ABSTRACT
Despite remarkable advances in diagnosis, prognosis and treatment, advanced or recurrent breast tumors have limited therapeutic approaches. Many treatment strategies try to explore the limitations of DNA damage response (DDR) in tumor cells to selectively eliminate them. BRCT (BRCA1 C-terminal) domains are present in a superfamily of proteins involved in cell cycle checkpoints and the DDR. Tandem BRCT domains (tBRCT) represent a distinct class of these domains. We investigated the expression profile of 7 tBRCT genes (BARD1, BRCA1, LIG4, ECT2, MDC1, PAXIP1/PTIP and TP53BP1) in breast cancer specimens and observed a high correlation between PAXIP1 and TP53BP1 gene expression in tumor samples. Tumors with worse prognosis (tumor grade 3 and triple negative) showed reduced expression of tBRCT genes, notably, PAXIP1 and TP53BP1. Survival analyses data indicated that tumor status of both genes may impact prognosis. PAXIP1 and 53BP1 protein levels followed gene expression results, i.e., are intrinsically correlated, and also reduced in more advanced tumors. Evaluation of both genes in triple negative breast tumor samples which were characterized for their BRCA1 status showed that PAXIP1 is overexpressed in BRCA1 mutant tumors. Taken together our findings indicate that PAXIP1 status correlates with breast cancer staging, in a manner similar to what has been characterized for TP53BP1.
KEYWORDS: BRCT, breast cancer, DNA repair, PAXIP1, TP53BP1
Introduction
Among women, breast cancer is the most commonly diagnosed cancer after non-melanoma skin cancer, and the leading cause of cancer deaths.1 Breast cancer is a heterogeneous disease that varies widely in treatment response, presentation and biology.2 Efforts in establishing gene expression patterns of these tumors have had a considerable impact in the understanding of breast cancer biology, leading to more precise classification, prognosis and treatment.3-7 However, the biologic heterogeneity of tumors remains a challenge, especially for triple negative (TN) breast tumors, which present the poorest prognosis among different subtypes and have no standard targeted therapies.8-11 Efficient prognosis and treatment must consider specific molecular and cellular features of the disease, thus, understanding critical cellular pathways in tumor biology is of great importance. Exploring DNA damage response (DDR) pathways has been a promising strategy in the development of specific anti-tumoral agents; a remarkable example is the sensitivity of BRCA1/2 deficient cells to Poly (ADP ribose) Polymerase (PARP) inhibitors.12,13
To gain further insight into the determinants of the DDR pathway signal transduction, our group generated a human protein-protein interaction network (PIN) centered on interactions mediated by the BRCT domain.14 This domain was initially recognized in the C-terminal region of BRCA1, a protein encoded by the major breast and ovarian susceptibility gene, and endowed with multiple roles in DDR.15 The BRCT PIN encloses a subnetwork centered in 7 BRCT tandem domain (tBRCT) containing proteins (BARD1, BRCA1, ECT2, LIG4, MDC1, PAXIP1/PTIP and 53BP1). The tBRCT containing proteins are mainly associated with DDR and cell cycle regulation pathways.14
PAXIP1 (also known as PTIP) and 53BP1 act in DNA double strand breaks (DSB) promoting non-homologous end-joining (NHEJ) repair. 53BP1 prevents BRCA1 and CtIP recruitment to DSB sites, and PAXIP1 interacts with 53BP1, being required for 53BP1-mediated inhibition of homologous recombination (HR).16,17
In the present study, we sought to analyze the expression patterns of these tBRCT coding genes in a panel of breast cancer specimens. We observed that tumors with worst prognosis showed a clear trend for lower expression levels, notably for PAXIP1 and TP53BP1. Immunohistochemistry data corroborated the findings for PAXIP1 and 53BP1.
Results
Histopathological characteristics of a tumor group (N = 154) obtained from a cohort from the Brazilian National Cancer Institute (INCA) used in this study are presented in Table 1. The predominant histological type was invasive ductal carcinoma (87.7%), with most tumors at early stages (TNM 0, I or II: 77.9%), although 57.8% of samples represented poorly differentiated tumors (tumor grade 3). Regarding tumor subtypes, most samples were positive for ER (estrogen receptor) and/or PR (progesterone receptor), and negative for HER2, with a consequent predominance of the luminal subtypes (77.3%).
Table 1.
Histopathological characteristics and classification of breast tumor specimens (INCA, N = 154).
| Histopathological characteristics | N | Tumor classifications | N | ||
|---|---|---|---|---|---|
| Histological type | 154 | % | TNM classification | 154 | % |
| ductal in situ | 3 | 1.9 | 0 | 3 | 1.9 |
| ductal invasive | 135 | 87.7 | I | 28 | 18.2 |
| lobular in situ | 0 | 0.0 | II | 89 | 57.8 |
| lobular invasive | 7 | 4.5 | III | 34 | 22.1 |
| mixed | 2 | 1.3 | |||
| other | 7 | 4.5 | Tumor subtypes | 154 | % |
| luminal A | 76 | 49.4 | |||
| Tumor grade | 154 | % | luminal B | 38 | 24.7 |
| grade 1 | 10 | 6.5 | luminala | 5 | 3.2 |
| grade 2 | 54 | 35.1 | HER2 like | 13 | 8.4 |
| grade 3 | 89 | 57.8 | triple negative | 20 | 13.0 |
| missing | 1 | 0.6 | Missing | 2 | 1.3 |
| Tumor size | 154 | % | |||
| pTis | 3 | 1.9 | |||
| pT1 | 48 | 31.2 | |||
| pT2 | 100 | 64.9 | |||
| pT3 | 3 | 1.9 | |||
| pT4 | 0 | 0.0 | |||
| Lymph node status | 154 | % | |||
| pN0 | 73 | 47.4 | |||
| pN1 | 47 | 30.5 | |||
| pN2 | 23 | 14.9 | |||
| pN3 | 11 | 7.1 | |||
| Estrogen receptor | 154 | % | |||
| negative | 34 | 22.1 | |||
| positive | 119 | 77.3 | |||
| missing | 1 | 0.6 | |||
| Progesterone receptor | 154 | % | |||
| negative | 58 | 37.7 | |||
| positive | 95 | 61.7 | |||
| missing | 1 | 0.6 | |||
| HER2 | 154 | % | |||
| negative | 113 | 73.4 | |||
| positive | 34 | 22.1 | |||
| missing | 7 | 4.5 |
luminal category refers to samples which were positive for both ER and PR but which HER2 status was not assessed.
tBRCT genes expression and histopathological features
Breast tumor samples from INCA were evaluated for tBRCT genes expression (BRCA1, BARD1, LIG4, MDC1, ECT2, TP53BP1 and PAXIP1), relative quantification (RQ) values ranged between 0.018 and 22.9 (Fig. 1A), with ECT2, BRCA1 and LIG4 showing the highest medians (2.34, 2.05 and 1.23, respectively). Using Spearman's correlation matrix, we assessed putative associations between pairs of genes expression levels (Fig. 1B). PAXIP1 and TP53BP1 genes had the highest correlation value (ρ = 0.735, P < 0.001), which is depicted in Fig. 1C.
Figure 1.
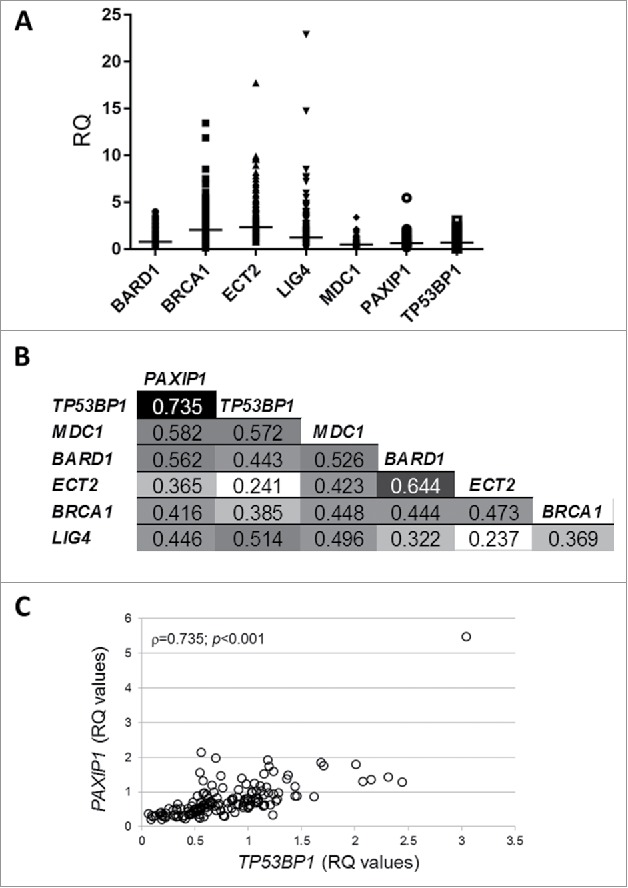
tBRCT genes expression from INCA cohort (N = 154). (A) Relative expression of the tBRCT genes in tumor specimens. mRNA relative quantification was measured by Taqman® real-time PCR using GAPDH and PPIA as housekeeping genes and a commercial normal breast tissue RNA as reference sample. The median expression is shown as a bar for each gene. (B) Spearman's correlation matrix for the tBRCT genes expression in the breast cancer specimens. The ρ values (Spearman's correlation coefficients) are indicated and a gray color scale designates the highest (black) and the lowest (white) values. (C) Correlation graph of PAXIP1 and TP53BP1 RQ values in the tumor specimens analyzed. Spearman's correlation ρ and P values are shown. RQ: relative quantification.
Table 2 shows samples median expression levels according to their histopathological features, which were dichotomized as better or worse prognosis. TNM classification was not included in the analysis because sample stratification as consequence of dichotomized TNM (0, I and II versus III) and of dichotomized lymph node status category were the same - node status is a component of TNM classification and this study does not include metastatic patients.
Table 2.
Distribution of breast tumors histopathological features and gene expression medians (INCA, N = 154).
|
BARD1 |
BRCA1 |
ECT2 |
LIG4 |
MDC1 |
PAXIP1 |
TP53BP1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (154) | Median | P | Median | P | Median | P | Median | P | Median | P | Median | P | Median | P | |
| Tumor grade | |||||||||||||||
| 1, 2 | 64 | 0.896 | 0.263 | 1.898 | 0.872 | 2.175 | 0.490 | 1.654 | 0.002 | 0.604 | 0.002 | 0.810 | <0.0001 | 0.996 | <0.0001 |
| 3 | 89 | 0.772 | 2.061 | 2.348 | 1.005 | 0.453 | 0.511 | 0.547 | |||||||
| Tumor size | |||||||||||||||
| pT ≤ 2 cm | 51 | 0.806 | 0.884 | 1.923 | 0.601 | 2.267 | 0.887 | 1.517 | 0.206 | 0.477 | 0.826 | 0.698 | 0.112 | 0.947 | 0.001 |
| pT > 2 cm | 103 | 0.779 | 2.162 | 2.348 | 1.183 | 0.486 | 0.587 | 0.612 | |||||||
| Lymph node status | |||||||||||||||
| pN0, pN1 | 120 | 0.812 | 0.288 | 2.107 | 0.775 | 2.477 | 0.087 | 1.368 | 0.042 | 0.488 | 0.131 | 0.661 | 0.829 | 0.692 | 0.991 |
| pN2, pN3 | 34 | 0.711 | 2.003 | 2.173 | 0.898 | 0.474 | 0.618 | 0.669 | |||||||
| ER status | |||||||||||||||
| Positive | 119 | 0.831 | 0.078 | 2.187 | 0.057 | 2.450 | 0.218 | 1.438 | 0.139 | 0.509 | 0.069 | 0.690 | <0.0001 | 0.851 | <0.0001 |
| Negative | 34 | 0.711 | 1.927 | 2.235 | 1.173 | 0.402 | 0.432 | 0.476 | |||||||
| PR status | |||||||||||||||
| Positive | 95 | 0.917 | 0.013 | 2.134 | 0.122 | 2.376 | 0.870 | 1.517 | 0.016 | 0.499 | 0.279 | 0.737 | <0.0001 | 0.874 | <0.0001 |
| Negative | 58 | 0.706 | 2.004 | 2.324 | 1.169 | 0.469 | 0.482 | 0.520 | |||||||
| HER2 status | |||||||||||||||
| Negative | 113 | 0.825 | 0.354 | 2.253 | 0.020 | 2.348 | 0.422 | 1.383 | 0.083 | 0.486 | 0.221 | 0.690 | <0.0001 | 0.809 | 0.0003 |
| Positive | 34 | 0.715 | 1.825 | 2.351 | 1.169 | 0.453 | 0.474 | 0.521 | |||||||
| Tumor subtypes | |||||||||||||||
| Luminal | 114 | 0.831 | 0.042 | 2.187 | 0.058 | 2.450 | 0.140 | 1.438 | 0.099 | 0.509 | 0.047 | 0.690 | <0.0001 | 0.852 | <0.0001 |
| HER2 like, TN | 33 | 0.709 | 1.923 | 2.218 | 1.168 | 0.389 | 0.429 | 0.469 | |||||||
| Tumor subtypes (exp.) | |||||||||||||||
| Luminal | 114 | 0.835 | — | 2.181 | — | 2.455 | — | 1.446 | — | 0.512 | — | 0.691 | — | 0.847 | — |
| HER2 like | 13 | 0.713 | ns | 1.923 | ns | 2.252 | ns | 1.168 | ns | 0.329 | ns | 0.393 | <0.001a | 0.439 | <0.001a |
| TN | 20 | 0.703 | ns | 1.921 | ns | 2.067 | ns | 0.976 | ns | 0.441 | ns | 0.469 | <0.001a | 0.476 | <0.0001a |
Mann-Whitney's U test P values are shown. The first line of each feature represents better prognosis category. ER: estrogen receptor, PR: progesterone receptor, exp: expanded, ns: not significant.
ANOVA in comparison with “luminal” category.
Our data show that tBRCT genes expression is reduced in histopathological categories of worse prognostic value in comparison with better prognostic categories, except for BRCA1 and ECT2 regarding tumor grade and size, and HER2 status for ECT2 (Table 2). However, the total of statistically significant associations observed showed a reduction of gene expression in worst prognosis characteristics. Reduced expression of BARD1 was significantly associated with negative PR status and with tumor subtypes HER2 like and TN, both of worse prognosis. BRCA1 expression was significantly lower in HER2-positive than in HER2-negative tumors and LIG4 was reduced in high grade tumors, worse lymph node status and negative for PR. MDC1 exhibited lower expression in high grade tumors and in worse prognosis tumor subtypes. ECT2 was the only gene that presented no statistical difference in expression levels according to histopathological features. PAXIP1 and TP53BP1 presented the most statistically significant differences for all characteristics, except for lymph node status.
As shown in Table 2, when tumor subtypes are stratified in 3 categories (“tumor subtypes expanded”), the significant association observed between reduced RQ values of PAXIP1 and TP53BP1 and worst prognosis tumor subtypes was preserved: HER2 like vs. luminal and TN vs. luminal tumors.
In a multivariate analysis, only MDC1, TP53BP1 and PAXIP1 remained significantly associated with histopathological features (Table 3). MDC1 and TP53BP1 were significantly associated with tumor grade, but only TP53BP1 remained associated with ER status. The 3 genes kept their association with tumor subtype, however, only TP53BP1 and PAXIP1 maintained the orientation of their associations, i.e., lower expression associated with worse prognosis. For MDC1, there was an apparent inversion, i.e., a tendency of higher expression associated with worse prognosis. The lack of an independent effect in multivariate models for PAXIP1 for tumor grade and ER status was probably due to its strong correlation with TP53BP1 (as seen in Fig. 1B and C). Indeed, when conducting the multivariate analysis without TP53BP1, a significant association with tumor grade, ER and HER 2 status was observed for PAXIP1 and its association with tumor subtype was enhanced (Table 3).
Table 3.
Multivariate model analysis (INCA, N = 154).
|
MDC1 |
TP53BP1 |
PAXIP1 |
||||||
|---|---|---|---|---|---|---|---|---|
| Logistic regression (all genes) | R2 | Coef. | P | Coef. | P | Coef. | P | |
| Tumor grade | ||||||||
| 1, 2 | 0.407 | 2.527 | 0.002 | −3.155 | <0.0001 | −1.139 | 0.08 | |
| 3 | ||||||||
| ER status | ||||||||
| Positive | 0.258 | — | — | −2.291 | 0.01 | −1.341 | 0.1 | |
| Negative | ||||||||
| Tumor subtypes | ||||||||
| Luminal | 0.347 | 2.210 | 0.003 | −2.836 | 0.005 | −3.0341 | 0.0372 | |
| HER2 like, triple negative | ||||||||
|
MDC1 |
PAXIP1 |
|||||||
| Logistic regression (excluding TP53BP1) |
R2 |
Coef. |
P |
|
|
Coef. |
P |
|
| Tumor grade | ||||||||
| 1, 2 | 0.273 | 1.550 | 0.02 | −2.528 | 0.0001 | |||
| 3 | ||||||||
| ER status | ||||||||
| Positive | 0.258 | — | — | −3.227 | 0.0003 | |||
| Negative | ||||||||
| HER2 status | ||||||||
| Negative | 0.165 | — | — | −2.148 | 0.007 | |||
| Positive | ||||||||
| Tumor subtypes | ||||||||
| Luminal | 0.274 | 2.080 | 0.003 | −5.392 | 0.0002 | |||
| HER2 like, triple negative | ||||||||
Only genes which had significant results are shown. Logistic regression (P < 0.05) was made for the statistically different results on Mann-Whitney's U test (univariate analysis) regarding the distribution of breast tumors histopathological features and gene expression.
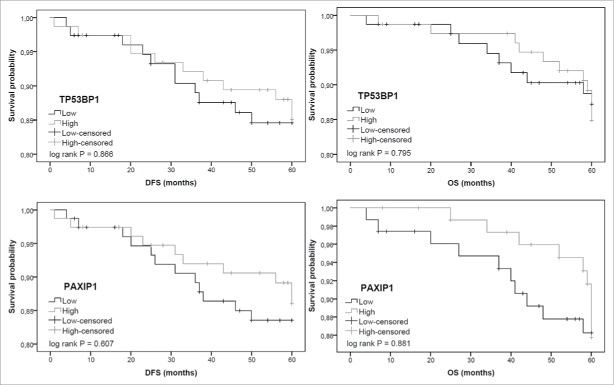
Survival analyses
For the most significant altered genes regarding histopathological features associations, TP53BP1 and PAXIP1, we evaluated the impact of their expression on patient prognosis. We estimated survival curves for 5-year DFS (disease-free survival) and OS (overall survival) regarding TP53BP1 and PAXIP1 for the INCA cohort. Gene expression status was divided between high and low according to their median values. For both outcome measures, tumors with lower expression values either for TP53BP1 or PAXIP1 appear to favor worst prognosis, although there was no significant association (Fig. 2).
Figure 2.
Disease-free (DFS) and overall (OS) survival curves in breast cancer INCA cohort (N = 154) according to TP53BP1 and PAXIP1 genes expression status (low x high). Gene expression status was divided according to their median values.
To compare our data with major breast cancer data sets, we assessed survival curves using a publically available gene expression array database (GEO, www.ncbi.nlm.nih.gov/geo). Both DFS and OS outcomes are in accordance to those from our cohort, DFS curves showed significant differences (TP53BP1: P < 0.0001; PAXIP1: P = 0.0022) (Fig. S1). We also estimated the impact of both genes expression in OS using TCGA breast cancer data set, results also supported our data; OS regarding PAXIP1 showed significantly reduced survival associated to low expression levels (P = 0.01).
These results suggest that both TP53BP1 and PAXIP1 tumor status may impact on prognosis, with lower gene expression values indicating a worst prognosis scenario. Similar results for TP53BP1 were already reported by Bouwman et al.18
53BP1 and PAXIP1 immunohistochemistry (IHC) evaluation
To find out if PAXIP1 and TP53BP1 tumor gene expression could reflect protein profile, we decided to conduct IHC analyses in a subset of tumor samples from INCA. Specimens with the highest or lowest RQ values (20 each) for both genes were chosen for IHC evaluation (a complete sample description is listed in Table S1). All specimens showed nuclear staining for both proteins in tumor and non-tumor areas, differences were observed only in intensity, depicted higher in non-neoplastic cells, as expected.
53BP1 staining was exclusively nuclear (Fig. 3B, D and F), whereas some PAXIP1 samples showed a marked cytoplasmic staining (Fig. 3A and C). Nuclear staining in tumor areas for both proteins exhibited a predominantly homogeneous pattern, which was characterized as “strong” (Fig. 3C and D) or “weak” (Fig. 3E and F), in comparison with the positive control sample used as a reference of strong immunostaining reaction (Fig. 3A and B).
Figure 3.
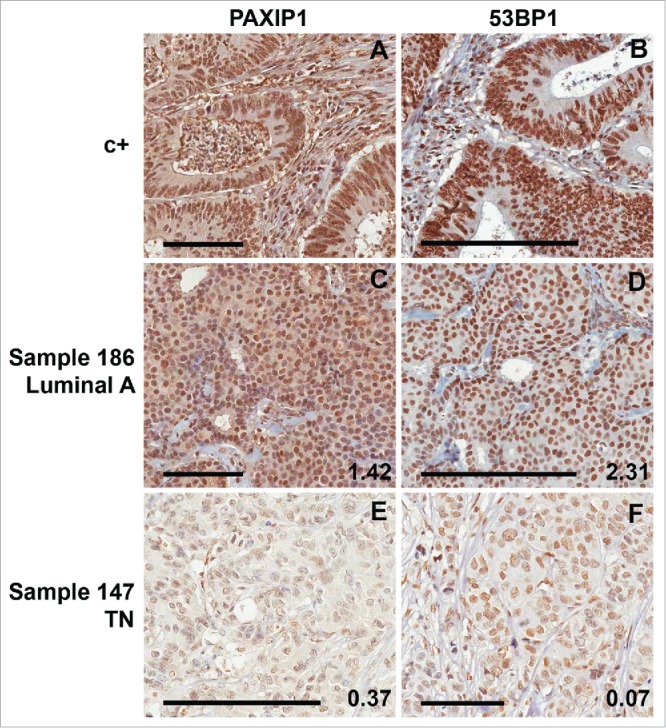
Immunohistochemistry for PAXIP1 and 53BP1. Samples from INCA cohort (N = 40). Representative slides of tumor samples for PAXIP1 and 53BP1 staining. A human colon adenocarcinoma sample was used as a positive control (c+) for immunostaining (A) for PAXIP1 and (B) 53BP1. Representative sample (#186, a luminal A tumor) of strong immunostaining score for either (C) PAXIP1 and (D) 53BP1. Representative sample (#147, a TN tumor) of weak immunostaining score for either (E) PAXIP1 and (F) 53BP1. The samples' RQ values for PAXIP1 and TP53BP1 genes are depicted in their respective slides. Scale bars: 100 μm (small) and 200 μm (large).
Table 4 shows the distribution of samples considering their IHC score (weak/strong) according to gene expression levels (low/high) and histopathological features. Strong IHC staining of PAXIP1 and 53BP1 were significantly associated with respective high RQ values. Interestingly, the IHC staining intensity of 53BP1 and PAXIP1 was significantly associated, i.e., samples with strong staining intensity for 53BP1 also tended to a strong staining for PAXIP1, in accordance with the observed correlation between their RQ values (Fig. 1C). In addition, samples with strong staining for PAXIP1 and 53BP1 were also associated with low tumor grades (1 and 2) and better prognosis tumor subtypes (luminal types).
Table 4.
Distribution of immunostained samples for PAXIP1 and 53BP1 according to their scores (weak/strong), RQ values (low/high) and histopathological features (INCA, N = 40).
| IHC score | high RQ value | low RQ value | OR | 95% CI | low grades (1, 2) | high grade (3) | OR | 95% CI | ER+ | ER- | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAXIP1 | strong | 12 | 2 | 13.1 | 2.4–74.8 | 11 | 3 | 15.4 | 3.09–76.8 | 14 | 0 | 17.5 | 2–152.6 |
| weak | 8 | 18 | 5 | 21 | 12 | 14 | |||||||
| 53BP1 | strong | 20 | 7 | 40 | 4.4–362 | 16 | 11 | 20.3 | 2.3–178.2 | 22 | 5 | 9.9 | 2.1–45.5 |
| |
weak |
0 |
13 |
|
|
0 |
13 |
|
|
4 |
9 |
|
|
| |
|
PR+ |
PR- |
OR |
95% CI |
HER2- |
HER2+ |
OR |
95% CI |
Luminal |
HER2 like, TN |
OR |
95% CI |
| PAXIP1 | strong | 14 | 0 | 50 | 4.4–460 | 12 | 1 | 5.3 | 0.5–48.3 | 14 | 0 | 17.5 | 2–153 |
| weak | 6 | 20 | 18 | 8 | 12 | 14 | |||||||
| 53BP1 | strong | 18 | 9 | 11 | 1.9–60.6 | 22 | 5 | 2.7 | 0.6–12 | 21 | 6 | 7.8 | 1.8–34.8 |
| weak | 2 | 11 | 8 | 5 | 4 | 9 | |||||||
Taken together, our results show that PAXIP1 and 53BP1 correlate at protein and gene expression levels in breast cancer specimens, being reduced in more aggressive phenotypes.
PAXIP1 and TP53BP1 gene expression in TN tumors
In view of the strong correlation observed between PAXIP1 and TP53BP1 expression levels and the association between low expression and worse prognosis in breast tumors specimens, a second set of breast tumor specimens obtained from A. C. Camargo Cancer Center (ACCCC group) was evaluated. Histopathological characteristics of ACCCC breast tumors (N = 28) are presented in Table 5. ACCCC samples included only TN tumors, which were also characterized for the presence of pathogenic mutations in BRCA1. Fig. 4 shows the expression levels of PAXIP1 and TP53BP1 in ACCCC samples. In comparison with TN tumors from INCA there is no statistically significant difference between tumor groups (Fig. 4A). We also evaluated PAXIP1 and TP53BP1 expression levels according to BRCA1 mutation status (Fig. 4B). PAXIP1 showed significantly higher values in BRCA1 mutated tumors. TP53BP1 expression was not significantly affected, although levels were lower in BRCA1 mutated TN tumors.
Table 5.
Histopathological characteristics and classification of TN breast tumor specimens (ACCCC, N = 28).
| Histopathological characteristics | N | ||
|---|---|---|---|
| Histological type | 28 | % | |
| ductal invasive | 22 | 78.6 | |
| metaplastic | 3 | 10.7 | |
| medular | 2 | 7.1 | |
| lobular invasive | 1 | 3.6 | |
| Tumor grade | 28 | % | |
| grade 1 | 0 | 0.0 | |
| grade 2 | 1 | 3.6 | |
| grade 3 | 25 | 89.3 | |
| missing | 2 | 7.1 | |
| Lymph node status | 28 | % | |
| negative | 12 | 42.9 | |
| positive | 15 | 53.6 | |
| missing | 1 | 3.6 | |
| BRCA1 status | 28 | % | |
| pathogenic mutation | 7 | 25.0 | |
| wild type | 21 | 75.0 | |
|
BRCA1 mutations |
Classification |
|
|
| 1 | c.181T>G; p.C61G | Pathogenic | |
| 2 | c.3331_3334delCAAG | Pathogenic | |
| 3 | c.1A>G; p.M1V | Pathogenic | |
| 4 | c.2405delTG; p. V801fs | Pathogenic | |
| 5 | c.4183C>T; p.G1395X | Pathogenic | |
| 6 | c.4408G>T; p.R1495M | Pathogenic | |
| 7 | c.1123delC; p.L375fs/c.1124T>A; p.L375G | Pathogenic | |
Figure 4.

Relative expression of the tBRCT genes PAXIP1 and TP53BP1 in TN tumor specimens analyzed from 2 independent cohorts. mRNA relative quantification was evaluated by Taqman® real-time PCR using GAPDH and PPIA as housekeeping genes and a commercial normal breast tissue RNA as reference sample. Tukey box-and-whiskers plot. (A) Relative quantification of tumor samples from INCA set (N = 20) and from A. C. Camargo Cancer Center set (ACCCC, N = 28). (B) Relative quantification of tumor samples from ACCCC. Samples were distributed by their BRCA1 mutation status. Mann-Whitney U test: ***P < 0.0001). RQ: relative quantification.
These data suggest that PAXIP1 is not only associated with histopathological features of breast cancer, but also associated with an intrinsic molecular characteristic: BRCA1 status.
Discussion
In attempt to characterize the involvement of tBRCT genes in breast cancer biology, we evaluated gene expression in a set of breast cancer specimens and correlated these values with histopathological features. Our data show that most tBRCT genes (BARD1, LIG4, MDC1, PAXIP1 and TP53BP1) have lower expression in breast tumors with worse prognosis when compared with those with better prognosis (Table 2), which is consistent with their roles in the maintenance of genome integrity.14
In our study, BRCA1 was the only tBRCT gene associated with HER2 status, showing lower gene expression values in HER2 positive tumors (Table 2). Consistently with our findings, BRCA1 loss (evaluated by IHC) has been associated with higher levels of HER2 in sporadic breast tumors.19,20
Curiously, MDC1 downregulation showed univariate association with high tumor grade, and also with HER2-like and TN subtypes (Table 2); however, in a multivariate analysis, such associations were apparently inverted, with higher MDC1 expression in the categories of worse prognosis (Table 3). Indeed, MDC1 role in tumor progression is still contradictory: it has been associated with more aggressive phenotypes of cervical and ovarian cancer,21,22 but it has been reported to be downregulated in breast tumor in comparison with benign hyperplasia.23
An important finding of this study is the correlation observed between PAXIP1 and TP53BP1 expression (Fig. 1B and C), and their downregulation association with worse prognosis (Table 2, Fig. 2). The role of 53BP1 in cancer biology as a tumor suppressor is consolidated; its increase in expression level inhibits invasion and metastasis in breast cancer,24 also, it was shown to inhibit growth and promote apoptosis in ovarian cancer.25 Likewise, Li et al.24 have demonstrated that protein levels of 53BP1 decrease as breast cancer progresses. In consonance with our study, Bouwman et al.18 demonstrated an association between low levels of TP53BP1 and TN breast tumors.
This is the first study to evaluate PAXIP1 expression in breast cancer. In 2016, a meta-analysis study described PAXIP1 downregulation associated with poor prognosis in ovarian cancer.26 PAXIP1 protein levels have been also evaluated in lung cancer specimens, and it was observed that a third of tumors were positive for its staining.27 PAXIP1 is known to play a role in transcriptional regulation through its association with MLL2/MLL3 histone methyltransferase complexes, and also involved in DNA repair.28-30 Callen et al.17 showed that ablation of PAXIP1 provides sustained DNA resection required for HR repair rescuing in BRCA1-deficient cells, indicating that PAXIP1 takes part in inhibiting HR repair, promoting NHEJ, similarly to what is observed for 53BP1.18,31 These observations show that PAXIP1 and 53BP1 play linked roles in DNA repair, supporting our findings that both PAXIP1 and TP53BP1 are correlated and associated with breast cancer prognosis (Tables 2 and 3). Loss of both genes have been demonstrated to contribute to resistance to HR-toxic DNA damaging agents (like PARP inhibitors and platinum compounds) in BRCA1-deficient tumor cells, by restoration of HR.16–18 It would be reasonable to infer that TN breast tumors expressing low levels of these genes could be resistant to HR-toxic therapies due to these observations.
Interestingly, in multivariate analysis, statistical significance of PAXIP1 associations with most categories were lost, whereas TP53BP1 maintained its association with tumor grade, ER status and tumor subtype (Table 3). Nevertheless, when TP53BP1 data are excluded of the multivariate model, PAXIP1 shows significant association with histopathological categories tumor grade, ER and HER2 status; also, an increase in its association with tumor subtype is observed (Table 3). These results suggest that PAXIP1 expression might be dependent on TP53BP1.
We also observed that gene expression of both PAXIP1 and TP53BP1 were associated with their correspondent protein levels, as evaluated by IHC staining (Fig. 3, Table 4). Thus, reduced protein levels of both PAXIP1 and 53BP1 were associated with high grade tumors and with HER2-like and TN subtypes, supporting the idea that loss of function of these genes may favor breast cancer progression and worse prognosis.
All tBRCT genes, except ECT2, were associated with histopathological features, but the multivariate model showed that their associations were not independent from each other, except for MDC1, TP53BP1 and PAXIP1 (Table 3). Thus, tBRCT genes behavior in breast cancer appears to be related to which other, with MDC1, TP53BP1 and PAXIP1 playing dominant roles. Actually, MDC1, 53BP1 and PAXIP1 are early mediators of DDR (reviewed in ref.16) and this could explain their observed notable impact on breast cancer prognosis.
We assessed the gene expression levels of PAXIP1 and TP53BP1 in a second population of breast cancer specimens (ACCCC), all of which were TN tumors tested for BRCA1 mutation status. PAXIP1 and TP53BP1 expression levels were similar to TN samples from INCA group (Fig. 4A), which are reduced when compared with luminal samples. Gene expression of PAXIP1, but not TP53BP1, was significantly higher in TN BRCA1 mutated samples than in BRCA1 wild type specimens; PAXIP1 levels in BRCA1 mutated samples were similar to the observed in normal breast reference sample (Fig. 4B). Thus, although PAXIP1 expression is diminished in TN breast cancer in general, its levels seem to vary according to BRCA1 status in these tumors. BRCA1 mutated cells rely on poor fidelity repair pathways (like the NHEJ) to repair DSB, as HR is lost in these cells due to loss of BRCA1 functions.13 This fact could explain higher levels of PAXIP1 expression in TN BRCA1 mutated tumors. It has been demonstrated that 53BP1 may promote NHEJ through 2 distinct pathways, one that would require PAXIP1 to inhibit HR and process DSB by NHEJ and another dependent on RIF1, which would be essential for immunoglobulin class switch recombination.17 This distinct aspect of PAXIP1 in NHEJ could explain the alteration of PAXIP1 levels in BRCA1 mutated tumors but not of TP53BP1 levels.
Jhuraney et al.27 showed that PAXIP1 status is involved in sensitizing lung cancer cells to a WEE1 kinase inhibitor in combination with platinum based chemotherapy. Therefore, it was proposed that PAXIP1 protein levels, in conjunction with WEE1, could be used as biomarkers of response to WEE1 inhibitor in combination with DNA damaging agents. These data support the idea that PAXIP1 is indeed associated with critical pathways in tumor biology and can be explored as a biomarker for tumor prognosis and treatment.
In summary, our data demonstrate that the tBRCT genes are associated with breast cancer prognosis, especially PAXIP1 and TP53BP1. Also, these data indicate that PAXIP1 is associated with breast cancer staging, in a similar manner to what has been characterized for TP53BP1.
These findings contribute to clarify the role of tBRCT genes in tumor biology, and provide supporting information that may help prognostic evaluation and therapy assessment.
Patients and methods
Patients and tumor samples
INCA tumor samples consisted of 154 sporadic breast tumor specimens collected prospectively, between February 2009 to April 2011, from patients of the Brazilian National Cancer Institute (INCA, Rio de Janeiro, Brazil). Inclusion criteria were: female patients with first diagnosis of unilateral non-metastatic breast cancer and assigned for tumor resection as first therapeutic approach. Tumors were resected during surgery and freshly stored at -80°C. The study protocol was approved by the Brazilian National Cancer Institute Research Ethics Committee (#129/08), and all patients gave written consent to participate.
ACCCC tumor samples consisted of 28 specimens exclusively composed of TN breast tumor obtained from A. C. Camargo Cancer Center (ACCCC, São Paulo, Brazil). Specimens were retrospectively selected in the ACCCC's database according to the following inclusion criteria: female breast tumors which were negative for estrogen receptor, progesterone receptor and HER2 expression. The study protocol was approved by the A. C. Camargo Cancer Center Research Ethics Committee (#1746/13), and all patients gave written consent to participate.
Histopathological characterization
Histopathological and clinical data were obtained from electronic medical records. Histopathological evaluation of the resected tumors was performed by clinical pathologists, following institutional routine procedures. The histopathological characterization was based on the TNM classification by the American Joint Committee on Cancer32 and on the Elston Ellis histological grading system.33 The following parameters were considered: histological type; tumor grade; tumor size; lymph node status; hormone receptors (ER and PR) and HER2 status.
The data on hormone receptors (ER and PR) and HER2 status were used to assess tumor subtypes, as proposed by Huober et al.,34 in the following categorical groups: Luminal A, defined as ER+, PR+ and HER2- tumors; Luminal B tumors were defined as ER+, PR- and HER2+/−, or ER-, PR+, and HER2-, or ER+, PR+ and HER2+; HER2 like tumors were defined as ER-, PR+ and HER2+, or ER-, PR- and HER2+; and TN as ER-, PR- and HER2-. Tumors positive for both ER and PR but which HER2 status was not assessed were defined as “Luminal” only.
RNA extraction and gene expression analysis
Fresh frozen breast tumors samples obtained from INCA were macrodissected and total RNA was extracted using RNeasy Mini Kit (QIAGEN, cat. #74104). ACCCC samples were performed according to the procedures adopted in A. C. Camargo Biobank (ACCB); total RNA preparation was done as described in Olivieri et al.35 1 µg of total RNA was used for cDNA synthesis using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, cat. #4368814).
Gene expression was assessed from 2 µL of cDNA (diluted 10x) using TaqMan Gene Expression Assays (Thermo Fisher Scientific) for BRCA1 (Hs01556193_m1), BARD1 (Hs00184427_m1), LIG4 (Hs01866071_u1), MDC1 (Hs00206182_m1), ECT2 (Hs00216455_m1), TP53BP1 (Hs00996818_m1) and PAXIP1 (Hs00697144_m1). GAPDH (Hs02758991_g1) and PPIA (Hs00216455_m1) were assayed as endogenous controls (reference genes). All samples were analyzed in triplicates and the comparative Ct (2−ΔΔCT) method36 was used for relative quantification. Reference genes were normalized by geometric mean. Human mammary gland total RNA (Clontech, cat. #636576) was used as reference sample.
Immunohistochemistry (IHC)
Paraffin-embedded specimens were cut into 4 µm sections and incubated at 60°C for at least 2h. Sections were deparaffinized with xylene and rehydrated. Heat-mediated antigen retrieval was performed in Trilogy commercial buffer (Cell Marque, cat #920P-06) for 30 minutes. Slides were allowed to cool to room temperature and then subjected to the IHC method using a peroxidase polymer-based commercial kit, according to the manufacturer's instructions (Novolink Max Polymer Detection System, Leica Biosystems, cat. #RE7280-K). Slides were incubated either with anti-53BP1 1:5000 (Bethyl Laboratories, cat. #A300–272A) or anti-PTIP (PAXIP1) 1:1000 (Abcam, cat. # ab168502) antibodies overnight at 4°C. A reference sample for both antibodies (human colon adenocarcinoma sample) was included in all routines as a positive control. As a negative control, the positive sample was assayed without primary antibody.
The immunostained tissue slides were evaluated by 2 independent observers (experienced pathologist and biologist); the whole slides' tumor areas were analyzed and staining intensity was scored as “weak” or “strong.” This evaluation was based upon the most representative area of the tumor sections analyzed, comparing its staining pattern with the positive control sample, taken as a “strong” staining pattern. Only the nuclear staining was considered for scoring.
DAB immunostaining was also quantified using the plugin IHC Profiler37 for ImageJ.38 The plugin scores the nuclear intensity and results are given as a percentage distribution among categories: negative, low positive, positive and high positive. We then dichotomized outputs as “weak” or “strong” as follows: “strong” - >55% of total score contribution in high positive and/or positive categories; “weak” - >55% of total score contribution in low positive and/or negative. For a final call, software's and observers'scores were compared. Eventual conflicting results were only observed for borderline samples (45–55%), in those cases the observer's score overruled.
BRCA1 sequencing
The tumor genomic DNA of ACCCC patients was submitted to BRCA1 sequencing. The entire coding sequence and exons boundaries of BRCA1 gene were sequenced by next generation sequencing (Ampliseq BRCA1 and BRCA2 panel, Ion PGM Torrent, Thermo Fisher Scientific). Data were analyzed in CLC Genomics Workbench 6 software, using NM_007294.3 (BRCA1) as reference sequence for alignment. As a variant calling criteria, identified variations must be identified in a region with >20x coverage and present at >5% frequency. Variants were searched and classified according to BIC (Breast Cancer Information Core; http://research.nhgri.nih.gov/bic) and LOVD-IARC (http://hci-exlovd.hci.utah.edu/home.php) databases and categorized as described previously.39 Pathogenic variants were validated in tumor DNA by Sanger sequencing (ABI 3130xl, BigDye Terminator v3.1, Thermo Fisher Scientific).
Statistical analysis
Histopathological features were dichotomized to represent categories for better and worse prognostic values. Comparison between gene expression and histopathological features was performed applying the non-parametric Mann–Whitney U-test and the Kruskal-Wallis one way analysis of variance using the GraphPad Prism 5.0 software (α = 0.05) (GraphPad Software, Inc.). A Spearman's correlation matrix was also constructed using GraphPad Prism 5.0. The statistical association between gene expression (explanatory variables) and the histopathological features (response variables), which were expressed as categorical binary variables according to intrinsic prognostic values, was inferred by fitting logistic regression models. This method, described by Harrell,40 is implemented as function ‘lrm’ available in the R package ‘rms’.41 Analysis of variance tables describe the Wald statistics for testing the model components.
Survival analyses were assessed by Kaplan-Meier curves using IBM SPSS Statistics v. 23 (IBM Corp) for disease free survival (DFS) and overall survival (OS) outcomes. Disease progression was characterized by the occurrence of loco-regional or contra-lateral recurrence of breast cancer, or by detection of distant metastasis. New primary cancer lesions, loss of patient follow-up, deaths by unrelated causes and patients achieving 5-year follow-up were censored in survival analyses. Survival plots of GEO deposited data were generated by the online software KM plotter (www.kmplot.com) using the filter for breast cancer42 and information regarding the investigated genes for OS (1402 samples) or DFS (3951 samples). OS plots from TCGA breast cancer dataset (1200 samples) were generated by UCSC Xena browser (xena.ucsc.edu).43
Supplementary Material
Ethical approval
All procedures performed in this study involving human participants were performed in accordance with the ethical standards of the institutional research committees (INCA #129/08, ACCCC #1746/13) and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from individual participants from the INCA cohort. For the ACCCC samples study formal consent is not required.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ackowledgments
The authors thank the Brazilian National Tumor Bank (BNT/INCA) and the A. C. Camargo Biobank (ACCB) for providing the breast tumor RNA samples used in this study.
Funding
This study was funded by Fundação de Amparo à Pesquisa do Rio de Janeiro – FAPERJ, Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPQ, Fundação de Amparo à Pesquisa de São Paulo – FAPESP (2013/23277–8) and the Florida Breast Cancer Foundation.
References
- 1.International Agency for Research on Cancer GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2013. [accessed 2016April13]. http://globocan.iarc.fr [Google Scholar]
- 2.Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol 2014; 5(3):412-24; PMID:25114856; https://doi.org/ 10.5306/wjco.v5.i3.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA et al.. Molecular portraits of human breast tumours. Nature 2000; 406:747-52; PMID:10963602; https://doi.org/ 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 4.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS et al.. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98:10869-74; PMID:11553815; https://doi.org/ 10.1073/pnas.191367098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT et al.. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415:530-6; PMID:11823860; https://doi.org/ 10.1038/415530a [DOI] [PubMed] [Google Scholar]
- 6.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 2003; 100:10393-8; PMID:12917485; https://doi.org/ 10.1073/pnas.1732912100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature 2012; 490:61-70; PMID:23000897; https://doi.org/110.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa A, Caruso D, Tomao S, Rossi L, Zaccarelli E, Tomao F. Triple-negative breast cancer: investigating potential molecular therapeutic target. Expert Opin Ther Targets 2015; 19(1):55-75; PMID:25307277; https://doi.org/ 10.1517/14728222.2014.970176 [DOI] [PubMed] [Google Scholar]
- 9.Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM. Therapies for triple negative breast cancer. Expert Opin Pharmacother 2015; 16(7):983-98; PMID:25881743; https://doi.org/ 10.1517/14656566.2015.1032246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK. Targeted therapies for triple-negative breast cancer: combating a stubborn disease. Trends Pharmacol Sci 2015; 36(12):822-46; PMID:26538316; https://doi.org/ 10.1016/j.tips.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Marmé F, Schneeweiss A. Targeted therapies in triple-negative breast cancer. Breast Care (Basel) 2015; 10(3):159-66; PMID:26557820; https://doi.org/ 10.1159/000433622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2 deficient tumours with inhibitors of poly(ADPribose) polymerase. Nature 2005; 434:913-7; PMID:15829966; https://doi.org/ 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 13.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C et al.. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005; 434:917-21; PMID:15829967; https://doi.org/ 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 14.Woods NT, Mesquita RD, Sweet M, Carvalho MA, Li X, Liu Y, Nguyen H, Thomas CE, Jr Iversen ES, Marsillac S et al.. Charting the landscape of tandem BRCT domain-mediated protein interactions. Sci signal 2012; 5:6; PMID:22990118; https://doi.org/ 10.1126/scisignal.2002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koonin EV, Altschul SF, Bork P. BRCA1 protein products…Functional motifs… Nat Genet 1996; 13:266-8; PMID:8673121; https://doi.org/ 10.1038/ng0796-266 [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann M, de Lange T. 53BP1: pro choice in DNA repair. Trends Cell Biol 2014; 24(2):108-17; PMID:24094932; https://doi.org/ 10.1016/j.tcb.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callen E, Di Virgilio M, Kruhlak MJ, Nieto-Soler M, Wong N, Chen HT, Faryabi RB, Polato F, Santos M, Starnes LM et al.. 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 2013; 153(6):1266-80; PMID:23727112; https://doi.org/ 10.1016/j.cell.2013.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, Hiddingh S, Thanasoula M, Kulkarni A, Yang Q et al.. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol 2010; 17:688-95; PMID:20453858; https://doi.org/ 10.1038/nsmb.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkadze G, Khardzeishvili O, Gudadze M, Tsikhiseli G, Turashvili G. Immunohistochemical expression of BRCA1 protein in invasive ductal carcinoma of the breast. Georgian Med News 2010; 7–8:(184–185): 51-60; PMID:20834076 [PubMed] [Google Scholar]
- 20.Partipilo G, Simone G, Scattone A, Scarpi E, Azzariti A, Mangia A. Expression of proteins involved in DNA damage response in familial and sporadic breast cancer patients. Int J Cancer 2016; 138(1):110-20; PMID:26205471; https://doi.org/ 10.1002/ijc.29699 [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Bu Y, Wang C, Yi F, Yang Z, Huang X, Cheng L, Liu G, Wang Y, Song F. NFBD1/MDC1 is a protein of oncogenic potential in human cervical cancer. Mol Cell Biochem 2012; 359(1–2):333-46; PMID:21853275; https://doi.org/ 10.1007/s11010-011-1027-7 [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Dong R, Jiang Z, Wei Y, Li Y, Wei L, Sun H, Li Y, Yang N, Yang Q et al.. MDC1 promotes ovarian cancer metastasis by inducing epithelial-mesenchymal transition. Tumour Biol 2015; 36(6):4261-9; PMID:25592380; https://doi.org/ 10.1007/s13277-015-3063-5 [DOI] [PubMed] [Google Scholar]
- 23.Zou R, Zhong X, Wang C, Sun H, Wang S, Lin L, Sun S, Tong C, Luo H, Gao P et al.. MDC1 Enhances Estrogen Receptor-mediated Transactivation and Contributes to Breast Cancer Suppression. Int J Biol Sci 2015; 11(9):992-1005; PMID:26221067; https://doi.org/ 10.7150/ijbs.10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Xu B, Moran MS, Zhao Y, Su P, Haffty BG, Shao C, Yang Q. 53BP1 functions as a tumor suppressor in breast cancer via the inhibition of NF-kappaB through miR-146a. Carcinogenesis 2012; 33:2593-600; PMID:23027628; https://doi.org/ 10.1093/carcin/bgs298 [DOI] [PubMed] [Google Scholar]
- 25.Hong S, Li X, Zhao Y, Yang Q, Kong B. 53BP1 suppresses tumor growth and promotes susceptibility to apoptosis. Oncol Rep 2012; 27:1251-7; PMID:22266878; https://doi.org/ 10.3892/or.2012.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willis S, Villalobos VM, Gevaert O, Abramovitz M, Williams C, Sikic BI, Leyland-Jones B. Single Gene Prognostic Biomarkers in Ovarian Cancer: A Meta-Analysis. PLoS One 2016; 11(2):e0149183; PMID:26886260; https://doi.org/ 10.1371/journal.pone.0149183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhuraney A, Woods NT, Wright G, Rix L, Kinose F, Kroeger JL, Remily-Wood E, Cress WD, Koomen JM, Brantley SG et al.. PAXIP1 potentiates the combination of WEE1 inhibitor AZD1775 and platinum agents in lung cancer. Mol Cancer Ther 2016; 15(7):1669-81; PMID:27196765; https://doi.org/ 10.1158/1535-7163.MCT-15-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M et al.. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem 2007; 282(28):20395-406; PMID:17500065; https://doi.org/ 10.1074/jbc.M701574200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz IM, Jowsey PA, Toth R, Rouse J. Phospho-epitope binding by the BRCT domains of hPTIP controls multiple aspects of the cellular response to DNA damage. Nucleic Acids Res 2007; 35:v5312-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Z, Cho YW, Kim JE, Ge K, Chen J. Accumulation of Pax2 transactivation domain interaction protein (PTIP) at sites of DNA breaks via RNF8-dependent pathway is required for cell survival after DNA damage. J Biol Chem 2009; 284:7284-93; PMID:19124460; https://doi.org/ 10.1074/jbc.M809158200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L et al.. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 2010; 141(2):243-54; PMID:20362325; https://doi.org/ 10.1016/j.cell.2010.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lester S, Bose S, Chen Y, Connolly J, de Baca M, Fitzgibbons P, Hayes DF, Kleer C, O'Malley FP, Page DL et al.. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch Pathol Lab Med 2009; 133:1515-1538; PMID:19792042; https://doi.org/ 10.1043/1543-2165-133.10.1515 [DOI] [PubMed] [Google Scholar]
- 33.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19(5):403-10; PMID:1757079; https://doi.org/ 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 34.Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C et al.. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010; 124(1):133-40; PMID:20697801; https://doi.org/ 10.1007/s10549-010-1103-9 [DOI] [PubMed] [Google Scholar]
- 35.Olivieri EH, Franco Lde A, Pereira RG, Mota LD, Campos AH, Carraro DM. Biobanking practice: RNA storage at low concentration affects integrity. Biopreserv Biobank 2014; 12(1):46-52; PMID:24620769; https://doi.org/ 10.1089/bio.2013.0056 [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4):402-8; PMID:11846609; https://doi.org/ 10.1006/meth.2001.1260 [DOI] [PubMed] [Google Scholar]
- 37.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 2014; 9(5):e96801; PMID:24802416; https://doi.org/ 10.1371/journal.pone.0096801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7):671-5; PMID:22930834; https://doi.org/ 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carraro DM, Koike Folgueira MA, Garcia Lisboa BC, Ribeiro Olivieri EH,Vitorino Krepischi AC, de Carvalho AF, de Carvalho Mota LD, Puga RD, do Socorro Maciel M, Michelli RA et al.. Comprehensive analysis of BRCA1, BRCA2 and TP53 germline mutation and tumor characterization: a portrait of early-onset breast cancer in Brazil. PLoS One 2013; 8(3):e57581; PMID:23469205; https://doi.org/ 10.1371/journal.pone.0057581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 41.Development Core Team R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 42.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treatment 2010; 123(3):725-31; PMID:20020197; https://doi.org/ 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- 43.Goldman M, Craft B, Swatloski T, Cline M, Morozova O, Diekhans M, Haussler D, Zhu J. The UCSC Cancer Genomics Browser: update 2015. Nucleic Acids Res. 2015 Jan; 43(Database issue): D812-7; PMID: 25392408; https://doi.org/ 10.1093/nar/gku1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.