Abstract
Objective
Assessment of the mobilization of non-hematopoietic very small embryonic-like stem cells (VSEL) in acute myocardial infarction (MI).
Background
Acute MI induces mobilization of bone marrow stem cells. Recently rare population of VSELs, expressing markers of embryonic pluripotent stem cells (PSC) was identified in adult murine bone marrow and human umbilical cord blood.
Methods
31 pts with acute MI and 30 healthy subjects (CTRL) were enrolled. Blood was sampled on admission, after 24 hours and 5 days later. Erythrocytes were lysed and lin-CD45- VSELs were isolated using a live cell sorting system (FACSAria).
Results
In healthy subjects the median number of circulating VSEL was very low [0.8 (0-1.3] cells/μL. In acute MI VSELs were mobilized early [2.7 (0.2-3.9) cells/μL; p<0.001), and remained elevated after 24 hrs and 5 days [4.7 (0.2-6.4); p<0.003 and 2.6 (0.3-3.6) cells/μL; p<0.03, respectively). The mobilization of VSEL was significantly reduced in patients older than 50 years and with diabetes in comparison to younger and non-diabetic patients. Circulating VSELs were small (7-8 μm) and enriched in mRNA of PSC markers (Oct-4, Nanog), cardiac lineage (GATA-4, Nkx2.5/Csx, MEF2C) and endothelial (VE-cadherin) markers. The presence of PSC markers (Oct-4, SSEA-4) and chemokine receptor CXCR4 in circulating VSELs was confirmed at the protein level by immunofluorescent staining and ImageStream system (ISS) analysis.
Conclusion
Acute MI induced mobilization of very small embryonic-like stem cells expressing pluripotent markers, early cardiac and endothelial markers and chemokine receptor CXCR4.
Keywords: very small embryonic-like stem cells, pluripotent stem cells, acute myocardial infarction, mobilization
Acute myocardial infarction (MI) induces generalized inflammatory response reflected by increased plasma levels of chemoattractants leading to subsequent mobilization of stem and progenitor cells from the bone marrow (BM) [1] [2] [3] [4] [5]. These circulating cells represent predominantly committed lineages such as granulocytes, lymphocytes and monocytes, but also much smaller subpopulations of mono- and multipotent cells which may play a role in cardiac and endothelial repair. Earlier studies showed that these cells released into peripheral blood following the acute MI consist of hematopoietic stem cells (HSC), endothelial progenitor cells (EPC) and multipotent mesenchymal stromal cells (MSC) [2] [3] [6]. In patients with acute MI we found significant up-regulation of early cardiac and endothelial lineage markers in circulating mononuclear cells (MNC) [3]. Kucia et al. showed that adult murine BM harbors a population of non-hematopoietic Sca-1+lin−CD45− cells enriched for GATA-4 and Nkx2.5/Csx that also expressed CXCR4, which migrated to stromal-derived factor-1 (SDF-1) gradient and underwent rapid mobilization into peripheral blood in experimental MI [1]. Our recently published data confirmed that murine BM-derived Sca-1+lin−CD45−CXCR4+ cells express not only cardiac and endothelial, but also several developmental markers characteristic for embryonic pluripotent stem cells (PSC), such as Oct-4, Nanog and SSEA-1. Based on their small size, morphology and presence of PSC markers these cells were named very small embryonic-like (VSEL) stem cells. Murine VSELs can differentiate into cell lineages from all three germ layers, including mesoderm-derived cardiomyocytes. This population of primitive cells can be isolated from BM and peripheral blood using multiparameter live cell sorting technique [7] [8] [9]. We hypothesized that VSELs are the progeny of epiblast-derived stem cells and form a population of quiescent PSC deposited early in organogenesis in developing BM and other organs (brain, heart) [10]. As mentioned above experimental MI in mice is associated with the mobilization of VSELs into peripheral blood, however there is no data confirming the presence of such primitive stem cells in patients with acute MI [11] [12] [13].
In the present study, we provide evidence for the first time in humans employing several experimental strategies showing that acute MI induces mobilization of VSELs expressing PSC markers.
Methods
Patient population
We studied 31 patients with acute ST-segment elevation MI referred within 12 hours after the symptoms onset for primary percutaneous coronary intervention (PCI) and 30 healthy age- and sex-matched subjects (CTRL) (table 1).
Table 1. Demographic, clinical and laboratory characteristics of patients with acute myocardial infarction (MI) and control group (CTRL).
| Acute MI (n=31) | Control (n=30) | |
|---|---|---|
| Age, (mean) | 62.5±12.1 | 40.4±9.4* |
| Males, n (%) | 20 (64) | 19 (63) |
| History of CAD, n (%) | 16 (51) | (-) |
| Hypertension, n (%) | 18 (58) | 16 (53) |
| Diabetes, n (%) | 8 (25) | 7 (23) |
| Family history of CAD, n (%) | 7 (22) | 6 (20) |
| Smoking, n (%) | 11 (35) | 10 (33) |
| Total cholesterol [mmol/L] | 4.9 (4.1-5.7) | 4.8 (4.0-5.5) |
| Creatinine [μmol/L) | 112 (97-125) | 109 (92-123) |
| Mean LVEF, % (range) | 45.5 (25-60) | 56.7 (50-62)* |
| prior statins (%, n) | 10 (32) | 9 (30) |
| prior ACEI (%, n) | 12 (38) | 11 (36) |
| max. Troponin I [ng/mL] | 6.4 (1.4-100) | (-) |
| max. CK-MB [U/L] | 124 (37-324) | (-) |
| 12 hrs Leukocytes, ×103 cells /μL (range) | 11.2 (6.3-15) | 5.9 (4.0-8.8) † |
| 24 hrs Leukocytes, ×103/μL (range) | 12.7 (7.4-16.2) | (-) |
| 5 day Leukocytes, ×103 cells/μL (range) | 10.9 (5.1-14.2) | (-) |
| Lymphocytes, ×103/μL (range) | 2.28 (0.8-3.9) | 2.1 (0.7-3.7) |
| Granulocytes, ×103/μL (range) | 7.6 (4.0-14.7) | 2.7 (2.1-4.3) |
| Hemoglobin, g/dL (mean±SD) | 14.6±1.2 | 14.7±1.1 |
Values of expressed as: percentage and numbers, medians with quartiles, means with standard deviation.
p<0.03 CTRL vs. MI group.
p<0.0001 CTRL vs. MI group.
FACS analysis and sorting of circulating VSELs
Figure 1 (panel A) shows the protocol of VSEL isolation and analysis. Peripheral blood (PB) samples were drawn on admission, 24 hours after PCI and 5 days later. VSELs (lin−CXCR4+CD45− cells that co-express CD133 and CD34 antigens) were sorted using multiparameter, live sterile cell sorting system (FACSAria) [Figure 1, panel B] and used for real-time RT-PCR and immunofluorescence and ImageStream system (ISS) analysis [2] [7] (more details are available in the online-only Data Supplement).
Figure 1. Procedure of isolation of VSEL from peripheral blood.
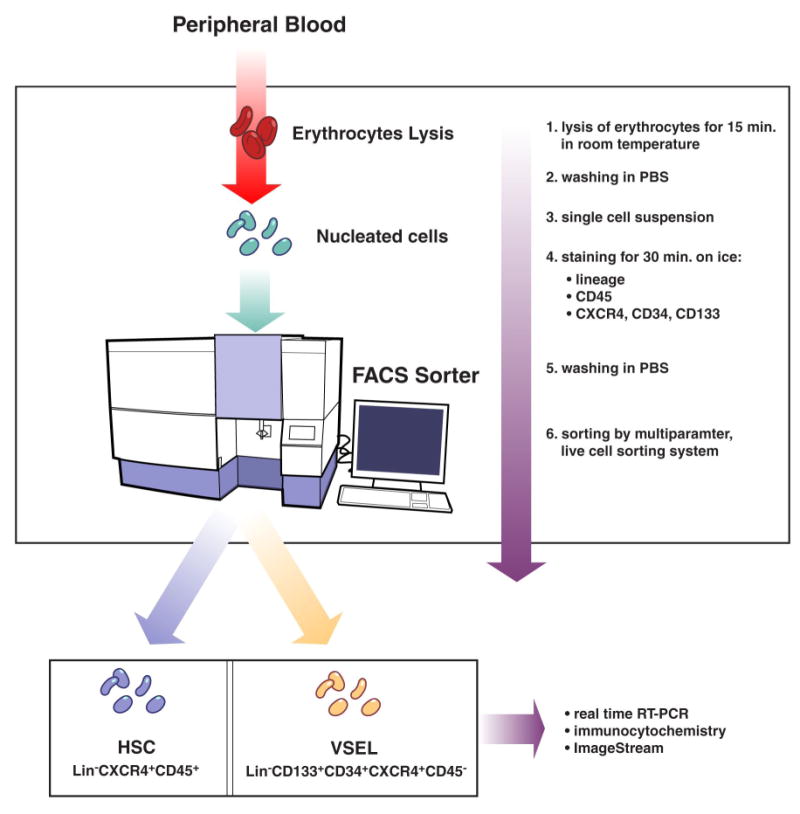
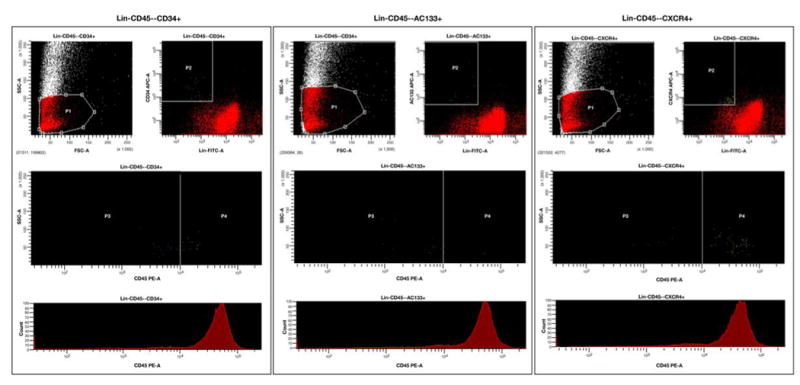
Isolation of VSEL from peripheral blood nucleated cells by hypotnic lysis of erythrocytes and sterile, live cell sorting using FACSAria cell sorting system (Panel A). Sorting was based on the immunophenotype (lin−CXCR4+CD34+CD133+CD45−) of VSEL. Gating strategy enabled to include small events consistent with the size range of VSEL (5-7 μm). Events contained by gate P1 were characterized by the absence of lineage markers and presence of CD34, CD133 and CXCR4 (gate P2) and absence of CD45 (gate P3) which represent the population of VSEL (Panel B).
Panel A. Procedure flowchart
Panel B. Sorting of subpopulation of non-hematopoietic CD45− lin−CD34+, lin−CD45− CD133+ and lin−CD45−CXCR4+ VSEL.
Real-time RT-PCR (RQ-PCR)
Real-time RT-PCR was used for analysis of mRNA levels for PSC (Oct-4, Nanog), early myocardial (Nkx2.5/Csx, GATA-4, MEF2C) and endothelial (VE-cadherin) markers (more details are available in the online-only Data Supplement).
Immunofluorescent staining of VSELs
VSELs were stained with anti-SSEA-4, Oct-4 and CXCR4 monoclonal antibodies (MoAb) (more details are available in the online-only Data Supplement).
ImageStream system (ISS) analysis
After lysis of red blood cells (RBC) and washing, the nucleated fraction of PB cells was fixed, permeabilized and stained for Oct-4, CD45, lineage markers and CXCR4. PB-derived granulocytes and erythrocytes were stained for their specific markers. Samples were analyzed with ISS 100 (more details are available in the online-only Data Supplement).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Mobilization of Oct-4+ VSELs in acute MI
In healthy subjects the number of circulating VSELs was very low [median (range) 0.8 (0-1.3) cells/μL]. In acute MI by contrast, the number of VSEL was higher than in CTRL early (<12 hrs) after the onset of symptoms [median 2.7 (0.2-3.9) cells/μL; P<0.001). Further mobilization was observed after 24 hrs [4.7 (0.2-7.4) cells/μL; P<0.003] (figure 2) and 5 days [2.6 (0.3-3.6) cells/μL; P<0.03]. Populations of CD34+CD133+VEGFR2+ EPC and CD34+CXCR4+ cells increased significantly in acute MI, however the absolute numbers of cells were higher than VSELs (table 2).
Figure 2. Mobilization of lin−CD133+CD45− VSEL.
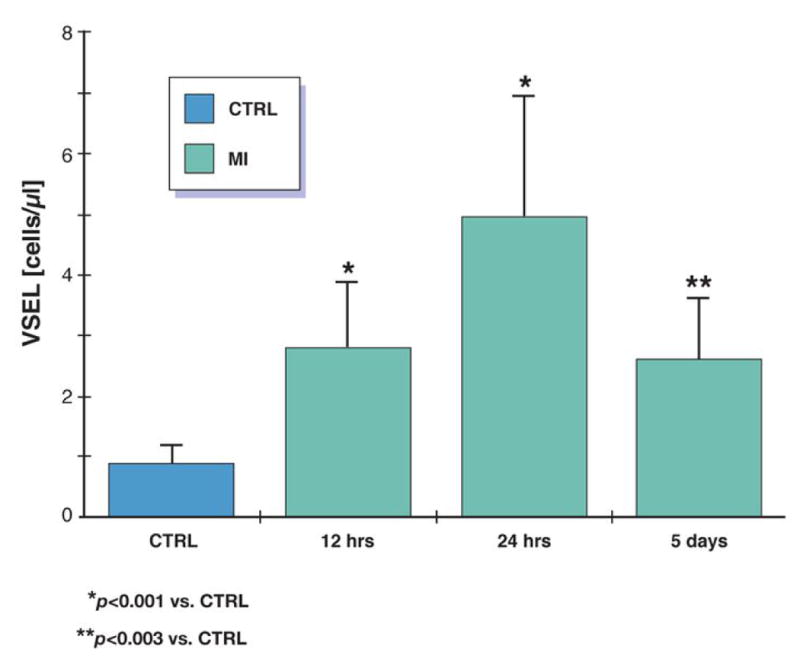
Mobilization of cells shown as change in absolute number of cells per μL of peripheral blood in patients with acute MI in comparison to healthy controls.
Table 2.
Mobilization of CD34+CD133+VEGFR2+ EPC, CD34+CXCR4+ cells and lin−CD133+CD45− VSELs in patients with acute myocardial infarction in comparison to healthy controls. Data expressed as median±range.
| CONTROL | acute MI admission | P-value (vs. CTRL) | acute MI 24 hrs | P-value (vs. CTRL) | P-value (vs. admission) | acute MI day 5 | P-value (vs. CTRL) | P-value (vs. admission) | |
|---|---|---|---|---|---|---|---|---|---|
| EPC | 1.6 | 3.7 | <0.003 | 5.7 | <0.0001 | <0.003 | 5.1 | <0.0001 | <0.03 |
| [cells/μL] | (0-2.1) | (0.1-5.6) | (0-8.1) | (0.2-7.3) | |||||
| CD34+CXCR4+ | 1.9 | 5.4 | <0.0001 | 7.3 | <0.0001 | <0.003 | 6.8 | <0.0001 | <0.03 |
| [cells/μL] | (0-3.2) | (0.4-7.7) | (0.3-9.7) | ||||||
| VSEL | 0.8 | 2.7 | <0.001 | 4.7 | <0.001 | <0.003 | 2.6 | <0.03 | NS |
| [cells/μL] | (0-1.3) | (0.2-3.9) | (0.3-7.4) | (0.3-3.6) |
EPC – endothelial progenitor cells
Expression of pluripotent stem cells markers by RQ-PCR and immunofluorescent staining
We found that the expression of PSC markers was significantly higher 12 hours after MI (Oct-4: 17.9±4.3-fold; Nanog: 18.2±3.7-fold) than in CTRL. The highest levels of mRNA were detected 24 hrs after acute MI, at the same time when the most significant mobilization of VSELs occurred (Oct-4: 206±32-fold; Nanog: 282±41-fold). After 5 days levels of mRNA were not different from CTRL (Oct-4: 2.3±0.4-fold; Nanog: 2.3±0.38-fold) [figure 3].
Figure 3. Expression of pluripotent stem cells markers in circulating VSEL.
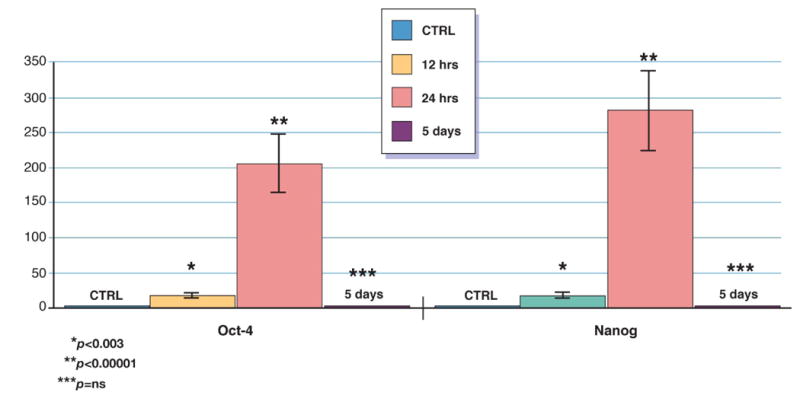
Relative changes in expression of pluripotent stem cells markers in circulating VSEL isolated by sterile, live cell sorting from peripheral blood of patients with acute MI and healthy controls measured by real time RT-PCR. Expression of each gene showed as fold difference results compared to healthy subjects (n=15).
The presence of PSC markers Oct-4, SSEA-4 and CXCR4 was subsequently confirmed at the protein level using immunofluorescent (IF) staining. VSELs sorted from PB were positive for nuclear transcription factor Oct-4, cell surface antigen SSEA-4 and CXCR4 receptor by direct immunofluorescence staning (figure 4, panel A). We noticed that 54±11.2 % sorted VSELs express Oct-4 antigen. This corresponds to our previously published data on mobilization of VSELs in murine model [8].
Figure 4. Analysis of VSELs by immunofluorescent staining and ImageStream System.
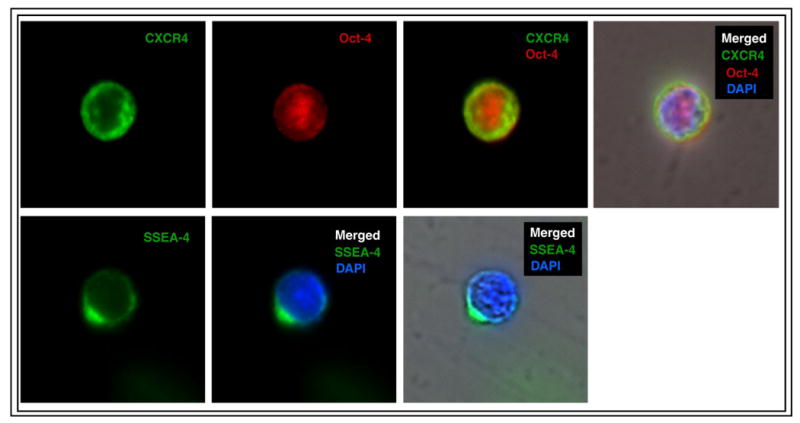
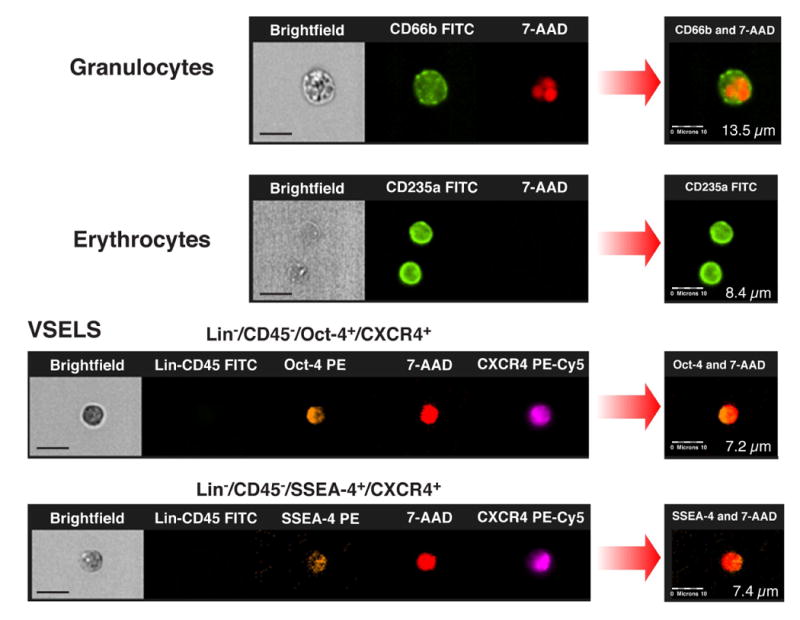
Immunofluorescent staining of peripheral blood-derived lin−CD45−CD133+ VSELs sorted with FACSAria (Panel A). VSELs express SSEA-4, Oct-4 and CXCR4 at the protein level. CXCR4 and SSEA-4 are visualized in the cellular membrane and Oct-4 in the nucleus. Nuclei are stained with DAPI. Staining was performed on cells isolated from four independent sorts. (Panel B) Circulating human VSELs analyzed by ImageStream system. Expression of Oct-4 and SSEA-4 in lin−CD45−CXCR4+ VSELs was confirmed at the single cell level. The size of VSELs was compared to granulocytes and erythrocytes derived from peripheral blood of patients with acute MI. The size of the cells was calculated by IDEAS software. Scale bars indicate 10μm.
Size and immunophenotype of VSELs
The population of VSELs consists of non-hematopoietic cell negative for lineage markers and CD45 antigen (lin− CD45−) and positive for CXCR4, CD133 and CD34 antigens as showed by IF and ISS. Expression of PSC markers Oct-4 and SSEA-4 was confirmed in population of small cells (∼7-8 μm). Direct ISS-based comparison of mobilized VSELs to other populations of circulating nucleated cells (monocytes, granulocytes) showed that VSEL are significantly smaller and have distinct morphology (figure 4 panel B).
Expression of early cardiac and endothelial markers in circulating VSELs by RQ-PCR
The highest relative expression level of early cardiac markers was detected 24 hours post acute MI for GATA-4 (210±34-fold), Nkx2.5/Csx (174±28-fold), MEF2C (209±32-fold) and VE-cadherin (3884±37-fold). After 5 days relative expression levels of GATA 4 (1.7±06-fold), Nkx2.5/Csx (2±0.5-fold) and VE-cadherin (3.3±0.9-fold) were comparable to CTRL while expression of MEF2C remained higher (97.5±19-fold) [figure 5].
Figure 5. Expression of early cardiac and endothelial markers in circulating VSELs.
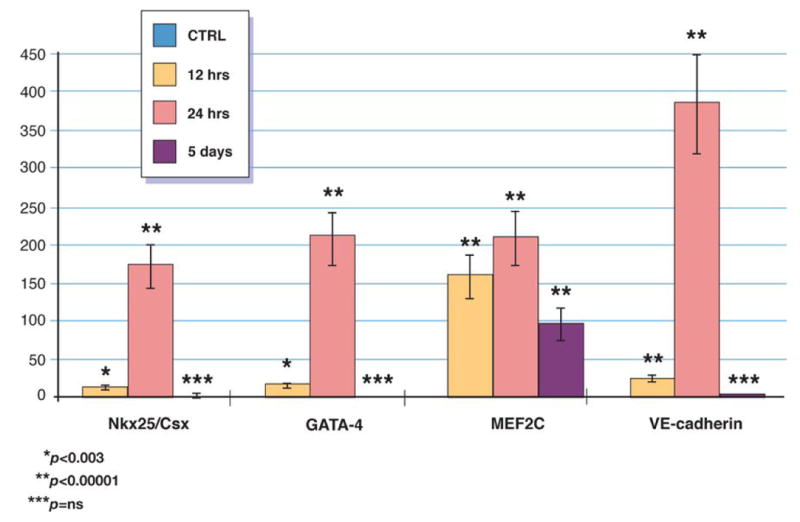
Relative changes in expression of early cardiac and endothelial markers in circulating VSEL isolated by sterile, live cell sorting from peripheral blood of patients with acute MI and healthy controls measured by real time RT-PCR. Expression of each gene showed as fold difference results compared to healthy subjects (n=15).
Plasma concentrations of chemokines, growth factors, cytokines and inflammatory markers
In acute MI the upregulation of plasma levels of chemoattractants (SDF-1, HGF, SCF, G-CSF and VEGF) was found (Table 3). The number of circulating VSELs in MI patients positively correlated with levels of SDF-1. Available in the online-only Data Supplement.
Clinical and demographic correlates of VSELs mobilization
The mobilization of VSELs was significantly higher in younger patients (<50 years), compared to older ones [4.9 (0-6.4) vs. 2.7 (0.2-5.1) cells/μL; P<0.05]. In diabetic patients the number of circulating VSELs was significantly lower than in non-diabetics [1.9 (0-2.3) vs. 4.8 (0.2-6.2) cells/μL; P<0.05]. In addition, mobilization of VSELs was impaired in patients with an LVEF <40%, compared to an LVEF of >40% [3.3 (0-4.9) vs. 4.7 (0.4-6.4) cells/μL; P<0.05]. The differences were significant after 12 and 24 hrs, but not after 5 days. The number of VSELs had a significantly negative correlation maximum levels of troponin I on admission (R=-0.41; p=0.03) and after 24 hours (R=-0.34; p=0.04, but not after 5 days (R=-0.31; p=0.45). Similar negative correlations were found in relation to maximum activity of CK-MB [on admission: R=-0.45; P=0.033; after 24 hours: R=-0.39; P=0.042, after 5 days R=-0.21; P=0.23).
Discussion
Adult bone marrow harbors various populations of cells that can potentially contribute to myocardial and endothelial repair [7] [14, 15] [16] [17] [18]. Data from the experimental model of acute MI in mice showed that VSELs are mobilized into peripheral blood in response to myocardial ischemia [11]. Furthermore, intramyocardial injection of VSELs was more efficient than HSC and placebo at improving global and regional left ventricular contractility, LVESD and systolic thickening as well as reducing myocardial hypertrophy. This effect was obtained using small total number of VSELs (10,000 cells), while a much higher number of hematopoietic stem cells (100,000 cells) was not effective [19].
VSELs circulate in peripheral blood in adult humans
Recently VSELs were identified in human cord blood, but there were no data on their presence in adults [20]. In the present work we demonstrated for the first time that VSELs are present in the peripheral blood in healthy adult subjects and undergo significant mobilization in patients with acute MI. We employed a FACS live cell sorting system to define the immunophenotype of human VSELs and to isolate these cells for subsequent analyses. We showed that the number of circulating VSELs increases 12 hrs after the onset of the symptoms reaching maximum after 24 hours and remaining elevated after 5 days. The time-course of the VSEL mobilization is similar to EPCs, however the absolute cell number is significantly lower and decrease more rapidly following acute MI.
Characteristics of murine and human VSELs
In adult murine BM VSELs comprise a rare population (∼0.01%) of mononuclear cells [7] [21]. In mice VSELs are nonhematopoietic Sca-1+lin−CD45− cells expressing markers of pluripotent stem cells Oct-4, Nanog, SSEA-1 at the mRNA and protein levels. Data from electron microscopy and ISS showed that VSEL have a morphology consistent with embryonic stem cells. These cells are small (∼3.6 μm in diameter) and have a large nucleus surrounded by a narrow rim of cytoplasm with numerous mitochondria and open-type chromatin [7] [21]. Approximately ∼5-10% of purified VSELs form in co-culture with C2C12 myoblast line spheres that resemble embryoid bodies. Cells derived from these spheres can be expanded if replated over C2C12 feeder layer or differentiated after replating into tissue specific differentiation media into cells representing all three germ layers including cardiac myocytes [7] [9] [21]. In present work the expression of mRNA for early developmental markers was shown in circulating VSELs by employing real-time RT-PCR which confirmed their enrichment in mRNA for Oct-4 and Nanog. Highest relative expression level of mRNA for these genes level was detected 24 hrs after acute MI, at the same time when the most significant mobilization of VSELs occurred. The presence of PSC markers Oct-4 and SSEA-4 was subsequently confirmed at the protein level using IF staining and ISS analysis. ISS is an imaging system that integrates the information obtained by FACS and immunofluorescence, allowing combination of the data on cell morphometry and quantitative image analysis, eg. information on colocalization of multiple cellular markers [21]. VSEL were positive for nuclear transcription factor Oct-4 and cell surface antigen SSEA-4. These small (∼ 7 μm) Oct-4 and SSEA-4 positive cells coexpressed CD34, CD133 and CXCR4 and were negative for lineage markers and the panhematopoietic marker CD45. The presence of CXCR4 on the surface of the cells was consistent with previously described VSEL profile [7]. Moreover, we noticed that ∼50-60% of circulating VSELs in PB in AMI patients express Oct-4 at protein level what corroborates with our previously published data on mobilization of VSELs in mice [8]. Comparison of mobilized VSEL to other populations of circulating nucleated cells (monocytes, granulocytes) showed that VSEL are not only significantly smaller, but they have a distinct morphology. We noticed, that similarly as in mice the size of human VSELs are slightly smaller than erythrocyte. Nevertheless human circulating VSELs in adult patients are larger than those isolated from human neonatal umbilical cord blood (3-5 μm) as well as murine (3-4 μm) BM-derived VSELs [11] [20].
Mobilization of VSELs as a hypothetical reparatory mechanism in acute MI
It is hypothesized that stem cells residing in BM and other tissues in order to reduce the cardiac ischemic injury the BM cells must undergo mobilization and subsequently home and engraft into the myocardium [22] [23-26]. Since BM is also a potential source of cardiac stem cells (CSC) and the mobilization of CSC from the BM may be additional physiological pathway to replenish these stem cells [27]. Shintani et al. described for the first time rapid increase of EPC number after the acute MI [5]. Massa et al. documented mobilization of both HSC and EPC in patients with acute MI. The number of circulating cells reached maximum early, within few hours after the onset of symptoms, decreased after 1 week and returned to level comparable with healthy subjects within 2 months. In addition to EPC and HSC other less well defined subpopulatons (c-met+, c-kit+, MSC) were also mobilized in acute MI [2] [28].
Circulating human VSELs are enriched in mRNA for early cardiac and endothelial markers
We found that circulating human VSELs are significantly enriched for mRNA for cardiac lineage (GATA-4, Nkx2.5/Csx, MEF2C) and endothelial (VE-cadherin) markers. The highest relative expression level of mRNA for these genes was detected 24 hours after acute MI. The decrease in mRNA expression probably could be related to “back-homing” to the BM of mobilized cells not incorporated to the myocardium. Also other possible mechanisms could be involved such as: 1) an increase in expression of cardiac committed genes in VSELs by signals released from damaged myocardium, 2) it is some point selective mobilization of VSELs already enriched in cardiac markers (committed to myocardiac lineage). Our previous data from patients with acute MI showed significant mobilization of CD34+/CXCR4+ cells early in acute MI. Parallel to the release of CD34+CXCR4+ cells we found significant increase of the mRNA for early myocardial, muscle and endothelial markers in the PB MNC, but we were not able to show which population of circulating MNC was responsible for the enrichment in the tissue specific markers [3]. In a murine model of MI, it was consistently shown that stem cells are mobilized into peripheral blood and the relative increase in expression levels of cardiac and endothelial markers correlates with stem cell mobilization [1].
The presence of Oct-4+ cells in adult murine BM raises an important question about their ability to differentiate into cardiomyocytes. Pallante et al. showed that the murine BM contains cells capable of cardiogenic differentiation that give rise to the population of spontaneously contracting cardiomyocytes expressing the cardiac structural proteins, beta-adrenergic receptors and connexins. These cells were identified as rare (0.05% of BMMNC) Oct3/4+c-kit+CXCR4+Sca-1-CD34-CD45- cells localized adjacent to the osteoblastic niche. Thus, this study somehow supports our results showing that the population of mobile non-hematopoietic Oct-4+CXCR4+CD133+ cells is enriched for the cardiac markers and capable of cardiac differentiation [1] [7] [29].
The number of circulating VSELs is correlated with plasma SDF-1 levels
We found that while the number of circulating VSEL in acute MI was positively correlated with levels of SDF-1, there was no significant correlation with other cytokines, growth factors, chemokines and inflammatory markers. This correlation between SDF-1 level and the circulating VSEL expressing SDF-1-binding receptor CXCR4 seems to be biologically important. Murine BM-derived VSELs express the CXCR4 as well as other receptors for cytokines and growth factors (c-met and leukemia inhibitory factor receptor, LIFR). We reported in the past that the population of murine BM-derived cells expressing cardiac markers migrated to the homogenates prepared from the infarcted myocardium in the SDF-1/CXCR4, LIF/LIFR and HGF/c-met dependent manner [1] [4] [7].
Mobilization of VSELs is decreased in older patients with diabetes and reduced LVEF
Several factors including age, presence of diabetes and the use of statins can influence the mobilization of cells into the peripheral blood. In murine models the age seems to be the most important determinant of mobilized VSEls and level of expression of PSC markers in these cells [7]. We report here that the mobilization of VSELs was significantly more efficient in younger patients than in older. This finding is consistent with our previous data showing that the number of circulating CD34+CXCR4+ cells is significantly reduced in patients older than 50 years [12]. To support this clinically relevant observation we already reported that in 1-year old mice mice the number of Sca-1+lin−CD45− VSEL in the bone marrow is significantly decreased as compared to young 1-month old animals. Data from animal model showed that both younger and older mice had similar time course and number of circulating VSEL following the acute MI, however the level of expression of PSC markers in circulating VSEL was significantly lower in older animals [11]. In diabetic patients the number of mobilized pluripotent VSELs is lower than in non-diabetics. It is worth mentioning that so far no study has investigated the influence of diabetes on tissue distribution and mobilization of pluripotent stem cells. However, diabetes is associated with reduced mobilization, functional capacity and survival of EPC cells as well as promotes the senescence of CSC [30] [31].
Mobilization of VSEL was also impaired in our study in patients with reduced LVEF. In addition, the number of VSELs showed significant negative correlations with cardiac necrosis markers, which suggest that reduced mobilization of VSEL is associated with larger infarct size and higher degree of impairment of the left ventricular contractility. This could be potentially explained by the fact that proteases are probably more abundantly released from the larger necrotic areas of myocardium and may negatively affect stability of certain stem cell mobilizing chemoattractants. To support this SDF-1 is a very well known substrate for several tissue proteases [32]. Current findings are consistent with our previous data suggesting that the numbers of circulating CD34+CXCR4+ cells are positively correlated with LVEF and negatively with markers of myocardial necrosis and NT-proBNP in patients with acute MI [13]. In the prospective 1-year follow-up of 55 patients with acute MI we showed that the mobilization of CD34+CXCR4+ cells in the acute phase is positively correlated with LVEF after 1 year [12]. In 15 patients followed-up using cardiac MRI we showed that mobilization of CD34+CXCR4+ cells in acute MI is inversely correlated with the parameters of left ventricular remodeling (LVESV, LVEDV), infarct area (delayed enhancement) and number of segments showing impaired perfusion after infusion of adenosine [3] [12]. The association between the mobilization of stem and progenitor cells was confirmed by Leone et al. who showed that higher number of CD34+ cells assessed 1 year after the MI is associated with better improvement of LVEF [33]. Numaguchi et al. demonstrated that in patients with acute MI the capability of mobilized EPC to differentiate into mature endothelial cells may be associated with improved recovery of LVEF, lower end-systolic volume and decreased infarct size [34]. However, these findings need confirmation in larger populations of patients before use of the stem cell number as the cardiac recovery marker can be recommended.
The search for the most suitable cell for cardiac repair continues, and cells displaying the features of pluripotent stem cells (e.g., VSELs) are good candidates for further studies in this area. VSELs can be easily isolated from the BM using live cell sorting system. In addition murine VSELs can be expanded and differentiated into cardiomyocytes. We are aware that the obvious limitation of our current study is the lack of direct proof that the human peripheral blood-derived VSELs can differentiate into the cardiac myocytes similarly to their murine counterparts. Indeed, this issue needs further investigations [8]. In conclusion we believe that human VSELs might be potentially useful in future clinical studies in patients with ischemic cardiomyopathy providing the technical issues with their large scale isolation, expansion and differentiation are resolved.
This study supports the hypothesis that rare population of very small embryonic-like cells expressing markers of pluripotent stem cells (Oct-4, Nanog, SSEA-4) as well as early cardiac and endothelial markers is mobilized to the peripheral blood in acute MI. Mobilization of VSELs is reduced in older patients with high levels of cardiac necrosis markers, reduced LVEF and diabetes.
Supplementary Material
Acknowledgments
Funding: Grants NIH R01 CA106281-01, Polish Ministry of Science and Higher Education PBZ-KBN-099/P05/2003, 0651/P01/2007/32, 2422/P01/2007/32 and European Vascular Genomics Network.
Abbreviations
- MI
myocardial infarction
- HSC
hematopoietic stem cells
- EPC
endothelial progenitor cells
- PSC
pluripotent stem cells
- VSEL
very small embryonic-like stem cells
- LVEF
left ventricular ejection fraction
- ISS
ImageStream System
- MNC
monononuclear cells
Footnotes
Disclosure: None of the authors have any conflict of interests in regard to the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 3.Wojakowski W, Tendera M, Michalowska A, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 4.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 5.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–9. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 7.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 8.Kucia M, Wysoczynski M, Wu W, Zuba-Surma E, Ratajczak J, Ratajczak MZ. Evidence that Very Small Embryonic-Like Stem Cells are Mobilized into Peripheral Blood. Stem Cells. 2008;26:2083–92. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 9.Wojakowski W, Kucia M, Zuba-Surma E, et al. Cardiogenic differentiation of very small embryonic-like cells isolated from adult bone marrow. J Am Coll Cardiol (Suppl A) 2007;49:43A. [Google Scholar]
- 10.Ratajczak MZ, Machalinski B, Wojakowski W, Ratajczak J, Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–7. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 11.Zuba-Surma E, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiology. 2008;44:865–73. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojakowski W, Kucia M, Wyderka R, et al. Mobilization of CXCR4+ Stem Cells in Acute Myocardial Infarction is Correlated with Left Ventricular Ejection Fraction and Myocardial Perfusion Assessed by MRI in 1 year follow-up (REGENT Trial) Circulation. 2006;114:II_669. [Google Scholar]
- 13.Wojakowski W, Tendera M, Zebzda A, et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–9. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- 14.Swynghedauw B. Phenotypic plasticity of adult myocardium: molecular mechanisms. J Exp Biol. 2006;209:2320–7. doi: 10.1242/jeb.02084. [DOI] [PubMed] [Google Scholar]
- 15.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation. 2003;107:3093–100. doi: 10.1161/01.CIR.0000074242.66719.4A. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 17.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow) Blood. 2007;110:3438–46. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 19.Dawn B, Tiwari S, Kucia M, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–55. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2008;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 21.Zuba-Surma EK, Kucia M, Abdel-Latif A, et al. Morphological characterization of very small embryonic- like stem cells (VSELs) by image stream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucia M, Reca R, Jala VR, Dawn B, Ratajczak J, Ratajczak MZ. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–27. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 23.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 24.Yoon YS, Lee N, Scadova H. Myocardial regeneration with bone-marrow-derived stem cells. Biol Cell. 2005;97:253–63. doi: 10.1042/BC20040099. [DOI] [PubMed] [Google Scholar]
- 25.Werner N, Nickenig G. Clinical and therapeutical implications of EPC biology in atherosclerosis. J Cell Mol Med. 2006;10:318–32. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U SA. 2005;102:8692–7. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard VLT, Edelberg JM. Stem Cells and the Regeneration of the Aging Cardiovascular System. Circ Res. 2007;100:1116–1127. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Johnsen HE, Mortensen S, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768–74. doi: 10.1136/hrt.2005.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pallante BA, Duignan I, Okin D, et al. Bone Marrow Oct3/4+ Cells Differentiate Into Cardiac Myocytes via Age-Dependent Paracrine Mechanisms. Circ Res. 2007;100:e1–e11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 30.Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Diabetes Care. 2007;30:1305–13. doi: 10.2337/dc06-2305. [DOI] [PubMed] [Google Scholar]
- 31.Fadini GP, Sartore S, Albiero, et al. Number and Function of Endothelial Progenitor Cells as a Marker of Severity for Diabetic Vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 32.Kucia M, Reca R, Miekus R, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 33.Leone AM, Rutella S, Bonanno G, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 34.Numaguchi Y, Sone T, Okumura K, et al. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114:I114–9. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


