.W.H.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.156000.supplemental
References
- Aarestrup K., Nielsen C. and Koed A. (2002). Net ground speed of downstream migrating radio-tagged Atlantic salmon (Salmo salar L.) and brown trout (Salmo trutta L.) smolts in relation to environmental factors. In Aquatic Telemetry (ed. Thorstad E. B., Fleming I. A. and Naesje T. F.), pp. 95-102. Netherlands: Springer. [Google Scholar]
- Aarestrup K., Baktoft H., Koed A., del Villar-Guerra D. and Thorstad E. B. (2014). Comparison of the riverine and early marine migration behaviour and survival of wild and hatchery-reared sea trout Salmo trutta smolts. Mar. Ecol. Prog. Ser. 496, 197-206. 10.3354/meps10614 [DOI] [Google Scholar]
- Aldvén D., Hedger R., Økland F., Rivinoja P. and Höjesjö J. (2015). Migration speed, routes, and mortality rates of anadromous brown trout Salmo trutta during outward migration through a complex coastal habitat. Mar. Ecol. Prog. Ser. 541, 151-163. 10.3354/meps11535 [DOI] [Google Scholar]
- Ando M. and Nagashima K. (1996). Intestinal Na+ and Cl- levels control drinking behavior in the seawater-adapted eel Anguilla japonica. J. Exp. Biol. 199, 711-716. [DOI] [PubMed] [Google Scholar]
- Ando M., Mukuda T. and Kozaka T. (2003). Water metabolism in the eel acclimated to sea water: from mouth to intestine. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 621-633. 10.1016/S1096-4959(03)00179-9 [DOI] [PubMed] [Google Scholar]
- Aviv R., Policker S., Brody F., Bitton O., Haddad W., Kliger A., Sanmiguel C. P. and Soffer E. E. (2008). Circadian patterns of gastric electrical and mechanical activity in dogs. Neurogastroenterol. Motil. 20, 63-68. 10.1111/j.1365-2982.2007.00992.x [DOI] [PubMed] [Google Scholar]
- Bath R. N. and Eddy F. B. (1979a). Salt and water balance in rainbow trout (Salmo gairdneri) rapidly transferred from freshwater to seawater. J. Exp. Biol. 83, 193-202. [Google Scholar]
- Bath R. N. and Eddy F. B. (1979b). Ionic and respiratory regulation in rainbow trout during rapid transfer to seawater. J. Comp. Physiol. 134, 351-357. 10.1007/BF00710003 [DOI] [Google Scholar]
- Brijs J., Hennig G. W., Axelsson M. and Olsson C. (2014). Effects of feeding on in vivo motility patterns in the proximal intestine of shorthorn sculpin (Myoxocephalus scorpius). J. Exp. Biol. 217, 3015-3027. 10.1242/jeb.101741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brijs J., Axelsson M., Grans A., Pichaud N., Olsson C. and Sandblom E. (2015). Increased gastrointestinal blood flow: An essential circulatory modification for euryhaline rainbow trout (Oncorhynchus mykiss) migrating to sea. Sci. Rep. 5, 10430 10.1038/srep10430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brijs J., Gräns A., Ekström A., Olsson C., Axelsson M. and Sandblom E. (2016). Cardiorespiratory upregulation during seawater acclimation in rainbow trout: effects on gastrointestinal perfusion and postprandial responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R858-R865. 10.1152/ajpregu.00536.2015 [DOI] [PubMed] [Google Scholar]
- Brijs J., Hennig G. W., Kellermann A.-M., Axelsson M. and Olsson C. (2017a). The presence and role of interstitial cells of Cajal in the proximal intestine of shorthorn sculpin (Myoxocephalus scorpius). J. Exp. Biol. 220, 347-357. 10.1242/jeb.141523 [DOI] [PubMed] [Google Scholar]
- Brijs J., Sandblom E., Dekens E., Näslund J., Ekström A. and Axelsson M. (2017b). Cardiac remodelling and increased central venous pressure underlie elevated stroke volume and cardiac output of seawater-acclimated rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R31-R39. 10.1152/ajpregu.00374.2016 [DOI] [PubMed] [Google Scholar]
- Bueno L., Ferre J.-P. and Ruckebusch Y. (1978). Effects of anesthesia and surgical procedures on intestinal myoelectric activity in rats. Am. J. Dig. Dis. 23, 690-695. 10.1007/BF01072353 [DOI] [PubMed] [Google Scholar]
- Bush T. G., Spencer N. J., Watters N., Sanders K. M. and Smith T. K. (2000). Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton. Neurosci. 84, 162-168. 10.1016/S1566-0702(00)00201-0 [DOI] [PubMed] [Google Scholar]
- Costa M. and Furness J. B. (1976). The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch. Pharmacol. 294, 47-60. 10.1007/BF00692784 [DOI] [PubMed] [Google Scholar]
- D'Antona G., Hennig G. W., Costa M., Humphreys C. M. and Brookes S. J. H. (2001). Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol. Motil. 13, 483-492. 10.1046/j.1365-2982.2001.00282.x [DOI] [PubMed] [Google Scholar]
- Deloose E., Janssen P., Depoortere I. and Tack J. (2012). The migrating motor complex: control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 9, 271-285. 10.1038/nrgastro.2012.57 [DOI] [PubMed] [Google Scholar]
- Eddy F. B. (1982). Osmotic and ionic regulation in captive fish with particular reference to salmonids. Comp. Biochem. Physiol. B - Biochem. Mol. Biol. 73, 125-141. 10.1016/0305-0491(82)90205-X [DOI] [Google Scholar]
- Edwards S. L. and Marshall W. S. (2013). Principles and patterns of osmoregulation and euryhalinity in fishes. In Euryhaline Fishes (ed. McCormick S. D., Farrell A. P. and Brauner C. J.), pp. 2-45. San Diego: Academic Press. [Google Scholar]
- Evans D. H. (2008). Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R704-R713. 10.1152/ajpregu.90337.2008 [DOI] [PubMed] [Google Scholar]
- Fioramonti J. and Bueno L. (1984). Relation between intestinal motility and mesenteric blood flow in the conscious dog. Am. J. Physiol. 246, G108-G113. [DOI] [PubMed] [Google Scholar]
- Fujimiya M. and Inui A. (2000). Peptidergic regulation of gastrointestinal motility in rodents. Peptides 21, 1565-1582. 10.1016/S0196-9781(00)00313-2 [DOI] [PubMed] [Google Scholar]
- Gräns A. (2012). The effects of temperature on gut blood flow and gut motility in fish. PhD thesis, University of Gothenburg, Gothenburg, Sweden. [Google Scholar]
- Gräns A. and Olsson C. (2011). Gut motility. In Encyclopedia of Fish Physiology: From Genome to Environment (ed. Farrell A. P.), pp. 1292-1300. San Diego: Academic Press. [Google Scholar]
- Gräns A., Albertsson F., Axelsson M. and Olsson C. (2009). Postprandial changes in enteric electrical activity and gut blood flow in rainbow trout (Oncorhynchus mykiss) acclimated to different temperatures. J. Exp. Biol. 212, 2550-2557. 10.1242/jeb.030593 [DOI] [PubMed] [Google Scholar]
- Gräns A., Seth H., Axelsson M., Sandblom E., Albertsson F., Wiklander K. and Olsson C. (2013). Effects of acute temperature changes on gut physiology in two species of sculpin from the west coast of Greenland. Polar Biol. 36, 775-785. 10.1007/s00300-013-1301-0 [DOI] [Google Scholar]
- Gregory R. S. (1993). Effect of turbidity on the predator avoidance behaviour of juvenile chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 50, 241-246. 10.1139/f93-027 [DOI] [Google Scholar]
- Grosell M. (2006). Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 209, 2813-2827. 10.1242/jeb.02345 [DOI] [PubMed] [Google Scholar]
- Grosell M. (2011). The role of the gastrointestinal tract in salt and water balance. In The Multifunctional Gut of Fish (ed. Grosell M., Farrell A. P. and Brauner C. J.), pp. 135-164. San Diego: Academic Press. [Google Scholar]
- Gross M. R., Coleman R. M. and McDowall R. M. (1988). Aquatic productivity and the evolution of diadromous fish migration. Science 239, 1291-1293. 10.1126/science.239.4845.1291 [DOI] [PubMed] [Google Scholar]
- Grzesiuk E., Laubitz D., Wójcik-Sikora A., Zabielski R. and Pierzynowski S. G. (2001). Influence of intestinal myoelectrical activity on the growth of Escherichia coli. Bioelectromagnetics 22, 449-455. 10.1002/bem.72 [DOI] [PubMed] [Google Scholar]
- Hansen L. P. and Jonsson B. (1994). Homing of Atlantic salmon: effects of juvenile learning on transplanted post-spawners. Anim. Behav. 47, 220-222. 10.1006/anbe.1994.1027 [DOI] [Google Scholar]
- Hennig G. W., Costa M., Chen B. N. and Brookes S. J. H. (1999). Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J. Physiol. Lond. 517, 575-590. 10.1111/j.1469-7793.1999.0575t.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. (1974). Some factors regulating water-intake by eel, Anguilla-Japonica. J. Exp. Biol. 61, 737-747. [DOI] [PubMed] [Google Scholar]
- Holmberg A., Schwerte T., Fritsche R., Pelster B. and Holmgren S. (2003). Ontogeny of intestinal motility in correlation to neuronal development in zebrafish embryos and larvae. J. Fish Biol. 63, 318-331. 10.1046/j.1095-8649.2003.00149.x [DOI] [Google Scholar]
- Holmberg A., Schwerte T., Pelster B. and Holmgren S. (2004). Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 207, 4085-4094. 10.1242/jeb.01260 [DOI] [PubMed] [Google Scholar]
- Holmberg A., Olsson C. and Holmgren S. (2006). The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 209, 2472-2479. 10.1242/jeb.02272 [DOI] [PubMed] [Google Scholar]
- Holmberg A., Olsson C. and Hennig G. W. (2007). TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J. Exp. Biol. 210, 1084-1091. 10.1242/jeb.000935 [DOI] [PubMed] [Google Scholar]
- Houston A. H. (1959). Osmoregulatory adaptation of steelhead trout (Salmo gairdneri Richardson) to seawater. Can. J. Zool. 37, 729-748. 10.1139/z59-074 [DOI] [Google Scholar]
- Huizinga J. D. and Lammers W. J. E. P. (2009). Gut peristalsis is governed by a multitude of cooperating mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1-G8. 10.1152/ajpgi.90380.2008 [DOI] [PubMed] [Google Scholar]
- Husebye E. (1999). The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol. Motil. 11, 141-161. 10.1046/j.1365-2982.1999.00147.x [DOI] [PubMed] [Google Scholar]
- Lee J. S. (1983). Effects of stretching and stirring on water and glucose absorption by canine mucosal membrane. J. Physiol. 335, 335-341. 10.1113/jphysiol.1983.sp014537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W. S. and Grosell M. (2005). Ion transport, osmoregulation and acid-base balance. In Physiology of Fishes (ed. Evans D. and Claiborne J. B.), pp. 177-230. Boca Raton, FL: CRC Press. [Google Scholar]
- McCormick S. D. and Saunders R. L. (1987). Preparatory physiological adaptations for marine life of salmonids: osmoregulation, growth and metabolism. Am. Fish. Soc. Symp. 1, 211-229. [Google Scholar]
- McDowall R. M. (1993). A recent marine ancestry for diadromous fishes - sometimes yes, but mostly no. Environ. Biol. Fishes 37, 329-335. 10.1007/BF00005199 [DOI] [Google Scholar]
- McDowall R. M. (2001). Anadromy and homing: two life-history traits with adaptive synergies in salmonid fishes? Fish Fish. 2, 78-85. 10.1046/j.1467-2979.2001.00036.x [DOI] [Google Scholar]
- Neilson J. D., Geen G. H. and Bottom D. (1985). Estuarine growth of juvenile chinook salmon (Oncorhynchus tshawytscha) as inferred from otolith microstructure. Can. J. Fish. Aquat. Sci. 42, 899-908. 10.1139/f85-114 [DOI] [Google Scholar]
- Nieuwenhuijs V. B., Verheem A., van Duijvenbode-Beumer H., Visser M. R., Verhoef J., Gooszen H. G. and Akkermans L. M. A. (1998). The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann. Surg. 228, 188-193. 10.1097/00000658-199808000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C. (2011). Gut anatomy. In Encyclopedia of Fish Physiology: From Genome to Environment (ed. Farrell A. P.), pp. 1268-1275. San Diego, CA: Academic Press. [Google Scholar]
- Olsson C. and Holmgren S. (2001). The control of gut motility. Comp. Biochem. Physiol. A - Mol. Integr. Physiol. 128, 481-503. 10.1016/S1095-6433(00)00330-5 [DOI] [PubMed] [Google Scholar]
- Perkins W. E. (1971). Method for studying electrical and mechanical activity of isolated intestine. J. Appl. Physiol. 30, 768. [DOI] [PubMed] [Google Scholar]
- Perry S. F., Shahsavarani A., Georgalis T., Bayaa M., Furimsky M. and Thomas S. L. Y. (2003). Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid-base regulation. J. Exp. Zool. A Comp. Exp. Biol. 300A, 53-62. 10.1002/jez.a.10309 [DOI] [PubMed] [Google Scholar]
- Rich A., Gordon S., Brown C., Gibbons S. J., Schaefer K., Hennig G. and Farrugia G. (2013). Kit signaling is required for development of coordinated motility patterns in zebrafish gastrointestinal tract. Zebrafish 10, 154-160. 10.1089/zeb.2012.0766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Membrilla A., Martinez V., Jimenez M., Gonalons E. and Vergara P. (1995). Is nitric oxide the final mediator regulating the migrating myoelectric complex cycle. Am. J. Physiol. Gastrointest. Liver Physiol. 268, G207-G214. [DOI] [PubMed] [Google Scholar]
- Rönnestad I., Rojas-Garcia C. R. and Skadal J. (2000). Retrograde peristalsis; a possible mechanism for filling the pyloric caeca? J. Fish Biol. 56, 216-218. 10.1111/j.1095-8649.2000.tb02098.x [DOI] [Google Scholar]
- Sanders K. M., Koh S. D., Ro S. and Ward S. M. (2012). Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 9, 633-645. 10.1038/nrgastro.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmiguel C. P., Aviv R., Policker S., Haddad W., Brody F. and Soffer E. E. (2007). Association between gastric electromechanical activity and satiation in dogs. Obesity 15, 2958-2963. 10.1038/oby.2007.353 [DOI] [PubMed] [Google Scholar]
- Schulze-Delrieu K., Brown B. P., Lange W., Custer-Hagen T., Lu C., Shirazi S. and Lepsien G. (1996). Volume shifts, unfolding and rolling of haustra in the isolated guinea pig caecum. Neurogastroenterol. Motil. 8, 217-225. 10.1111/j.1365-2982.1996.tb00260.x [DOI] [PubMed] [Google Scholar]
- Shehadeh Z. H. and Gordon M. S. (1969). The Role of the intestine in salinity adaptation of the rainbow trout, Salmo Gairdneri. Comp. Biochem. Physiol. 30, 397-418. 10.1016/0010-406x(69)92011-8 [DOI] [Google Scholar]
- Smith H. W. (1930). The absorption and excretion of water and salts by marine teleosts. Am. J. Physiol. 93, 480-505. [Google Scholar]
- Smith T. K., Bornstein J. C. and Furness J. B. (1990). Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J. Autonom. Nerv. Syst. 29, 203-217. 10.1016/0165-1838(90)90146-A [DOI] [PubMed] [Google Scholar]
- Smith T. K., Oliver G. R., Hennig G. W., O'Shea D. M., Berghe P. V., Kang S. H. and Spencer N. J. (2003). A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. J. Physiol. 551, 955-969. 10.1113/jphysiol.2003.049163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini M., Costa M., Brookes S. J. H. and Humphreys C. M. S. (1996). Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neurosci. 73, 287-297. 10.1016/0306-4522(96)00040-1 [DOI] [PubMed] [Google Scholar]
- Trendelenburg P. (2006). Physiological and pharmacological investigations of small intestinal peristalsis. Naunyn Schmiedebergs Arch. Pharmacol. 373, 101-133. 10.1007/s00210-006-0052-7 [DOI] [PubMed] [Google Scholar]
- Vantrappen G., Janssens J., Hellemans J. and Ghoos Y. (1977). Interdigestive motor complex of normal subjects and patients with bacterial overgrowth of small intestine. J. Clin. Invest. 59, 1158-1166. 10.1172/JCI108740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman S. A. and Costa M. (1994). The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J. Physiol. 477, 459-468. 10.1113/jphysiol.1994.sp020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman S. A., Tonini M. and Costa M. (1994). The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. J. Physiol. Lond. 481, 223-232. 10.1113/jphysiol.1994.sp020433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. W. and Grosell M. (2003). Intestinal bicarbonate secretion in marine teleost fish-source of bicarbonate, pH sensitivity, and consequences for whole animal acid-base and calcium homeostasis. Biochim. Biophys. Acta Biomemb. 1618, 163-174. 10.1016/j.bbamem.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Wilson J. M. and Grosell M. (2002). Intestinal bicarbonate secretion by marine teleost fish—why and how? Biochim. Biophys. Acta Biomemb. 1566, 182-193. 10.1016/S0005-2736(02)00600-4 [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Millero F. J., Taylor J. R., Walsh P. J., Christensen V., Jennings S. and Grosell M. (2009). Contribution of fish to the marine inorganic carbon cycle. Science 323, 359-362. 10.1126/science.1157972 [DOI] [PubMed] [Google Scholar]
- Won K.-J., Sanders K. M. and Ward S. M. (2005). Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl. Acad. Sci. USA 102, 14913-14918. 10.1073/pnas.0503628102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. M. and Bucking C. (2011). The role of feeding in salt and water balance. In The Multifunctional Gut of Fish (ed. Grosell M., Farrell A. P. and Brauner C. J.), pp. 165-212. San Diego: Academic Press. [Google Scholar]
- Yin J., Levanon D. and Chen J. D. Z. (2004). Inhibitory effects of stress on postprandial gastric myoelectrical activity and vagal tone in healthy subjects. Neurogastroenterol. Motil. 16, 737-744. 10.1111/j.1365-2982.2004.00544.x [DOI] [PubMed] [Google Scholar]