Abstract
Introduction
Atypical asymmetry in brain activity has been implicated in the behavioral and attentional dysregulation observed in ADHD. Specifically, asymmetry in neural activity in the right versus left frontal regions has been linked to ADHD, as well as to symptoms often associated with ADHD such as heightened approach behaviors, impulsivity and difficulties with inhibition. Clarifying the role of frontal asymmetry in ADHD-like traits, such as disinhibition, may provide information on the neurophysiological processes underlying these behaviors.
Method
ADHD youth (ADHD: n=25) and healthy, typically developing controls (TD: n=25) underwent an electroencephalography (EEG) recording while completing a go/no-go task—a commonly used test measuring behavioral inhibition. In addition, advanced signal processing for source localization estimated the location of signal generators underlying frontal alpha asymmetry (FA) during correct and incorrect trials.
Results
This is the first study in ADHD to demonstrate that the dorsal-lateral prefrontal cortex (DLPFC) may be responsible for generating frontal alpha. During failed inhibition trials, ADHD youth displayed greater FA than TD youth. In addition, within the ADHD group, frontal asymmetry during later processing stages (i.e., 400–800ms after stimulus) predicted a higher number of commission errors throughout the task.
Conclusions
These results suggest that frontal alpha asymmetry may be a specific biomarker of cognitive disinhibition among youth with ADHD.
Keywords: ADHD, Frontal Asymmetry, Disinhibition, EEG, Source Localization
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder associated with cognitive and behavioral dysregulation. This dysregulation has been ascribed to brain abnormalities in ADHD individuals. In particular, structural and functional hemispheric asymmetries and atypical brain laterality have been purported as potential biomarkers of ADHD-like traits (Bradshaw and Sheppard, 2000; Hale et al., 2009a; Hale et al., 2009b; Hale et al., 2007; Stefanatos and Wasserstein, 2001; van Ewijk et al., 2012). One trait often observed among individuals with ADHD is behavioral disinhibition—the inability to stop distractions, control behaviors, and self-regulate (Barkley, 1997). However, whether there is a relationship between hemispheric asymmetry and disinhibited behaviors in ADHD is less clear. With compromised inhibitory functioning purported as a foundational deficit in ADHD (Barkley, 1997), increased understanding of the neurobiological mechanisms contributing to such behaviors is warranted.
Behavioral disinhibition and impulsivity have recently been linked to abnormalities in the approach system (Neal and Gable, 2016). Similarly, ADHD has been conceptualized as a disorder of excessive approach-related tendencies (Keune et al., 2011, 2015; Mitchell, 2010). Consistent with this idea, approach-related tendencies, as assessed with self-report measures, have been shown to predict hyperactive—impulsive symptoms in a sample of young adults (Mitchell, 2010). Adults with an ADHD diagnosis have demonstrated greater behavioral approach in experimental and self-report measures as compared with unaffected adults (Mitchell et al., 2011). Using this theoretical framework to understand ADHD behaviors, frontal alpha asymmetry (FA), a neurophysiological (EEG) index of approach-related sensitivity (Coan and Allen, 2003; Harmon-Jones and Allen, 1997), must be considered as a viable biomarker for examining disinhibition in individuals with ADHD. Frontal alpha EEG asymmetry is the difference in alpha-band (8–12 hertz [Hz]) activity over right vs. left frontal hemispheres of the brain. Because alpha power is thought to be inversely related to cortical activation, higher left cortical activity is associated with elevated right frontal alpha power (Allen et al., 2004; Davidson, 1988; Laufs et al., 2003). For the sake of clarity in this manuscript, all FA findings will represent greater right alpha power (i.e., greater left frontal activation). Increased FA has been linked to self-reported approach motivation, whereas decreased FA has been linked with withdrawal or avoidance (Davidson, 1998; Depue and Iacono, 1989). In addition to approach-related behaviors, FA has also been linked to traits common to ADHD such as reduced reward responsiveness, aggression, and difficulties with inhibition (Davidson, 1998; Rybak et al., 2006), providing additional support for the potential role of FA in ADHD.
Only a few studies have directly examined FA in ADHD, particularly in children. In a study of 4–8 year-old males, those with ADHD during an eyes-open resting condition showed higher right alpha power, consistent with greater FA, than those without ADHD (Baving et al., 1999). Similar findings of higher alpha power in the right hemisphere were reported in a broader (i.e., 6–17 years old), co-ed sample of ADHD youth. These findings showed high specificity in distinguishing ADHD youth from healthy, unaffected controls (Chabot and Serfontein, 1996). Additionally, ADHD youth with a parent who also has ADHD (i.e., greater familial loading for ADHD), versus those with an unaffected parent, showed greater frontal alpha asymmetry during an eyes closed condition (Hale et al., 2010), indicating that there may be a genetic component of FA.
Adult studies have also shown similar findings where greater frontotemporal asymmetry (FT8-FT7), in the 8–10Hz range of alpha (low-alpha), during an eyes-closed condition was associated with ADHD group status, versus healthy controls (Hale et al., 2009). In a replication study that used a composite measure of frontal alpha asymmetry during eyes open and closed, ADHD adults also displayed higher right frontal alpha power, and those with greater FA had more ADHD symptoms (Keune et al., 2011). Taken together with the youth samples, these results suggest that FA may be a heritable, trait-like feature of ADHD that persists throughout the lifetime of the disorder.
While FA, measured during resting states, appears to be a robust correlate of ADHD, these studies provide no information on how, or whether, frontal alpha asymmetry affects inhibitory behavior. One study of adults with and without ADHD, collected EEG data during a continuous performance task (i.e., go/no-go task) and showed that the ADHD group displayed higher FA during the task than non-ADHD controls, specifically in the lower alpha band (8–10Hz) and in the FT8-FT7 region (Hale et al., 2009). Importantly, however, the EEG data was averaged across the 14-min task, providing no differential information on the relationship between FA and specific events such as commission errors, which indicate disinhibited behaviors. While there seems to be strong support for a relationship between ADHD and FA, there is no research linking FA in ADHD youth with in the moment behaviors such as inhibition.
Not only is there a paucity of work examining FA response to stimuli, many studies average FA across a long time-interval providing limited information on whether FA is a dynamic process or where in the inhibitory processing stream it may have effects. The first second following a stimulus has been highlighted as the critical time window for when the majority of inhibitory processes are thought to unfold (Pires et al., 2014), where early stages (0–200ms) are considered to reflect automatic processes of inhibition and later stages (400–800ms) reflect top down, controlled processes of inhibition. Using this standard convention, examining FA at different time points during an inhibitory trial could clarify its role in the information processing stream.
Additionally, to our knowledge, all of the work examining frontal asymmetry in ADHD has used channel-based indices such as the lateral frontal sites (F7/F8) and mid-frontal sites (F3/F4) to calculate asymmetry that has revealed little consistency in which channels are related to ADHD. Frontal asymmetry in lateral-frontal regions (Hale et al., 2009; Keune et al., 2015), mid-frontal regions (Keune et al., 2011), and in averages from the frontal regions of each hemisphere (Hale et al., 2010) have all been reportedly associated with ADHD. The use of differing methods for data analysis and processing likely contributes to the inconsistencies in the literature and further complicates the interpretations gleaned from the data.
Rather than selecting electrode sites that have demonstrated equivocal results in the literature, source-resolved analyses may provide more robust findings. Because of volume conduction and smearing of brain signals by the scalp, more commonly used channel signals contain mixtures of both relevant and irrelevant brain and non-brain sources. These “mixing” limits the effective signal to noise ratio and reduces the statistical value within the data. To improve the quality of the data, source localization uses independent components analysis (ICA) to “un-mix” brain from non-brain (artifacts such as muscle, eye movements, line noise) signals and localizes the cortical sources from which the scalp signal emanates. Using ICA and source analyses for identification of relevant information (e.g., the source generator of frontal alpha) reduces the likelihood that the resulting measures contain an admixture of information—thus increasing the effective signal-to-noise ratio of the information gleaned from the data. In fact, recent work has suggested that alternative methods that reduce the contributions of non-frontal sources in FA may provide a better index of FA and increase the quality of results. Suggested methods include current source density (CSD), source localization and ICA (Smith et al., 2017).
While scalp/channel-based analyses of EEG are commonly used in psychiatric research, the use of source-resolved time-frequency analysis allows one to capture neural processes with greater spatial resolution and specificity to specific anatomic regions. Indeed, a recent review demonstrated that using unmixed signals from source-resolved analyses can be more powerful in unmasking clinical group differences (Loo et al., 2015; Stewart et al., 2010). Importantly, recent research in psychopathology and behavior has begun to move in the direction of source-localization to clarify the functional role of these brain processes (e.g., Auerbach et al., 2015; Schiller et al., 2014).
Using ICA and source localization methods, the current study aimed to identify the location of the cortical generators of frontal asymmetry—a purported neurophysiological correlate of psychopathology, and examine the role of FA in inhibitory processing across youth with ADHD as compared to those who are typically developing (TD). Specifically, we were interested in two questions. First, we examined whether FA could distinguish ADHD from TD youth. Given the lack of work examining evoked (i.e., stimulus-locked) FA in youth with ADHD, we initially examined whether ADHD and TD youth differed in brain activity across trials. We anticipated that youth with ADHD would exhibit greater FA than TD, but it was unclear whether group differences would be present during both correct and incorrect trials, suggesting a trait-like difference between the groups, or if ADHD and TD youth would only differ during failed inhibition trials, suggesting state-like differences in brain activity during disinhibition. To further characterize the role of FA in the inhibitory process, we segmented trials into 3 time windows to capture both the “automatic” (0–200ms, 200–400ms) and “controlled” (400–800ms) phases of inhibitory processes (Pires et al., 2014).
Second, we examined whether FA could be a biomarker of disinhibited behavior. That is, we examined the effects of FA at each time point, as well as the potential moderating effects of clinical group status (i.e., the interaction of FA x group) on behavior (i.e., commission errors). If FA is associated with greater approach-related behaviors, we would expect that, regardless of group status, higher FA would predict more disinhibition, as demonstrated by higher number of commission errors. To examine the specificity of this relationship we tested FA during correct trials and FA during failed inhibition trials as predictors of the number of commission errors throughout the task. With the research in event-related FA in ADHD versus TD youth so limited, it remained unclear whether the individual effects of FA and ADHD would interact (i.e., be potentiated).
Method and Materials
Participants
Participants were recruited from a large medical center and the surrounding community. They were recruited to participate in two larger ADHD studies that used identical research methods, but recruited a broader age range of ADHD and typically developing individuals (age range from 5–64yrs). At the baseline appointment for participation, eligibility requirements were assessed.
Those individuals between ages 7–14 where clinically interviewed to determine the presence (ADHD: n=25) or absence (TD: n=25) of ADHD. Youth with ADHD met DSM-IV criteria for any of the ADHD subtypes and were free of psychosis, autism spectrum disorder, or any current (prior 6 months) anxiety or mood disorder. TD youth had no history of ADHD, nor did they have a history of mood, psychotic, oppositional defiant disorder or anxiety (except simple phobia) diagnoses. An additional criterion for the sample was that participants must have had non-clinical range scores (T-scores <70) across subscales of the Child Behavior Checklist (Auchenbach, 1991) (excluding the Attention subscale for the ADHD group). Restricting comorbidities across the samples provided a more refined method (i.e., the groups were “clean”) of examining ADHD vs. TD differences. Exclusion criteria included a history of seizures or head injury or loss of consciousness. IQs were greater than 80, measured by the Wechsler Abbreviated Scale of Intelligence (WASI)(Wechsler, 1999) and all families were English speaking. Written and verbal permission and consent were obtained from parents and youth prior to beginning study procedures, which were approved by the University of California-Los Angeles Institutional Review Board.
The groups did not differ in race (88% Caucasian; χ2=3.36, p=0.34), age (mean: 10.65, SD: 2.16; F(1, 49)=0.33, p=0.57), or sex (χ2=0.33, p=0.57). Given the large age-range and studies showing that frontal asymmetry can differ by sex (Baving et al., 2003), age and sex were both examined as predictors of FA. There were no sex differences in FA across any of the measures (all ps>0.24), nor was there a relationship between frontal asymmetry and age.1 Thus, no demographic covariates were included in the analyses and genders were combined across groups.
Diagnostic Assessments
The Kiddie Schedule of Affective Disorders and Schizophrenia (KSADS-PL) (Kaufman et al., 1997) was used for the clinical interview, in combination with symptom rating scales provided to parents and teachers, to arrive at the final diagnoses. Interviewers were clinical psychologists or highly trained interviewers, and final diagnoses were confirmed by senior clinicians. Overall reliability of the interviews was excellent (mean weighted kappa=0.95, SD=0.03).
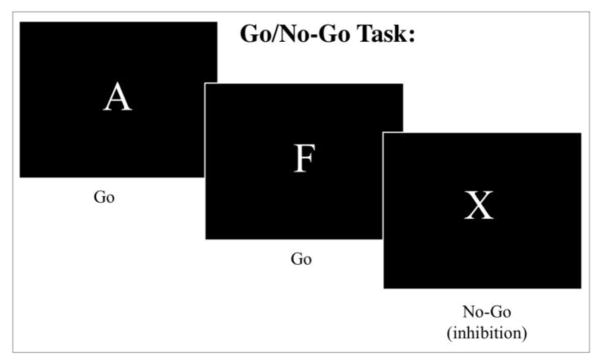
Go/No-go Task
To assess response inhibition, a computerized version of a continuous performance go/no-go task (Conners, 2004) was administered to participants utilizing E-Prime software (v1.1b5; Psychology Software Tools). During this task, participants were required to respond to target stimuli (“Go” trials) but told to inhibit their responses to an inhibitory stimulus (“No-Go” trials). There were 360 total trials with 90% “Go” (324 trials) and 10% “No-Go” (36 trials).
They were instructed to press the spacebar after viewing each letter (“Go” trial) except for the letter X (“No-Go” trial) where they were not to press the spacebar, inhibiting the response. There were 3 different inter-stimulus interval (ISI) times used for each trial: 1000ms, 2000ms, or 4000ms. Each letter was always presented for 250ms, leaving either 750ms, 1750ms, or 3750ms of the remainder response time per given fixed ISI. The different ISIs were presented in blocks of 20 trials. The order of ISI blocks was random and determined by the program. ISI type was randomly presented and equally distributed across the trials. (Go: 108 trials at each ISI; No-Go: 12 trials at each ISI).
Dependent variables yielded from the task were accuracy and reaction time (RT). Omission errors were used to measure inattentive behaviors, while errors of commission represented impulsivity and motor disinhibition (see Table 1).
Table 1.
Means and standard errors for the typically developing (TD) and ADHD youth across behavioral measures and FA values
| TD | ADHD | |||
|---|---|---|---|---|
|
| ||||
| Mean | SE | Mean | SE | |
| Commission Errors* | 21.08 | 6.21 | 25.84 | 5.96 |
| Hit Rate (log transformed) | 2.6 | 0.02 | 2.55 | 0.02 |
| Omission Errors (sqrt)* | 4.5 | 0.49 | 6.08 | 0.42 |
| Incorrect Trials | ||||
| FA early* | 0.12 | 0.23 | 0.99 | 0.27 |
| FA mid | 0.12 | 0.28 | 0.81 | 0.29 |
| FA late | −0.09 | 0.36 | 0.41 | 0.32 |
| Correct Trials | ||||
| FA early | 0.72 | 0.37 | −0.06 | 0.37 |
| FA mid | 0.38 | 0.31 | 0.3 | 0.36 |
| FA late | −0.24 | 0.37 | 0 | 0.42 |
Significant group differences (i.e., p<.05) are designated using an asterisk (*).
Electroencephalography (EEG)
EEG recording utilized 40 Ag/AgCL surface electrodes embedded into the cap using an extended international 10/20-location system (ElectroCap, Eaton, OH). Individualized electrode locations were recorded and digitized using Fowler calipers to produce 3-D spherical coordinates. MANSCAN (Sam Technology, San Francisco, CA) hardware and software were used to record EEG data. Impedance was set below 10 κOhms. EEG data was recorded at a rate of 256 samples per second and referenced to linked-ears. EEG data was continuously recorded throughout completion of the Go/No-Go task, and event markers were embedded in the task to denote stimulus type (Go or No-Go) and response (correct versus incorrect).
EEG Signal Processing
EEG data was processed offline within Matlab (Mathworks) utilizing EEGLab functions (v12.0). We used an identical processing pipeline as previously published and outlined in detail (Lenartowicz et al., 2014). However, we provide a summary here. Our data cleaning process had multiple steps that continued to refine the data as it progressed. Our first data cleaning step removed the data with excessive noise or artifact. First a high-pass filter at <1hz was used followed by manual removal of any bad channels (i.e., broken or disconnected channels). Then noisy segments of the data were removed by segmenting the data into 0.5 second time bins, and any segment outside of 5 standard deviations of the overall data was immediately removed up to a maximum of 10% of the data. These “bad” segments were visually confirmed as “noise” (i.e., major movement and muscle artifacts) by experienced technicians blind to diagnosis. These initial segments were only used for the cleaning purposes described in this initial step. We describe our classification of epoching to behavioral events below.
Consistent with recent suggestion, Independent Component Analysis (ICA) was then run on individual subjects to separate neural and artifact signals (Smith et al., 2017; Makeig et al., 1996). Because a greater amount of data included enhances ICA, our initial step, described above, was intended only to remove the worst noise, which can negatively impact the ICA solution. ICA was then run on the remaining data from individual subjects to separate neural and artifact signals. ICA creates components that contain EEG data with similar spatial-temporal patterns of voltage change. We used the parameters set by binica in EEGLAB, which allows for up to a 1,000 maximum learning steps and a stopping weight change of 1e-7. ICs representing non-brain activity (i.e., ocular activity, muscular activity, EMG, EKG, and line noise) were then removed to further clean the data. Two trained researchers who were blind to diagnosis identified these types of artifacts. They visually inspected all ICs and examined their spatial, temporal and spectral characteristics to further determine whether the IC reflected brain or non-brain activity.
To further exclude processes that could not be attributed to cortical source, we calculated an estimated equivalent dipole model for each participant by running the DIPFIT protocol from the toolbox in EEGLAB (http://sccn.ucsd.edu/wiki/A08:_DIPFIT). The DIPFIT protocol registers each participant’s electrode positions to the Montreal Neurological Institute (MNI) head model. Using the boundary element method, it then calculates an electrical forward solution (Kybic, Clerc, Faugeras, Keriven & Papadopoulo, 2006). For each IC, a source model consisting of a single-equivalent current dipole is estimated. Using the projection pattern of the equivalent dipole model, the scalp electrodes are linearly fit to a scalp map. Only ICs whose residual variance of dipole model location was less than 15% were retained for analyses.
From the remaining individual ICA solutions, we clustered ICs across participants utilizing a k-means solution (k=11 with threshold for outliers of 3 SD), which resulted in similar cortical sources of activity being grouped together within each cluster. It should be noted that this method reduced the number of subjects included in the analysis by omitting subjects that did not have sources of activity included in clusters of interest.2 However, this ensures that similar sources are being compared across subjects. The 50 subjects presented here are those who remained in the clusters. The data were then epoched 2 seconds before and after each presentation of the Go and No-Go stimulus events. We specifically examined trials for which there was a response, i.e., correct “Go” trials and incorrect “No-Go” trials. Following ICA, 97% of incorrect trials and 93% of correct trials were retained within the ADHD group, and 98% of incorrect and 91% of correct trials were retained within the HC group.
Following epoching to behavioral events, using Morlet wavelet decomposition, spectral power was computed separately for correct and incorrect trials across the entire 4-second window, using a pre-stimulus baseline of 500ms. Within each trial type (correct vs. incorrect), spectral power was averaged together and log transformed into decibel (dB) units. We calculated mean event-related spectral perturbations (ERSPs) with a baseline of −300 to −100ms preceding stimulus onset from the correct and incorrect trials. From the ERSPs, spectral power (dB) was extracted for our time-points of interest.
The 11-cluster solution provided clear identification of individual left and right frontal clusters and maximized our number of participants in each cluster. We visually examined the dipoles within each of these frontal clusters (right and left) and removed mis-clustered dipoles (e.g., dipole was outside of the brain or located in the opposite hemisphere). Three outliers from the left frontal cluster and two from the right frontal cluster were removed. The centroid dipole location for the right and left clusters was in the dorsal lateral prefrontal cortex (DLPFC), BA46. The talairach coordinates for the right cluster were X=46, Y=36, Z=9 and for the left cluster were X=−47, Y=42, Z=3.
In order to examine FA during initial (FAearly), middle (FAmid) and later (FAlate) stages of the inhibitory process similar to methods used with ERPs (Pires et al., 2014), we calculated FA using spectral power averaged across each of the time intervals from the right and left cluster. Data was extracted from the following stimulus-locked time points during correct and incorrect trials: 0–200ms (FAearly), 200–400ms (FAmid), and 400–800ms (FAlate). Given that ERSP data is already log transformed, no additional transformations were performed prior to calculating FA. FA was then calculated by subtracting the left frontal activity from right frontal activity (R-L=FA). Following the calculation of FA, the data were examined for outliers based on alpha power beyond 2 standard deviations of the mean within each processing time window (early, mid, and late). There were no outliers in the data during correct trials. However, during incorrect trials, one FA outlier (TD) was identified in the late phase; this reduced the TD sample by one during the late phase (n=24). Analyses of variance were employed to examine group differences in neurophysiological activity and mixed effects general linear modeling was used to examine the effect of FA on behavior. Additional details are provided below.
Behavioral Data
One-way analysis of variance (ANOVA) was employed to assess differences between groups in task accuracy. Given skewed data, hit rate variables were normalized with log transformations and the square root was taken of the number of omissions. No group differences were revealed for reaction time measures, (Hit rate: F(50, 1)=3.12, p=.08; Hit rate standard error: F(50, 1)=2.31, p=.14; and Hit rate standard deviation: F(50, 1)=2.16, p=.15). Accuracy measures revealed differences between groups in omission errors (F(50, 1)=6.06, p=.02, partial η2=.11) with the ADHD group displaying significantly more omission errors than TD. Significant group differences in commission errors (F(50, 1)=7.65, p<.01, partial η2=.14) also revealed that the ADHD group displayed significantly more errors of commission than TD (See Table 1).
Group Differences in Frontal Asymmetry During Correct and Incorrect Trials
Univariate analyses of variance determined whether clinical group status was associated with differing patterns of neural activation across early, mid, and late processing after stimulus onset. Table 1 contains means and standard errors of the frontal asymmetry and behavioral measures for the ADHD and TD groups.
For correct “Go” trials, the ANOVA revealed non-significant group differences across all stages of processing (FAearly: F(1, 49)=2.13, p=.15, partial η2=.04; FAmid: F(1, 49)=.03, p=.87, partial η2<.01; and FAlate: F(1, 49)=.18, p=.67, partial η2=.00), suggesting that the groups displayed similar neurophysiological activity on trials associated with a correct response.
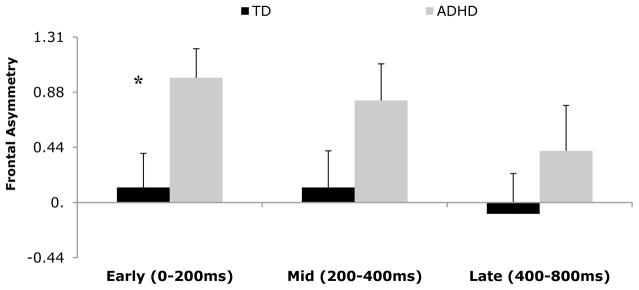
Next, we examined whether our groups differed in FA across these time segments following stimuli that were associated with error trials (i.e., commissions during “No-Go” trials). During the early stage of processing, for stimuli associated with a commission error a significant group effect emerged (FAearly: F(1, 49)=5.81, p=.02 partial η2=.11). The ADHD group had significantly higher FAearly during error trials than did TD. During middle and late stages of processing no group differences were revealed in FA between ADHD and TD, (FAmid: F(1, 49)=2.84, p=.10 partial η2=.06, and FAlate: F(1, 48)=.33, p=.57 partial η2=.01) (See Figure 2).3
Figure 2.
Frontal asymmetry means (y-axis) on incorrect trials for ADHD and TD youth over time (x-axis). Error bars represent the standard errors of the mean. * Denotes significant group difference.
Frontal Asymmetry and Disinhibition
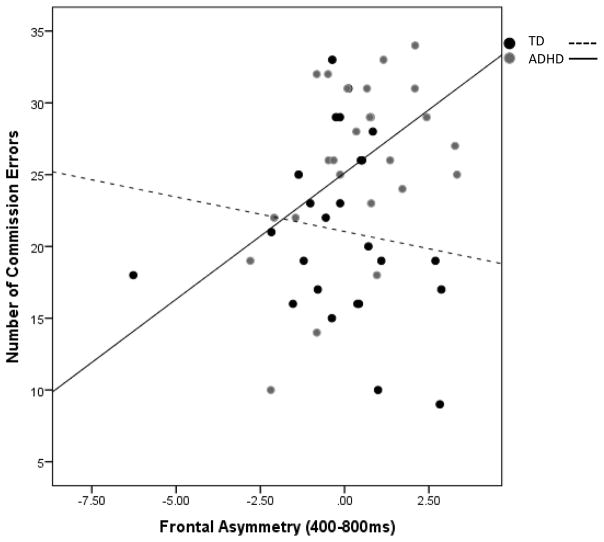
To specifically examine the effects of FA on disinhibited behavior, a mixed effects general linear model was employed for incorrect trials to examine the individual effects of FA and clinical group status, as well as the interaction effect of FA x group, on commission responses (i.e., number of commission errors). The main effect of group on number of commission errors was reported in the Behavioral Data section (see Table 1) and will not be repeated here. For early stages of processing, the interaction of group x FAearly and the main effect of FAearly were both non-significant, F(1, 49)=.52, p=.47 and F(1, 49)=.09, p=.77, respectively. During middle stages of processing, there was no effect of FAmid (F(1, 49)<.00, p=.99) or the interaction of group x FAmid (F(1, 49)=1.33, p=.26). Finally, for the late stages of processing, a non-significant effect of FA was revealed, (F(1, 48)=.07, p=.79). The interaction of FAlate x group (F(1, 48)=7.06, p=.01) was significant. Follow-up analyses indicated that within the TD group, FAlate was not associated with total number of commission errors (B=−.1.45, t=−1.49, p=.15, R2=.09). Within the ADHD group, however, greater FAlate significantly predicted greater number of commission errors (B=1.76, t=2.61, p=.02, R2=.23) (see Figure 3).
Figure 3.
The interaction of group status x frontal asymmetry (400–800ms) on total commission errors. Greater FA in ADHD youth was associated with greater number of total commission errors. There was no relationship in TD youth.
To determine whether FA during incorrect trials was a unique, trial-specific predictor of overall behavioral disinhibition (i.e., predicted a greater total number of commission errors) we also examined FA during correct trials as a predictor of total commission errors throughout the task. This allowed us to rule-out whether general FA (regardless of correct/incorrect trial-type) predicted disinhibition, or if it was only FA during incorrect trials that was associated with this behavior. The mixed-effects model revealed no effects of FAcorrect (ps>.29), nor interaction effects of FAcorrect x group (ps>.32) on behavioral disinhibition (i.e., number of commission errors), indicating that the effect of FALate in ADHD is a specific neurophysiological marker of the brain state associated with disinhibition.
Discussion
The current study sought to identify whether youth with ADHD and TD differed in their frontal alpha asymmetry during an inhibition task and examine the role of FA across these groups in predicting commission errors. Results were two-fold. First, youth with ADHD were distinguished from TD during early stages of processing prior to making a disinhibited response, but not middle and later stages. Specifically, ADHD youth displayed higher FA immediately following stimulus onset (i.e., early stages of processing) in the pre-error phase than TD. These effects were unique to error trials, as no group differences in FA emerged for correct trials. Measures of effect size suggest that these group effects decrease substantially across each time segment suggesting that group differences in FA are specific to greater FA during the automatic processing stages of stimuli. This result indicates that greater approach-oriented brain activation, particularly early in the processing stages, distinguishes ADHD youth from TD following stimulus onset.
Second, to test whether higher FA was an underlying, non-disorder specific brain state associated with behavior, we examined the role of FA as a biomarker of disinhibited behavior (i.e., total commission errors). While FA alone did not predict disinhibited behavior (i.e., commission errors), an approach-related brain state potentiated the effects of ADHD on inhibitory errors. Specifically, within the ADHD group, FA explained nearly a quarter of the variance in predicting disinhibited behavior—more than two and a half times the variance that FA explained in TD. Further, these results were specific to brain activation during incorrect trials only. That is, brain activity during correct trials was not associated with group status, nor did it predict disinhibited behavior. This highlights the unique, response-specific contribution of FA to the behavioral disinhibition observed in ADHD and provides additional support for the idea that ADHD is a disorder of excessive approach tendencies (Keune et al., 2011).
While differences in the neural processes underlying errors have been used to distinguish ADHD from TD youth using fMRI (Spinelli et al., 2011), this is the first study to distinguish ADHD vs. TD youth using approach-related brain state differences during error-trials. Given that fMRI methodology is unable to capture the rapid neural changes associated with event-related activity, the current results provide additional evidence of pre-error brain-state differences, specifically in the automatic processing stages, in ADHD and TD youth.
The study also demonstrated, in ADHD individuals only, a relationship between brain-state and behavior. Greater approach-related brain activity during later stages of processing on error-trials predicted more inhibitory errors throughout the task in ADHD youth. Heightened approach-related activity may weaken the top-down control necessary for behavioral inhibition, resulting in more errors. The process of inhibition involves a period following the stimulus of “interference control” that protects individuals from inappropriate responses (Barkley, 1997). Accordingly, these results may imply that the influence of a greater “approach-related” brain state later in the processing stages disrupts future interference control during subsequent trials. Future work should aim to examine trial-by-trial relationships between FA and commission errors to test this hypothesis. However, these results suggest that FA is, indeed, a complex process that unfolds over-time.
Our results are consistent with work using resting FA to distinguish ADHD vs. TD youth, as well as expand on the role of frontal asymmetry as a predictor of behaviors—disinhibition—in ADHD (Baving et al., 1999; Hale et al., 2009; Keune et al., 2015, 2011). While resting FA, regardless of the situation, emphasizes dispositional differences in approach versus withdrawal tendencies, task-elicited EEG asymmetry, which depends on the demands of the situation, measures the degree to which people are capable of approach versus withdrawal responses, such as inhibition. This “capability model” of FA posits that measuring FA during a challenge may reflect a capacity for emotion regulation and reduce the uncontrolled variance seen in the resting state literature (Coan et al., 2006). Indeed, individual differences in FA are more pronounced during emotional challenges, and these differences are thought to reflect a more sensitive indicator of traits and behaviors (Coan et al., 2006; Harmon-Jones et al., 2002; Harmon-Jones and Sigelman, 2001). Accordingly, these results suggest that ADHD individuals are less capable of regulating (i.e., inhibiting) approach-related behaviors.
Importantly, this study represents the first use of source analyses to identify the dorsolateral prefrontal cortex (DLPFC) as a cortical generator underlying FA and must be considered in the interpretation of results. Understanding brain processes in terms of their functional network increases the meaningfulness of EEG biomarker research. The DLPFC has been associated with various executive functions (EF), particularly “cool” EFs (i.e., processing of non-emotional information related to the exteroceptive environment) (Petrovic and Castellanos, 2016), but also those related to response selection, inhibition of pre-potent responses and inhibitory stimulus control (Aron et al., 2004; Floden and Stuss, 2006; Oldrati et al., 2016). The go/no-go task elicits both inhibitory and response-selection processes on the less frequent No-Go trials, consistent with the potential involvement of the DLPFC found in the current study. Indeed, ADHD youth, as compared to TD, have shown greater activity in areas associated with response selection and control (i.e., cerebellum, DLPFC, and ventrolateral PFC) during the pre-error phase (Spinelli et al., 2011). Unfortunately, it remains unclear whether an approach-related brain state is reflective of disinhibition specifically or whether it predicts a broader state associated with response selection, particularly in the early/automatic stages of processing. It is also not clear whether the DLPFC is a task-specific source of FA (i.e., related specifically to the go/no-go task) or a constant generator (i.e., at rest) of FA. Future work should aim to clarify these issues.
Despite the questions that still remain, the study provides further support for FA to be considered an underlying transdiagnostic marker of approach-related behaviors such as disinhibition (Davidson, 1998; Harmon-Jones et al., 2002, 2003, 2008, 2010). Clinically, FA has been used as a marker for illness course and symptom presentation, particularly in mood disorders where depressed individuals show lower FA, and bipolar individuals show greater FA, similar to ADHD (Davidson, 1998; Harmon-Jones et al., 2002, 2010). Greater FA may be a mechanism contributing to heightened approach behaviors and difficulties with inhibition observed across both of these disorders and help to explain the high comorbidity and overlap in symptom presentation (Brody, 2001; Weintraub et al., 2014). To test this, future work should examine FA during failed inhibition in ADHD and bipolar youth to determine whether the groups display similar increases in FA during disinhibition.
Our ADHD sample was unique in that it did not have comorbidities typically observed in ADHD, which may be considered a limitation since it reduces the generalizability of the results. However, we view this as a considerable strength of the study. There were no significant symptoms of mood or aggression—symptoms often associated with FA. As such, the findings are free of the potential confound of overlapping symptoms and can be considered specific to the core features of ADHD. Another potential limitation is our use of source-resolved analyses that reduced our overall sample when generating our final clustering solution. Despite this, the use of source localization analyses to identify frontal clusters, rather than utilizing scalp electrodes, improved the quality of the data by improving spatial resolution and increasing the effective signal-to-noise ratio of the information. The quality of this data was essential to examine the time-course of the inhibition process. This provided a more fine-grained analysis of the role of FA on inhibitory processing and identified where in the information processing stream FA was associated with diagnosis or behaviors.
In summary, these results further contribute to the robust literature implicating the role of cerebral asymmetries in the ADHD presentation (Bradshaw and Sheppard, 2000; Hale et al., 2009; Stefanatos and Wasserstein, 2001), and provide additional support for the role of FA as a potential biomarker of disinhibition in ADHD.
Figure 1.
Go/No-Go Task Schematic.
Highlights.
Greater frontal asymmetry during failed inhibition distinguished ADHD from healthy youth.
ADHD youth with greater frontal asymmetry had a greater number of inhibitory errors.
Frontal asymmetry may be a biomarker of cognitive disinhibition among ADHD youth.
The dorsal-lateral prefrontal cortex may be responsible for generating frontal alpha.
Findings support conceptualization of ADHD as an approach-related disorder.
Acknowledgments
Alissa Ellis, Chantelle Kinzel, Giulia Salgari, and Sandra Loo, Semel Institute of Neuroscience and Human Behavior, Department of Psychiatry, University of California-Los Angeles. The authors have no disclosures to report. Correspondence concerning this article should be addressed to Alissa Ellis, Semel Institute of Neuroscience and Human Behavior, University of California-Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90025, 58-225. This work was supported by grants from the National Institutes of Health: K23 MH106785-01 (PI: Ellis) and R01 NS054124, RO3 MH92829 (PI: Loo).
Footnotes
Primary results remained significant when age was included in analyses as a covariate. Group differences in FAearly during failed inhibition continued to distinguish ADHD individuals from TD (F(3, 47)=5.69, p=.021). Additionally, when controlling for age, the FA x diagnostic group interaction strengthened in its effect on number of commission errors (F(4, 44)=8.09, p<.01).
The full sample before clustering included 35 ADHD and 33 HC youth. That is, approximately 75% of individuals were retained in the final analyses.
We re-ran our initial analyses using channel-based data from the F3 and F4 electrode sites. To calculate FA, we used the following: (F4−F3)/(F4+F3). Results showed a similar pattern for mean FA across groups during early, middle, and late processing where the ADHD showed greater FA during each of these stages. The results, however, were non-significant due to excessive error variance. For our result of group differences in the early stages of processing our reported F-value was 5.81, versus F=.50 using channel based measures. In examining the role of FA on commission errors, channel-based FA was not related to commissions in the ADHD or TD groups (p’s>.20).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Front EEG Asymmetry Emot Psychopathol. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Stewart JG, Stanton CH, Mueller EM, Pizzagalli DA. Emotion-processing biases and resting EEG activity in depressed adolescents. Depress Anxiety. 2015;32:693–701. doi: 10.1002/da.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baving L, Laucht M, Schmidt MH. Frontal EEG correlates of externalizing spectrum behaviors. Eur Child Adolesc Psychiatry. 2003;12:36–42. doi: 10.1007/s00787-003-0307-5. [DOI] [PubMed] [Google Scholar]
- Baving L, Laucht M, Schmidt MH. Atypical Frontal Brain Activation in ADHD: Preschool and Elementary School Boys and Girls. J Am Acad Child Adolesc Psychiatry. 1999;38:1363–1371. doi: 10.1097/00004583-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Sheppard DM. The Neurodevelopmental Frontostriatal Disorders: Evolutionary Adaptiveness and Anomalous Lateralization. Brain Lang. 2000;73:297–320. doi: 10.1006/brln.2000.2308. [DOI] [PubMed] [Google Scholar]
- Brody JF. Evolutionary recasting: ADHD, mania and its variants. J Affect Disord. 2001;65:197–215. doi: 10.1016/S0165-0327(00)00170-1. [DOI] [PubMed] [Google Scholar]
- Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40:951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biol Psychol. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C. CPTII: Conners’ Continuous Performance Test. 2004. [Google Scholar]
- Davidson RJ. Affective Style and Affective Disorders: Perspectives from Affective Neuroscience. Cogn Emot. 1998;12:307–330. [Google Scholar]
- Davidson RJ. EEG Measures of Cerebral Asymmetry: Conceptual and Methodological Issues. Int J Neurosci. 1988;39:71–89. doi: 10.3109/00207458808985694. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Annual Review of Psychology, Vol. 40., Annual Review of Psychology. Annual Reviews; Palo Alto, CA: 1989. Neurobehavioral aspects of affective disorders; pp. 457–492. [DOI] [PubMed] [Google Scholar]
- Floden D, Stuss DT. Inhibitory Control is Slowed in Patients with Right Superior Medial Frontal Damage. J Cogn Neurosci. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- Hale TS, Loo SK, Zaidel E, Hanada G, Macion J, Smalley SL. Rethinking a Right Hemisphere Deficit in ADHD. J Atten Disord. 2009a;13:3–17. doi: 10.1177/1087054708323005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Dang J, Hanada G, Macion J, McCracken JT, McGough JJ, Loo SK. ADHD familial loading and abnormal EEG alpha asymmetry in children with ADHD. J Psychiatr Res. 2010;44:605–615. doi: 10.1016/j.jpsychires.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Hanada G, Macion J, McCracken JT, McGough JJ, Loo SK. Atypical alpha asymmetry in adults with ADHD. Neuropsychologia. 2009b;47:2082–2088. doi: 10.1016/j.neuropsychologia.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie LD, Alloy LB, Fearn M. Effect of Bipolar Disorder on Left Frontal Cortical Responses to Goals Differing in Valence and Task Difficulty. Neurobiol Ther Antidepressant-Resist Depress. 2008;63:693–698. doi: 10.1016/j.biopsych.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. J Pers Soc Psychol. 2002;82:610–618. doi: 10.1037/0022-3514.82.4.610. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol. 1997;106:159–163. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biopsychology Emot Curr Theor Empir Perspect. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J Pers Soc Psychol. 2001;80:797–803. doi: 10.1037/0022-3514.80.5.797. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD, Bohlig A, Harmon-Jones C. Anger, coping, and frontal cortical activity: The effect of coping potential on anger-induced left frontal activity. Cogn Emot. 2003;17:1–24. doi: 10.1080/02699930302278. [DOI] [PubMed] [Google Scholar]
- Jan Kybic, Maureen Clerc, Olivier Faugeras, Renaud Keriven, Théo Papadopoulo. Generalized head models for MEG/EEG: boundary element method beyond nested volumes. Phys Med Biol. 2006;51:1333. doi: 10.1088/0031-9155/51/5/021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keune PM, Schönenberg M, Wyckoff S, Mayer K, Riemann S, Hautzinger M, Strehl U. Frontal alpha-asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biol Psychol. 2011;87:306–310. doi: 10.1016/j.biopsycho.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Keune PM, Wiedemann E, Schneidt A, Schönenberg M. Frontal brain asymmetry in adult attention-deficit/hyperactivity disorder (ADHD): Extending the motivational dysfunction hypothesis. Clin Neurophysiol. 2015;126:711–720. doi: 10.1016/j.clinph.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/S1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Delorme A, Walshaw PD, Cho AL, Bilder RM, McGough JJ, McCracken JT, Makeig S, Loo SK. Electroencephalography Correlates of Spatial Working Memory Deficits in Attention-Deficit/Hyperactivity Disorder: Vigilance, Encoding, and Maintenance. J Neurosci. 2014;34:1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Lenartowicz A, Makeig S. Use of EEG biomarkers in child psychiatry research: current state and future directions. J Child Psychol Psychiatry. 2015 doi: 10.1111/jcpp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, AnlloVento L, Jung P, Bell AJ, Sejnowski TJ, Hillyard SA. Independent component analysis of event-related potentials during a selective attention task. Soc Neurosci Abstr. 1996;22:1698. [Google Scholar]
- Mitchell JT. Behavioral Approach in ADHD: Testing a Motivational Dysfunction Hypothesis. J Atten Disord. 2010;13:609–617. doi: 10.1177/1087054709332409. [DOI] [PubMed] [Google Scholar]
- Mitchell JT, Robertson CD, Kimbrel NA, Nelson-Gray RO. An Evaluation of Behavioral Approach in Adults with ADHD. J Psychopathol Behav Assess. 2011;33:430–437. doi: 10.1007/s10862-011-9253-6. [DOI] [Google Scholar]
- Neal LB, Gable PA. Neurophysiological markers of multiple facets of impulsivity. Biol Psychol. 2016;115:64–68. doi: 10.1016/j.biopsycho.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Oldrati V, Patricelli J, Colombo B, Antonietti A. The role of dorsolateral prefrontal cortex in inhibition mechanism: A study on cognitive reflection test and similar tasks through neuromodulation. Neuropsychologia. 2016;91:499–508. doi: 10.1016/j.neuropsychologia.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Castellanos FX. Top-Down Dysregulation—From ADHD to Emotional Instability. Front Behav Neurosci. 2016;10:70. doi: 10.3389/fnbeh.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires L, Leitão J, Guerrini C, Simões MR. Event-Related Brain Potentials in the Study of Inhibition: Cognitive Control, Source Localization and Age-Related Modulations. Neuropsychol Rev. 2014;24:461–490. doi: 10.1007/s11065-014-9275-4. [DOI] [PubMed] [Google Scholar]
- Rybak M, Crayton JW, Young IJ, Herba E, Konopka LM. Frontal Alpha Power Asymmetry in Aggressive Children and Adolescents with Mood and Disruptive Behavior Disorders. Clin EEG Neurosci. 2006;37:16–24. doi: 10.1177/155005940603700105. [DOI] [PubMed] [Google Scholar]
- Schiller B, Gianotti LRR, Nash K, Knoch D. Individual Differences in Inhibitory Control—Relationship Between Baseline Activation in Lateral PFC and an Electrophysiological Index of Response Inhibition. Cereb Cortex. 2014;24:2430–2435. doi: 10.1093/cercor/bht095. [DOI] [PubMed] [Google Scholar]
- Sigi Hale T, Bookheimer S, McGough JJ, Phillips JM, McCracken JT. Atypical Brain Activation During Simple & Complex Levels of Processing in Adult ADHD: An fMRI Study. J Atten Disord. 2007;11:125–139. doi: 10.1177/1087054706294101. [DOI] [PubMed] [Google Scholar]
- Smith EE, Reznik SJ, Stewart JL, Allen JJB. Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Rigor Replication Improv Best Pract Psychophysiological Res. 2017;111:98–114. doi: 10.1016/j.ijpsycho.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Joel S, Nelson TE, Vasa RA, Pekar JJ, Mostofsky SH. Different Neural Patterns Are Associated With Trials Preceding Inhibitory Errors in Children With and Without Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:705–715. e3. doi: 10.1016/j.jaac.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos GA, Wasserstein J. Attention Deficit/Hyperactivity Disorder as a Right Hemisphere Syndrome. Ann N Y Acad Sci. 2001;931:172–195. doi: 10.1111/j.1749-6632.2001.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WASI: Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- Weintraub M, Youngstrom EA, Marvin S, Podell J, Walshaw PD, Kim EY, Suddath R, Matkevich B, Miklowitz DJ. Diagnostic profiles and clinical characteristics of youth referred to a pediatric mood disorders clinic. J Psychiatr Sci. 2014;20:154–162. doi: 10.1097/01.pra.0000445251.20875.47. http://dx.doi.org/10.1097/01.pra.0000445251.20875.47. [DOI] [PMC free article] [PubMed] [Google Scholar]