Abstract
The major DNA photoproduct of dormant, UV-irradiated Bacillus subtilis spores is the thymine dimer 5-thyminyl-5,6-dihydrothymine [spore photoproduct (SP)]. During spore germination, SP is reversed to two intact thymines in situ by the DNA repair enzyme SP lyase, an S-adenosylmethionine (S-AdoMet)-dependent iron-sulfur ([Fe-S]) protein encoded by the splB gene. In the present work, cross-linking, SDS/PAGE, and size exclusion chromatography revealed that SplB protein dimerized when incubated with iron and sulfide under anaerobic reducing conditions. SplB isolated under aerobic conditions generated an EPR spectrum consistent with that of a partially degraded [3Fe-4S] center, and reduction of SplB with dithionite shifted the spectrum to that of a [4Fe-4S] center. Addition of S-AdoMet to SplB converted some of the [4Fe-4S] centers to an EPR-silent form consistent with electron donation to S-AdoMet. HPLC and electrospray ionization MS analyses showed that SP lyase cleaved S-AdoMet to generate 5′-deoxyadenosine. The results indicate that (i) SP lyase is a homodimer of SplB; (ii) dimer formation is coordinated by a [4Fe-4S] center; and (iii) the reduced [4Fe-4S] center is capable of donating electrons to S-AdoMet to generate a 5′-adenosyl radical that is then used for the in situ reversal of SP. Thus, SP lyase belongs to the “radical SAM” superfamily of enzymes that use [Fe-S] centers and S-AdoMet to generate adenosyl radicals to effect catalysis. SP lyase is unique in being the first and only DNA repair enzyme known to function via this novel enzymatic mechanism.
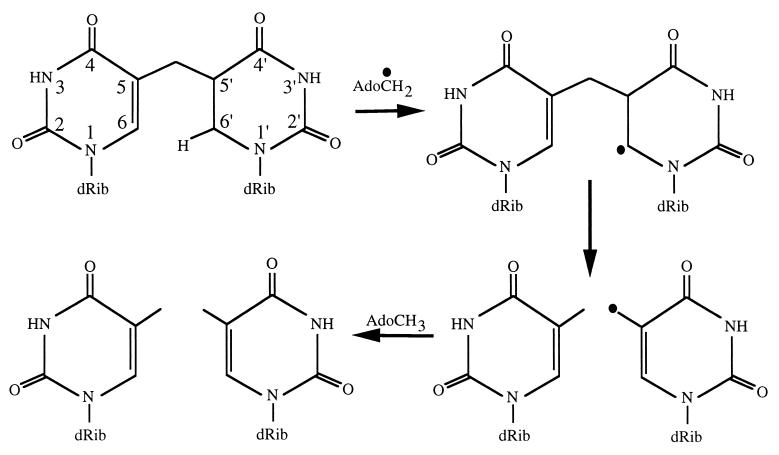
An important property of bacterial endospores is their ability to survive high doses of UV radiation, which is due to the packaging of DNA and the unique photochemistry that this packaging imparts on dormant spores (1–3). The major photoproduct in DNA of UV-irradiated dormant spores is the unique thymine dimer 5-thyminyl-5,6-dihydrothymine, informally called spore photoproduct or SP (4, 5). SP can be removed from DNA during spore germination by the general nucleotide excision repair (NER) pathway (6, 7). In addition to NER, spores possess an SP-specific enzyme called SP lyase that can directly reverse SP to two thymines in situ without excision from DNA (6, 8, 9). Analysis of the deduced amino acid sequence encoded by the SP lyase gene (splB) cloned from Bacillus subtilis revealed a limited region with similarity to DNA photolyases (10), although early indirect experiments indicated that SP lyase caused direct reversal of SP back to two thymines in situ in a light-independent process (8, 9). These observations led to the question as to the mechanism SP lyase utilizes to reverse SP to two thymines. Inspection of the 342-aa deduced SplB sequence (10) revealed that the protein contains four cysteines, three of which are clustered at residues 91, 95, and 98, and the fourth at residue 141. The region from C91 to C98 was similar to the signature cysteine clusters present in the recently dubbed “radical SAM” protein superfamily (11), which includes enzymes such as anaerobic ribonucleotide reductase (RNR), pyruvate formate-lyase (PFL), lysine-2,3-aminomutase (KAM), and biotin synthase (BioB) (12). In radical SAM enzymes, the clustered cysteines serve as ligands in the formation of iron-sulfur ([Fe-S]) centers, and these enzymes use S-adenosylmethionine (S-AdoMet) as a cofactor to generate an adenosyl radical (13–16). We constructed a version of SplB protein containing an N-terminal tag of 10 histidines [(10His)SplB] and subsequently determined that (10His)SplB (i) contained both iron and acid-labile sulfur in a stoichiometric ratio of 1–2 atoms of iron or sulfur per (10His)SplB subunit (17); (ii) exhibited a UV-visible absorption spectrum consistent with other [Fe-S] proteins (17); (iii) required both anaerobic reducing conditions and S-AdoMet for activity in vitro (17, 18); and (iv) was inactive for SP cleavage unless its (10His) tag was first removed proteolytically (17, 18). The above evidence led us to the hypothesis that SP lyase carries out catalysis using an [Fe-S] center to cleave S-AdoMet, thus generating a 5′-adenosyl radical that could participate either directly or indirectly in SP cleavage. Support for this hypothesis has recently been obtained by Mehl and Begley (19). Using synthetic analogues of SP, they proposed a mechanistic scenario for SP reversal to two thymines in which the radical generated from S-AdoMet cleavage by electron donation from the [4Fe-4S] center can abstract a proton from C6′ of SP, effectively generating an SP radical that fragments readily back to two thymines (Fig. 6) (19). The experiments presented in this communication support this model for catalysis, and provide evidence indicating that SP lyase is a dimer of SplB subunits associated through a single oxygen-labile [4Fe-4S] center.
Figure 6.
Proposed SP cleavage mechanism of SP lyase. See text and ref. 19 for details.
Experimental Procedures
Purification of (10His)SplB.
Strain WN434, an Escherichia coli strain that overexpresses B. subtilis (10His)SplB in response to isopropyl-β-d-galactopyranoside (IPTG) (17) was cultivated with shaking in 1 liter of LB medium (20) at 37°C to an OD at 660 nm of 0.6. IPTG was added to 1.0 mM, and cultivation was continued at room temperature (ca. 25°C) for 12 h. Purification of (10His) SplB was performed as in ref. 17 with modifications. IPTG-induced cells were harvested by centrifugation, and the cell pellet was washed once, resuspended in 40 ml of 50 mM sodium phosphate (pH 8.0)/300 mM NaCl/10 mM imidazole/1 mM PMSF/lysozyme (1 mg/ml), and incubated on ice for 30 min. After sonication and centrifugation (12,100 × g, 20 min, 4°C), the lysate on ice was introduced into an anaerobic chamber (Bactron IV, Sheldon Manufacturing, Cornelius, OR) containing a 5% CO2:5% H2:90% N2 atmosphere, and 25°C internal temperature. The lysate was passed through a nickel-nitrilotriacetic acid (Ni-NTA) agarose column (Superflow; Qiagen, Chatsworth, CA) previously equilibrated with 50 mM Tris⋅HCl (pH 8.0)/300 mM NaCl/10 mM imidazole, and the column was washed with 15 ml of the same buffer containing 30 mM imidazole; and (10His)SplB was eluted from the column using the same buffer containing 200 mM imidazole. All buffers and solutions were degassed under vacuum, introduced into the anaerobic chamber, and allowed to equilibrate with the internal atmosphere before use.
[Fe-S] Center Reconstitution.
Purified (10His)SplB was treated with DTT to a final concentration of 10 mM; then Fe(NH4)2(SO4)2 and Na2S were added to a final concentration of 0.1 mM each. Treated (10His)SplB was then incubated anaerobically for 12 or 24 h at 4°C.
Cross-Linking.
(10His)SplB cross-linking was performed as described (21). (10His)SplB protein (1 mg/ml) in 50 mM Hepes⋅HCl (pH 8.0)/300 mM NaCl/200 mM imidazole was treated with dimethyl suberimidate (DMS; 2.5 mg/ml, 20 min, 25°C). The reaction was stopped by the addition of 250 μl SDS/PAGE sample loading buffer and analyzed by SDS/PAGE and Western blotting as described (17).
Size Exclusion Chromatography.
Purified (10His)SplB was chromatographed through a Bio-Gel P-100 column (Bio-Rad, 1.0 cm × 50 cm) at 4°C in 50 mM Tris⋅HCl (pH 8.0)/300 mM NaCl/200 mM imidazole. The buffer was degassed under vacuum and sparged with argon gas before use. (10His)SplB elution was monitored with a flow-through 280-nm UV photodetector, relative to standards of BSA (66.2 kDa) and lysozyme (14.4 kDa). SplB antigen detection by Western blotting and iron assay of each fraction was performed as described (17).
EPR Spectroscopy.
Low temperature EPR spectral analyses were performed on an ESP 300E CW X-band Bruker (Billerica, MA) EPR spectrometer equipped with an Oxford Helium Flow Cryostat. Samples (0.2 ml) of purified (10His)SplB isolated under aerobic conditions in 50 mM Tris⋅HCl (pH 8.0)/300 mM NaCl/200 mM imidazole/10% glycerol were degassed under vacuum, placed into quartz EPR tubes, and frozen in liquid nitrogen before analysis.
Cleavage of S-AdoMet by SP Lyase.
SP lyase reaction conditions were performed as described (17) with the following modifications. Reaction mixtures (260 μl total volume) were prepared in the anaerobic chamber containing 400 μg of purified (10His)SplB, plus parallel control reactions lacking protein. All reaction mixtures and controls were then treated with iron, sulfide, and dithionite as described above. To one sample containing protein and one control were added 0.435 mM S-AdoMet (final concentration). To a second protein sample plus control were added 0.435 mM S-AdoMet and 1.63 μg spore chromosomal DNA containing ≈5% of total thymine as SP. The reactions and controls were incubated at ≈25°C for 60 min, frozen rapidly in a dry ice-ethanol bath, and stored at −20°C. All samples were thawed and centrifuged to remove precipitated protein before injection into the HPLC column.
HPLC Analysis.
Isocratic reverse phase HPLC was performed on a Luna 5μ C18 column (250 mm × 3 mm) (Phenomenex, Torrance, CA) at 38.5°C with 20% acetonitrile in water at a flow rate of 0.5 ml/min. Peaks were monitored by A260 and collected manually for further analysis.
Electrospray Ionization (ESI)-MS Analysis.
ESI-MS analysis was performed by using a Finnigan LCQ system fitted with an ion trap. The mass of the compound isolated was determined, as well as the mass of the fragments generated by collision. The mass and fragmentation patterns were then compared with those of purified S-AdoMet and 5′-dAdo (Sigma).
Results
SP Lyase Forms a Dimer of (10His)SplB Subunits Under Conditions Favoring [Fe-S] Center Formation.
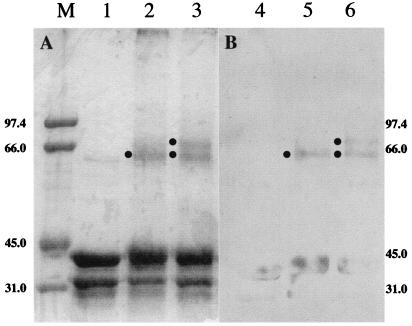
To elucidate the subunit structure of SP lyase and the role of the [Fe-S] center in its formation, purified (10His)SplB was incubated under conditions favoring [Fe-S] cluster formation (16) and then chemically cross-linked with DMS. Products of the reaction were analyzed after electrophoresis through denaturing SDS/PAGE and Coomassie blue staining (Fig. 1A) or Western blotting by using polyclonal antibodies directed against purified (10His)SplB (Fig. 1B). SDS/PAGE of untreated (10His)SplB showed SplB monomers (molecular mass ≈ 41 kDa), as well as an ≈34-kDa product in both the Coomassie-stained gel (Fig. 1, lane 1) and the Western blot (Fig. 1, lane 4). N-terminal amino acid sequencing of the ≈41-kDa protein confirmed that it was indeed (10His)SplB (data not shown). N-terminal amino acid sequencing of the ≈34-kDa protein revealed that this species was also (10His)SplB, lacking an ≈7-kDa fragment from its C terminus (data not shown). A minor band was detected in the Coomassie-stained gel that corresponded to a protein of ≈62 kDa (Fig. 1, lane 1), but which did not crossreact with anti-(10His)SplB antibodies (Fig. 1, lane 4). Treatment of (10His)SplB with DMS resulted in some smearing of the bands corresponding to the 41-kDa and 34-kDa forms of (10His)SplB, consistent with internal cross-linking. In addition, a new band was observed that migrated at ≈64 kDa (Fig. 1, lane 2) and that crossreacted with anti-(10His)SplB antibodies (Fig. 1, lane 5). Incubation of (10His)SplB under conditions that promote the formation of [Fe-S] centers followed by cross-linking with DMS showed a pattern very similar to (10His)SplB treated with only DMS, but with an additional new ≈72-kDa band in the Coomassie blue-stained gel (Fig. 1, lane 3) that crossreacted with anti-(10His)SplB antibodies (Fig. 1, lane 6). Because the new ≈72-kDa species migrated at approximately twice the predicted molecular weight of (10His)SplB monomers and its appearance was dependant on treatment of (10His)SplB under conditions favoring the formation of [Fe-S] centers, it is reasonable to propose that SP lyase is a homodimer of SplB and that dimer formation is coordinated by the [Fe-S] center. No higher molecular weight cross-linked species were detected that could correspond to a different subunit arrangement of SplB (Fig. 1 and data not shown). The species migrating at a calculated molecular mass of ≈64 kDa could be due to the formation of dimers of the ≈34-kDa degradation product (≈68 kDa predicted weight), because the cysteines potentially required for the coordination of the [Fe-S] center at residues 91, 95, 98, and 141 would still be present in the ≈34-kDa fragment and could still potentially form an [Fe-S] cluster, thus allowing dimerization to occur.
Figure 1.
Analysis of cross-linked SplB by SDS/PAGE. (10His)SplB was treated as described in Experimental Procedures, denatured, and electrophoresed through 10% SDS/PAGE. (A) Coomassie blue-stained gel. (B) Western blot. Lanes 1 and 4, untreated; lanes 2 and 5, cross-linked with DMS; lanes 3 and 6, cross-linked with DMS after incubation under conditions favoring [Fe-S] cluster formation. Filled circles indicate positions of cross-linked species at a molecular mass of ≈64 kDa and ≈72 kDa. The lane marked M contains molecular weight markers. Sizes are in kilodaltons.
To corroborate the results of the DMS cross-linking experiments described above, (10His)SplB was also analyzed by size exclusion chromatography. (10His)SplB protein chromatographed on Biogel P-100 immediately after its purification from Ni-NTA agarose exhibited a single UV-absorbing protein peak with a molecular mass of ≈50 kDa (Fig. 2A). The ≈50-kDa protein crossreacted with anti-(10His)SplB antibodies (data not shown), and presumably represented (10His)SplB monomers. Chromatography of (10His)SplB pretreated with 10 mM DTT/0.1 mM Na2S/0.1 mM Fe(NH4)2(SO4)2 for 24 h at 4°C revealed both the ≈50-kDa peak and a new peak of ≈65–70 kDa (Fig. 2B) that also crossreacted with anti-(10His)SplB antibodies (data not shown). Fractions from the chromatogram depicted in Fig. 2B were assayed for iron, and only the ≈65- to 70-kDa peak contained a significantly higher iron concentration (Fig. 2C). Thus, it appeared that the ≈ 65- to 70-kDa iron-containing peak consisted of (10His)SplB dimers associated through an [Fe-S] cluster. To test this notion, purified (10His)SplB was incubated under conditions favoring [Fe-S] cluster formation, cross-linked with DMS for 30 min, and then injected into the size exclusion column. The DMS-cross-linked (10His)SplB protein sample exhibited a chromatogram essentially identical to that shown in Fig. 2B (data not shown). Fractions corresponding to the ≈65- to 70-kDa peak were pooled, precipitated with ice-cold acetone, and analyzed on a 10% SDS/PAGE gel stained with Coomassie blue. The ≈65- to 70-kDa peak was found to be composed of cross-linked (10His)SplB species of ≈64 kDa and ≈72 kDa, as well as (10His)SplB monomers and some degradation products (data not shown). Taken together, the above experiments indicate that SP lyase consists of a homodimer of SplB subunits and that (10His)SplB dimerization occurs under conditions that favor [Fe-S] center formation.
Figure 2.
Analysis of (10His)SplB by size exclusion chromatography. (10His)SplB was chromatographed through Biogel P-100, immediately after its purification by Ni-NTA chromatography (A) and after incubation under conditions to reconstitute [Fe-S] clusters (B). The y axes (A280) in A and B are scaled identically. (C) Results of colorimetric iron assays (16) performed on fractions from the experiment depicted in B.
SP Lyase Is a [4Fe-4S] Protein.
To further probe the [Fe-S] configuration in SP lyase, (10His)SplB was analyzed by EPR spectroscopy. The EPR spectrum obtained from (10His)SplB purified under aerobic conditions (Fig. 3A) was virtually identical in shape and g-value (g = 2.025) to that of the oxidized form of the [3Fe-4S] center of the E. coli anaerobic RNR activase (16, 22). In contrast, purified (10His)SplB treated on ice for 20 min. with 10 mM sodium dithionite before EPR spectroscopy exhibited a spectrum characteristic of a reduced [4Fe-4S] center, with g∥ = 2.027 and g⊥ = 1.93 (Fig. 3B), in good agreement with the literature values for [4Fe-4S] clusters (16, 22, 23). Conversion of the [3Fe-4S] form to the [4Fe-4S] form was accomplished simply by dithionite treatment without exogenously added iron or sulfur.
Figure 3.
EPR analysis of (10His)SplB. EPR spectra of (10His)SplB isolated under aerobic conditions (protein concentration 6 mg/ml; A); (10His)SplB treated with 10 mM dithionite (protein concentration 12 mg/ml; B); and (10His)SplB treated with 10 mM dithionite and 1 mM S-AdoMet (protein concentration 12 mg/ml; C). In all three traces, relative signal strength (y axes) are depicted on identical scales.
Reversal of SP to two thymines in DNA by SP lyase is absolutely dependent on S-AdoMet (17, 18). A common theme in the reaction pathway of radical SAM enzymes is cleavage of S-AdoMet by electron donation from a [4Fe-4S] cluster to generate methionine and a 5′-adenosyl radical (12, 13–16), which would be predicted to result in a reduction in EPR signal strength. To test this notion, purified (10His)SplB was treated on ice for 20 min with 10 mM dithionite and 1 mM S-AdoMet before EPR spectroscopy. (10His)SplB again exhibited an EPR spectrum typical of a [4Fe-4S] cluster, with the same g∥ and g⊥ values as the protein incubated with only dithionite (Fig. 3B), except that the intensity of the signal was reduced by more than half (Fig. 3C), supporting the hypothesis that the [4Fe-4S] center of SP lyase donates an electron to S-AdoMet during catalysis.
SP Lyase Cleaves S-AdoMet To Generate 5′-Deoxyadenosine (5′-dAdo).
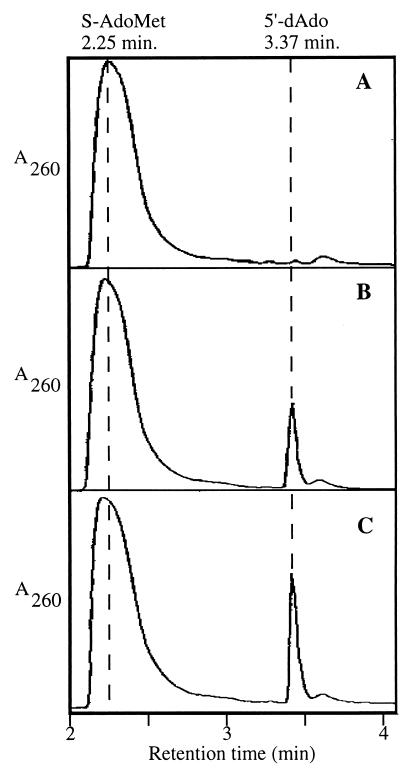
The current catalytic model of radical SAM enzymes (22, 24) predicts the formation of 5′-dAdo as a product of the S-AdoMet cleavage by SP lyase. To detect 5′-dAdo, isocratic reverse-phase HPLC was used. On HPLC, S-AdoMet and 5′-dAdo standards produced peaks with characteristic retention times (RT) of 2.25 min and 3.37 min, respectively (Fig. 4). Other components of the SP lyase reaction mixtures did not absorb significantly at 260 nm (data not shown). HPLC analysis of a control reaction containing all reagents except (10His)SplB protein exhibited only the absorption peak characteristic of S-AdoMet (RT = 2.25 min; Fig. 4A). Addition of (10His)Spl, pretreated to favor [4Fe-4S] formation, to the reaction mixture resulted in appearance of a new absorption peak on HPLC with an RT of 3.37 min, presumably 5′-dAdo (Fig. 4B). HPLC analysis of an SP lyase reaction containing (10His)SplB protein, S-AdoMet, and SP-containing B. subtilis chromosomal DNA exhibited slightly greater conversion of S-AdoMet to the new peak with an RT of 3.37 min (Fig. 4C).
Figure 4.
HPLC analysis of S-AdoMet conversion to 5′-dAdo by (10His)SplB. Standard SP lyase reactions were performed as described in Experimental Procedures and analyzed by HPLC. Reactions contained no (10His)SplB (A); 400 μg (10His)SplB (B); and 400 μg (10His)SplB and 1.63 μg B. subtilis SP-containing chromosomal DNA (C). The vertical dashed lines denote the RT at which purified S-AdoMet and 5′-dAdo elute from the column. In all three traces, the y axes (A260) are depicted on identical scales.
To confirm that the peak observed on HPLC with an RT of 3.37 min was indeed 5′-dAdo, ESI-MS analysis was performed. The peak with an RT of 3.37 min was obtained by HPLC and subjected to positive ESI-MS, resulting in a mass/charge (m/z) ratio of 252.0 (Fig. 5A). When this molecule was fragmented, ESI-MS revealed a predominant fragment with a m/z = 136.3 (Fig. 5B). ESI-MS analysis performed on purified 5′-dAdo (Sigma) also exhibited a strong predominant peak with an m/z = 252.0, and fragmentation of 5′-dAdo revealed a single fragment with an m/z = 136.1 corresponding to adenine (data not shown). [In positive ESI-MS, the amino group on adenine is the only charged group, resulting in only a single peak observed in the fragmentation analysis (Fig. 5).] Thus, it is likely that the identity of the compound comprising the peak in the HPLC experiment with an RT of 3.37 min (Fig. 4) is indeed 5′-dAdo.
Figure 5.
ESI-MS analysis of the peak (RT = 3.37 min) from Fig. 4. Displayed are the primary ESI-MS spectra of the unknown compound (A) and its fragmentation product (B).
Integration of the area under the peaks corresponding to S-AdoMet and 5′-dAdo from the HPLC chromatogram performed in the absence (Fig. 4B) or presence (Fig. 4C) of SP-containing DNA indicated that ≈8% and 10.6%, respectively, of the S-AdoMet present in each reaction was converted to 5′-dAdo. Based on the amount of (10His)SplB protein added to each reaction (400 μg), ≈1.8 and 2.4 molecules of 5′-dAdo were generated per molecule of (10His)SplB dimer (this value assumes that all of the protein present was in the form of a dimer and was capable of splitting S-AdoMet). Similarly, Ollagnier et al. (22) observed that ≈3 S-AdoMet molecules were cleaved per dimer of the activase subunit of E. coli type III RNR.
Discussion
Previous genetic evidence suggested that SP lyase was composed of more than one SplB subunit (12), and the present study provides clear evidence related to the homodimeric structure and assembly of SP lyase. Chemical cross-linking, SDS/PAGE (Fig. 1) and size exclusion chromatography (Fig. 2) revealed that, when (10His)SplB was incubated under anaerobic conditions that favor the formation of [Fe-S] centers, a new species with the apparent eletrophoretic mobility and elution characteristics of [(10His)SplB]2 was formed, supporting the model that SP lyase functions as a homodimeric enzyme and that SplB dimerization occurs concomitant with [Fe-S] center formation. In this respect, SP lyase resembles several other well-characterized radical SAM proteins that have also been demonstrated to dimerize via single [4Fe-4S] clusters, such as KAM, BioB, and the activase subunits of type III RNR and PFL (13–16). This conclusion is supported by previous chemical (17) and atomic absorption analyses (data not shown) that have indicated a stoichiometry of between 1 and 2 iron or sulfur atoms per (10His)SplB subunit.
Analysis of the deduced amino acid sequence of SplB (10, 12) revealed three closely spaced cysteine residues (C91, C95, and C98) within a short region demonstrating a high degree of similarity to proteins belonging to the radical SAM superfamily (11, 25–28). Radical SAM enzymes contain oxygen-labile [4Fe-4S] clusters that readily degrade to an oxidized [3Fe-4S] form; the oxidized and reduced forms generate distinct EPR spectra. UV-visible spectroscopy and chemical analyses suggested that SP lyase also may contain a [4Fe-4S] center (17), and the results obtained from EPR spectral analyses presented here (Fig. 3) are in agreement with this notion. It is interesting to note that conversion of the [3Fe-4S] cluster in SP lyase to a [4Fe-4S] cluster occurred simply by chemical reduction with 10 mM dithionite in the absence of exogenously added iron (Fig. 3), suggesting that the fourth iron atom lost from the cluster by oxidation may remain associated with the enzyme. An analogous EPR spectral shift has also been observed with the anaerobic RNR activase (23). Although there are no published data indicating the oxygen tension inside germinating spores, the presence of an oxygen-labile [4Fe-4S] cluster in SP lyase was originally unexpected, considering the fact that, to detect SP repair in vivo, spores are usually germinated with vigorous aeration (17). B. subtilis has also been shown to produce another radical SAM enzyme, KAM encoded by the yodO gene, which is 62% identical to its homologue found in the strictly anaerobic bacterium Clostridium subterminale; interestingly, the purified B. subtilis KAM appears to be much more aerotolerant than the C. subterminale enzyme (29).
Addition of S-AdoMet to (10His)SplB containing a reduced [4Fe-4S] center decreased its EPR signal intensity by more than one-half (Fig. 3), consistent with a model in which some of the reduced [4Fe-4S] centers donated electrons to S-AdoMet, thus yielding EPR-silent [4Fe-4S] clusters with all of the iron atoms in a Fe3+ state (23, 30). Fig. 3C represents only a single time point in the process of electron loss from the [4Fe-4S] cluster to S-AdoMet. Analogous experiments performed on the RNR activase showed that EPR signal loss over time continues when the enzyme is incubated with S-AdoMet until all [4Fe-4S] clusters are depleted of electrons and no EPR signal is detected (22). When treated under in vitro conditions that lead to repair of SP (17, 18), SP lyase generated from S-AdoMet a new molecule with HPLC elution (Fig. 4) and mass spectral (Fig. 5) characteristics essentially identical to those of of 5′-dAdo. Taken together, the HPLC and ESI-MS data demonstrate that SP lyase generates 5′-dAdo from S-AdoMet, because there are no degradation products or metabolites with similar m/z ratios to the compounds isolated (31).
What is the mechanistic pathway of SP lyase? The radical SAM enzymes characterized to date can be divided into two subgroups. In one group, typified by KAM and BioB, both [4Fe-4S] and catalytic domains are contained on the same dimeric enzyme, and reduction of S-AdoMet generates a 5′-dAdo radical that is used directly in catalysis. A second group, typified by enzymes like anaerobic RNR and PFL, are α2β2 enzymes in which the β2 “activase” subunit dimerizes via a [4Fe-4S] cluster and splits S-AdoMet to generate a 5′-dAdo radical. Radical transfer proceeds from 5′-dAdo to an active-site glycine carried on the α2 subunit within the amino acid sequence RV(C/S)GY (32); notably, this pentapeptide sequence is absent from the SplB amino acid sequence (10). The evidence presented above and in previous communications (12, 17, 18) that SP lyase is an (SplB)2 enzyme tends to place it into the BioB/KAM subgroup, but further studies are needed to support or refute this placement. The evidence to date led us to propose the following model for SP cleavage to two thymines in situ by SP lyase: (i) two subunits of SplB dimerize through the formation of a reduced [4Fe-4S] center to form SP lyase; (ii) the [4Fe-4S] center donates electron(s) to S-AdoMet, splitting it to generate methionine and a 5′-dAdo radical; and (iii) the 5′-dAdo radical is used either directly or indirectly to abstract a proton from C6′ of SP (Fig. 6), which Mehl and Begley (19) have proposed would lead to β-scission of the bond linking the thymines and completion of the cleavage reaction by back transfer of the proton.
In addition to the preceding series of events, SP lyase also specifically recognizes and binds to DNA containing SP (18). Binding of SP lyase to DNA causes significant distortion to the DNA surrounding the SP lesion, possibly because of “flipping out” of the thymine dimer from the interior of the helix (18). Future experiments are directed toward integrating the SP binding and catalytic aspects of the SP lyase reaction into a unified pathway. In particular, the data presented here indicate that SP lyase can cleave S-AdoMet in the absence of SP binding [although S-AdoMet cleavage may be somewhat stimulated in the presence of SP-containing DNA (Fig. 4)]. Conversely, previous experiments indicated that (10His)SplB can specifically bind to SP-containing DNA under conditions unfavorable to [4Fe-4S] center formation (18).
From the results of this communication, SP lyase can now be grouped confidently with an increasing number of radical SAM enzymes (11) that use [Fe-S] centers and S-AdoMet to generate adenosyl radicals to effect catalysis. SP lyase is unique in being the first and only DNA repair enzyme known to function via this novel enzymatic mechanism.
Acknowledgments
We thank Dana Perry, Arnold Raitsimring, Arpad Somogyi, and Ric Gonzalez for generous assistance with analytical techniques used. This research was supported by a grant from the Arizona Agricultural Experiment Station (United States Department of Agriculture-Hatch) to W.L.N.
Abbreviations
- 5′-dAdo
5′-deoxyadenosine
- [Fe-S]
iron-sulfur
- S-AdoMet
S-adenosylmethionine
- SP
spore photoproduct
- RNR
ribonucleotide reductase
- PFL
pyruvate formate-lyase
- KAM
lysine-2,3-aminomutase
- BioB
biotin synthase
- DMS
dimethyl suberimidate
- RT
retention time
- ESI
electrospray ionization
References
- 1.Setlow P. Comments Mol Cell Biophys. 1988;5:253–264. [Google Scholar]
- 2.Setlow P. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson W L, Munakata N, Horneck G, Melosh H J, Setlow P. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/mmbr.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnellan J E, Setlow R B. Science. 1965;149:308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- 5.Varghese A J. Biochem Biophys Res Commun. 1970;38:484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]
- 6.Munakata N. Mol Gen Genet. 1969;104:258–263. doi: 10.1007/BF02539290. [DOI] [PubMed] [Google Scholar]
- 7.Munakata N. Mol Gen Gent. 1977;156:49–54. [Google Scholar]
- 8.Munakata N, Rupert C S. J Bacteriol. 1972;111:192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munakata N, Rupert C S. Mol Gen Genet. 1974;130:239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- 10.Fajardo-Cavazos P, Salazar P, Nicholson W L. J Bacteriol. 1993;175:1735–1744. doi: 10.1128/jb.175.6.1735-1744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofia H J, Chen G, Hetzler B G, Reyes-Spindola J F, Miller N E. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson W L, Chooback L, Fajardo-Cavazos P. Mol Gen Genet. 1997;255:587–594. doi: 10.1007/s004380050532. [DOI] [PubMed] [Google Scholar]
- 13.Külser R, Pils T, Kappl R, Hüttermann J, Knappe J. J Biol Chem. 1998;273:4897–4903. doi: 10.1074/jbc.273.9.4897. [DOI] [PubMed] [Google Scholar]
- 14.Guianvarc'h D, Florentin D, Tse Sum Bui B, Nunzi F, Marquet A. Biochem Biophys Res Commun. 1997;236:402–406. doi: 10.1006/bbrc.1997.6952. [DOI] [PubMed] [Google Scholar]
- 15.Lieder K W, Booker S, Ruzicka F J, Beinert H, Reed G H, Frey P A. Biochemistry. 1998;37:2578–2585. doi: 10.1021/bi972417w. [DOI] [PubMed] [Google Scholar]
- 16.Ollagnier S, Mulliez E, Gaillard J, Eliasson R, Fontecave M, Reichard P. J Biol Chem. 1996;271:9410–9416. doi: 10.1074/jbc.271.16.9410. [DOI] [PubMed] [Google Scholar]
- 17.Rebeil R, Sun Y, Chooback L, Pedraza-Reyes M, Kinsland C, Begley T P, Nicholson W L. J Bacteriol. 1998;180:4879–4885. doi: 10.1128/jb.180.18.4879-4885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slieman T, Rebeil R, Nicholson W L. J Bacteriol. 2000;182:6412–6417. doi: 10.1128/jb.182.22.6412-6417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehl R A, Begley T P. Org Lett. 1999;1:1065–1066. doi: 10.1021/ol9908676. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 21.Hildebrand E L, Grossman L. J Biol Chem. 1999;274:27885–27890. doi: 10.1074/jbc.274.39.27885. [DOI] [PubMed] [Google Scholar]
- 22.Ollagnier S, Mulliez E, Schmidt T T, Eliasson R, Gaillard J, Deronzier C, Bergman T, Graslund A, Reichard P, Fontecave M. J Biol Chem. 1997;272:24216–24223. doi: 10.1074/jbc.272.39.24216. [DOI] [PubMed] [Google Scholar]
- 23.Liu A, Graslund A. J Biol Chem. 2000;275:12367–12373. doi: 10.1074/jbc.275.17.12367. [DOI] [PubMed] [Google Scholar]
- 24.Wong K K, Murray B W, Lewisch S A, Baxter M K, Ridky T W, Ulissi-DeMario L, Kozarich J W. Biochemistry. 1993;32:14102–14110. doi: 10.1021/bi00214a005. [DOI] [PubMed] [Google Scholar]
- 25.Tamarit J, Gerez C, Meier C, Mulliez E, Trautwein A, Fontecave M. J Biol Chem. 2000;275:15669–15675. doi: 10.1074/jbc.275.21.15669. [DOI] [PubMed] [Google Scholar]
- 26.Broderick J B, Henshaw T F, Cheek J, Wojtuszewski K, Smith S R, Trojan M R, McGhan R M, Kopf A, Kibbey M, Broderick W E. Biochem Biophys Res Comm. 2000;269:451–456. doi: 10.1006/bbrc.2000.2313. [DOI] [PubMed] [Google Scholar]
- 27.Hewitson K S, Baldwin J E, Shaw N M, Roach P L. FEBS Lett. 2000;466:372–376. doi: 10.1016/s0014-5793(00)01101-7. [DOI] [PubMed] [Google Scholar]
- 28.Petrovich R M, Ruzicka F J, Reed G H, Frey P A. Biochemistry. 1992;31:10774–10781. doi: 10.1021/bi00159a019. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Ruzicka F J, Frey P A. Biochem J. 2000;348:539–549. [PMC free article] [PubMed] [Google Scholar]
- 30.Beinert H, Sands R H. In: Foundations of Modern EPR. Eaton S S, Salikhov K M, editors. River Edge, NJ: World Scientific; 1998. pp. 396–397. [Google Scholar]
- 31.Matos J R, Wong C H. Bioorg Chem. 1986;15:71–80. [Google Scholar]
- 32.Sun X Y, Ollagnier S, Schmidt P P, Atta M, Mulliez E, Lepape L, Eliasson R, Graslund A, Fontecave M, Reichard P, Sjoberg B M. J Biol Chem. 1996;271:6827–6831. [PubMed] [Google Scholar]