Abstract
Objective
To identify the predictors of time from initial diagnosis of metastatic castration-resistance prostate cancer (mCRPC) to all-cause death within the Shared Equal Access Regional Cancer Hospital cohort.
Patients and Methods
We performed a retrospective analysis of 205 mCRPC men. Overall survival was estimated and plotted using the Kaplan-Meier method. The uni- and multivariable overall survival predictors were evaluated with Cox proportional hazards model. A nomogram was generated to predict overall survival at 1, 2, 3, and 5 years after mCRPC. Concordance index and calibration plot were obtained.
Results
A total of 170 men (83%) died over a median follow up of 41 months. In univariable analysis, older age, more remote year of mCRPC, nonblack race, greater number of bone metastasis, higher prostate-specific antigen (PSA) levels, shorter PSA doubling time, and faster PSA velocity at mCRPC diagnosis were significantly associated with shorter overall survival (all P<.05). In multivariable analysis, older age, more remote year of mCRPC, greater number of bone metastasis, higher PSA levels and shorter PSA doubling time at mCRPC diagnosis remained significantly associated with shorter overall survival (all P<0.05). On the basis of these variables a nomogram was generated yielding a concordance index of 0.67 and good calibration.
Conclusion
The use of clinical parameter such as age, disease burden, PSA levels and kinetics can be used to estimate overall survival in mCRPC patients.
Keywords: Disease-Free Survival, Metastasis, Mortality, Prostate Cancer, Prostatectomy, Prostate Specific Antigen
Introduction
Although metastatic castration-resistant prostate cancer (mCRPC) patients generally have an unfavorable prognosis, not all patients have an identical clinical course. Indeed, some patients quickly experience progression to widespread metastatic disease and die of cancer, while others have a much more indolent disease progression.1 Previously, several predictive models have been proposed to estimate the survival of mCRPC patients.2–5 However, most of these studies used data from clinical trials evaluating patients after chemotherapy. Given the availability of new non-chemotherapic agents to treat mCRPC in recent years, including sipuleucel T6, abiraterone7, enzalutamide8 and radium-2239, not all patients receive chemotherapy immediately after mCRPC diagnosis. The survival and predictors of mortality at the time of initial mCRPC diagnosis, before any treatment is received, have been evaluated in a few studies.10 Moreover, given that patients in clinical trials are generally a highly selected group that does not necessarily represent the average mCRPC population (i.e. healthy enough to undergo an experimental therapy) along with well-known low rates of trial participation among black men and men of lower socioeconomic status, survival studies using clinical databases which are more likely to reflect survival probabilities outside clinical trials are needed.11
We thus investigated the predictors of time from mCRPC diagnosis to all-cause mortality among patients within the Shared Equal Access Regional Cancer Hospital (SEARCH), a database of patients with prostate cancer (PC) treated at Veteran Affairs hospitals. We developed a nomogram to predict the survival probabilities at 1, 2, 3, and 5 years after mCRPC diagnosis.
Methods
Study Population
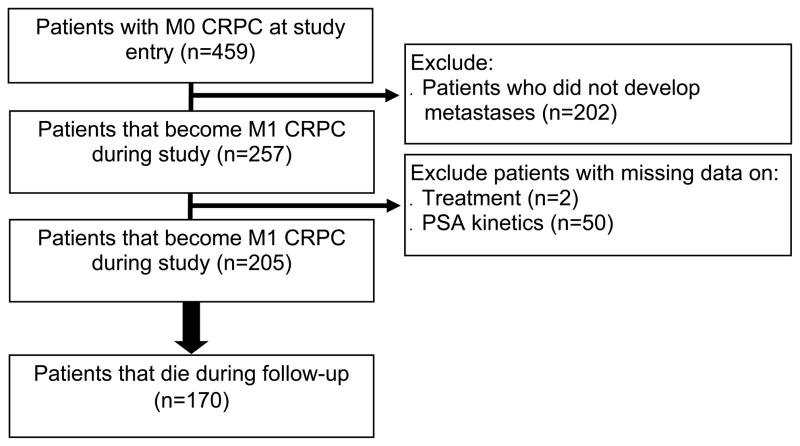
After obtaining institutional review board approval, data from PC patients who received androgen deprivation therapy (ADT) between 1983 and 2013 at 2 Veteran Affairs Medical Center (San Diego, CA and Durham, NC) and had prostate-specific antigen (PSA) levels ≥ 2 ng/mL after initiating ADT were abstracted into an electronic database. The database included information on patient age at time of mCRPC, race, PSA levels and kinetics (such as PSA doubling time [PSADT] and PSA velocity [PSAV]), tumor pathology (Gleason grade and stage), radiology studies (including simple x-rays, bone scans, computed tomography scans, magnetic resonance imaging), primary and secondary treatments for PC, and overall survival.12 A total of 7888 subjects on ADT for PC with PSA levels ≥2ng/mL after initiating ADT were entered in the database. Of these, 459 (9%) had documented CRPC as defined by PSA progression per PC Working Group 2 definition (relative increase of 25% and absolute increase of 2ng/mL or more above nadir) in patients receiving continuous ADT (gonadotropin-releasing hormone agonist, antagonist, or bilateral orchiectomy), with no evidence of metastatic disease prior to CRPC diagnosis (i.e. M0CRPC), and were diagnosed with CRPC in 2000 or later.13 The rationale to exclude men with metastasis prior to CRPC is to generate a cohort of men who all had initial diagnosis of mCRPC without having had previous metastases. We limited our analysis to men diagnosed with CRPC in the year 2000 or later, as electronic databases within the Veteran Affairs Health System were limited before 2000. The characteristics of these 459 have been described previously.14 Of these 459 men with non-metastatic CRPC, 202 (28%) did not develop metastatic disease during follow-up and were excluded. Of the remaining 257 patients who had developed documented mCRPC, 52 (20%) had missing PSA or treatment data and were excluded. This resulted in a final study sample of 205 (80%) patients (Figure 1). Primary and secondary treatments for PC were at the discretion of the patient and treating physician.
Figure 1.
Patient Consort Diagram
Statistical Analysis
Baseline patient and disease characteristics at the time of mCRPC diagnosis are presented as absolute numbers and percentages, and as median and interquartile range (IQR) for categorical and continuous variables, respectively (Table 1). Time from mCRPC diagnosis to all-cause death was evaluated and plotted using the Kaplan-Meier function. The association of patient and disease characteristics with time from mCRPC to all-cause mortality was evaluated with the Cox proportional hazards model in multivariable analysis. Variables analyzed included patient’s age at mCRPC (continuous, in years), year of mCRPC diagnosis (continuous), patient’s race (black or non-black), treatment center, biopsy Gleason score (2–6, 3+4, 4+3–10 or unknown/not available), localized treatment for PC (none, radical prostatectomy ± radiation ± ADT, radiation alone ± ADT, other), number of bone metastases (1, 2, 3–9, ≥ 10 or visceral/lymph node metastasis only), metastases to lymph nodes (yes or no), metastases in visceral tissue (yes or no), PSA at mCRPC (continuous and log-transformed, in log[ng/mL]) PSADT at mCRPC (continuous and log-transformed, in log[months]), and PSAV at mCRPC (continuous, in ng/mL/year). The proportional hazard assumption was addressed by examining Schoenfeld residuals of each variable and tested with Grambsch and Therneau’s statistic.15 For nomogram development, variable selection was conducted using Akaike information criterion stepwise algorithm.16 Multicollinearity was evaluated using variation inflation factor. The nomogram was created based on the Cox proportional hazards regression model and designed to predict the overall survival probabilities at 1, 2, 3, and 5 years after mCRPC diagnosis. We internally validated the nomogram by determining the overall unadjusted and bias-corrected concordance index using bootstrapping (200 repetitions) and generating a calibration plot comparing predicted and actuarial overall survivals 2 years after mCRPC diagnosis.
Table 1.
Baseline Patient and Disease Characteristics in 205 Subjects
| Characteristic | Value |
|---|---|
| Number of Deaths | 170 (83%) |
| Age at Metastases (years) | 74 (67, 81) |
| Year of Metastases | 2007 (2005, 2010) |
| Race | |
| Non-black | 136 (66%) |
| Black | 69 (34%) |
| Treatment Center | |
| Center 1 | 97 (47%) |
| Center 2 | 108 (53%) |
| Biopsy Gleason score | |
| 2–6 | 31 (15%) |
| 3+4 | 37 (18%) |
| 4+3, 8–10 | 65 (32%) |
| Unknown/No Biopsy | 72 (35%) |
| Local Treatment | |
| None (Watchful Waiting/ADT) | 85 (41%) |
| Radical Prostatectomy ± Radiation | 49 (24%) |
| Radiation Alone | 71 (35%) |
| Number of Bone Metastases | |
| Bone Metastases | 181 (88%) |
| 1 | 36 (18%) |
| 2 | 23 (11%) |
| 3–9 | 69 (34%) |
| ≥10 | 53 (26%) |
| Visceral/Lymph Node Only | 24 (12%) |
| Metastases in Lymph Nodes | 40 (20%) |
| Metastases in Visceral Tissue | 15 (7%) |
| Metastases in Liver | 7 (3%) |
| Metastases in Lung | 1 (<1%) |
| Metastases in Other Soft Tissue | 8 (4%) |
| PSA at Metastases (ng/mL) | 52.4 (19.6, 145.4) |
| PSADT at Metastases (months) | 5.4 (3.4, 10.1) |
| PSAV at Metastases (ng/mL/year) | 33.6 (10.0, 92.7) |
| Total Follow-up (months)* | 40.7 (23.7, 79.9) |
Data are presented as n(%) or median (interquartile range).
Abbreviations: ADT = androgen deprivation therapy; CI = confidence interval; CRPC = castration-resistant prostate cancer; PSA = prostate specific antigen; PSADT = PSA doubling time; PSAV = PSA velocity.
Follow-up reported among those alive at last follow-up.
All statistical analyses were performed using Stata 12.1 (StataCorp, College Station, TX) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P < .05 was considered to indicate statistical significance.
Results
The median (IQR) age, year of mCRPC diagnosis, PSA level, PSADT, and PSAV at mCRPC were, respectively, 74 (67–81) years, 2007 (2005–2010), 52.4 (19.6–145.4) ng/mL, 5.4 (3.4–10.1) months, 33.6 (10.0–92.7) ng/mL/year. A total of 69 patients (34%) were black. Biopsy Gleason score was 2–6 in 31 cases (15%), 3+4 in 37 (18%), 4+3–10 in 65 (32%), and unknown/not available in 72 (35%). Local treatment for PC was none in 85 cases (41%), radical prostatectomy ± radiation ± ADT in 49 (24%) and radiation ± ADT in 71 (35%). A total of 181 patients (88%) had at least one bone metastasis, 145 (71%) at least 2 bone metastases, and 53 (26%) had 10 or more bone metastases, while 24 (12%) had visceral/lymph node metastasis only. Metastasis to lymph nodes was present in 40 patients (20%), while visceral metastasis was observed in 15 patients (7%). Of these, metastasis to the liver, lung, and other organs was found in 7, 1 and 8 patients, respectively.
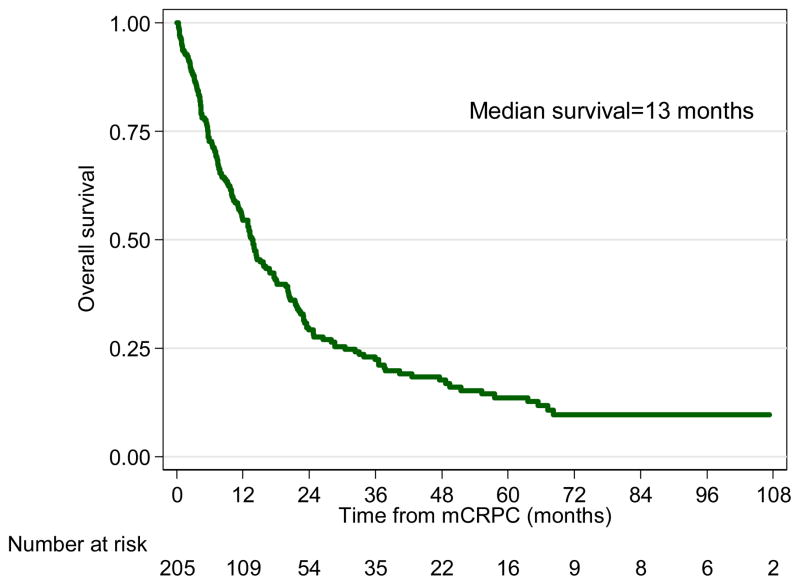
A total of 170 patients (83%) died over a median follow-up of 41 (IQR, 27–80) months. The median survival was 13 months (Figure 2). In univariable analysis, older age at mCRPC, more remote year of mCRPC diagnosis, nonblack race, greater number of bone metastasis, higher PSA levels, shorter PSADT and faster PSAV at mCRPC were significantly associated with shorter time from mCRPC diagnosis to all-cause death. In multivariable analysis, older age at mCRPC, more remote year of mCRPC diagnosis, greater number of bone metastasis, higher PSA levels, and shorter PSADT at mCRPC remained significantly associated with shorter time from mCRPC diagnosis to all-cause death (Table 2).
Figure 2.
Overall Survival of Metastatic Castration-Resistant Prostate Cancer Patients
Table 2.
Univariable and Multivariable Predictors of Overall Survival Among Metastatic Castration-Resistant Prostate Cancer Patients
| Predictor | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age at Metastases | 1.03 | 1.01–1.05 | <0.001 | 1.04 | 1.02–1.06 | <0.001 |
| Year of Metastases | 0.92 | 0.88–0.96 | <0.001 | 0.93 | 0.89–0.98 | 0.003 |
| Race | ||||||
| Non-black | ref. | ref. | ||||
| Black | 0.72 | 0.52–0.997 | 0.048 | 0.85 | 0.59–1.22 | 0.381 |
| Treatment Center | ||||||
| Center 1 | ref. | ref. | ||||
| Center 2 | 1.13 | 1.02–1.25 | 0.021 | 1.18 | 0.83–1.68 | 0.366 |
| Biopsy Gleason score | ||||||
| 2–6 | ref. | ref. | ||||
| 3+4 | 0.59 | 0.34–1.02 | 0.059 | 0.77 | 0.43–1.37 | 0.389 |
| 4+3, 8–10 | 0.94 | 0.59–1.49 | 0.784 | 0.99 | 0.60–1.63 | 0.966 |
| Unknown/No Biopsy | 0.91 | 0.58–1.43 | 0.671 | 0.73 | 0.44–1.23 | 0.239 |
| Local Treatment | ||||||
| Watchful Waiting/ADT | ref. | ref. | ||||
| Radical Prostatectomy ± Radiation ± ADT | 0.86 | 0.59–1.25 | 0.426 | 0.94 | 0.62–1.41 | 0.753 |
| Radiation ± ADT | 0.74 | 0.52–1.05 | 0.095 | 0.87 | 0.60–1.28 | 0.486 |
| Number of Bone Metastases | ||||||
| 1 | ref. | ref. | ||||
| 2 | 1.23 | 0.67–2.25 | 0.505 | 1.41 | 0.76–2.62 | 0.278 |
| 3–9 | 1.71 | 1.07–2.75 | 0.026 | 1.58 | 0.95–2.62 | 0.075 |
| ≥10 | 3.92 | 2.38–6.45 | <0.001 | 2.88 | 1.68–4.93 | <0.001 |
| Visceral/Lymph Node Only | 1.66 | 0.92–3.00 | 0.093 | 1.13 | 0.49–2.61 | 0.775 |
| Metastases in Lymph Nodes | 1.05 | 0.72–1.53 | 0.795 | 1.11 | 0.63–1.97 | 0.714 |
| Metastases in Visceral Tissue | 1.58 | 0.91–2.74 | 0.103 | 1.63 | 0.91–2.94 | 0.102 |
| PSA at Metastasesa | 1.22 | 1.11–1.35 | <0.001 | 1.16 | 1.04–1.29 | 0.010 |
| PSADT at Metastasesa | 0.78 | 0.67–0.92 | 0.003 | 0.81 | 0.69–0.95 | 0.010 |
| PSAV at Metastasesb | 1.02 | 1.01–1.04 | <0.001 | 1.01 | 1.00–1.03 | 0.081 |
PSA, PSADT, and PSAV are modeled separately in the multivariable model.
Abbreviations: ADT = androgen deprivation therapy; CI = confidence interval; CRPC = castration-resistant prostate cancer; HR = hazard ratio; PSA = prostate specific antigen; PSADT = PSA doubling time; PSAV = PSA velocity.
Log transformed.
Per 10 ng/mL/year change
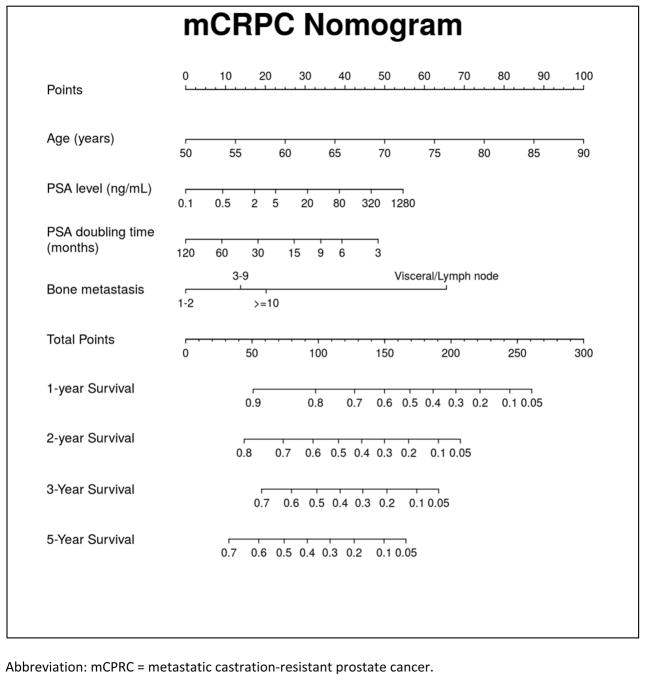
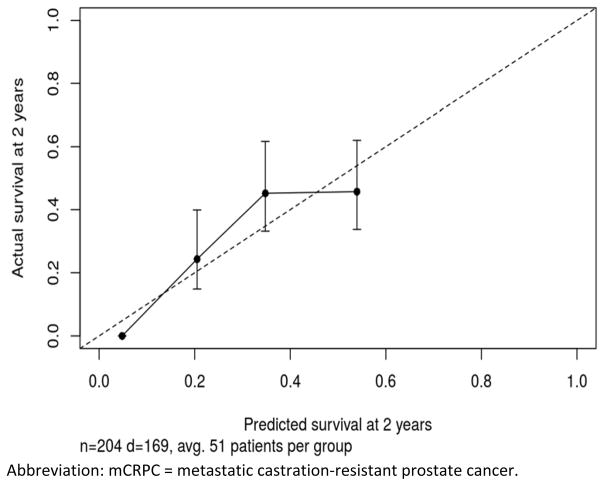
On the bases of the multivariable model, a nomogram predicting overall survival probabilities at 1, 2, 3, and 5 years after mCRPC diagnosis was generated (Figure 3, Supplemental Equation 1, and Supplemental Tables 1 and 2). Overall, the nomogram had an adequate performance with an unadjusted concordance index of 0.68 and bias-corrected concordance index of 0.67. The calibration for 2-year survival predictions was satisfactory (Figure 4, Supplemental Figure 1).
Figure 3.
Metastatic Castration-Resistant Prostate Cancer Nomogram
Abbreviation: mCPRC = metastatic castration-resistant prostate cancer.
Figure 4.
Calibration Plot Comparing Predicted and Actuarial Overall Survival at 2 Years After mCRPC Diagnosis
Abbreviation: mCRPC = metastatic castration-resistant prostate cancer.
Discussion
mCRPC is considered a late stage in the natural history of PC. Although mCRPC is usually associated with an ominous prognosis, many patients experience a more indolent disease progression. Indeed, although we found the median survival of mCPRC patients in our series with a median year of mCRPC diagnosis of 2007 to be only 13 months, our data suggest approximately 15% survived beyond 5 years. Given this heterogeneity in prognosis, we sought to identify the predictors of time from metastasis to mortality among mCRPC patients within the SEARCH cohort. We found older age at mCRPC, more remote year of mCRPC diagnosis, greater number of bone metastases, higher PSA levels, and shorter PSADT at mCRPC were independently associated with shorter time from mCRPC diagnosis to all-cause mortality. Using the variables above, we developed a nomogram to predict the survival probabilities at 1, 2, 3 and 5 years after mCRPC diagnosis. The nomogram showed an adequate concordance (concordance index=0.67) and good calibration. Thus, the nomogram can help identify those patients with mCRPC who have an even worse prognosis and those who are expected to have a more favorable disease progression. This risk stratification can help physicians identify very high-risk patients who theoretically may benefit from more aggressive approaches and should be enrolled on novel clinical trials. Likewise, men at lower risk of death may be able to avoid initial treatment.
Multiple clinical trials have reported varying overall survival times for men with “early stage” mCRPC. These estimates have ranged from a low of 16 to 19 months for men receiving first-line chemotherapy17 to 32 months in more recent trials of men receiving enzalutamide.8 In the current study, median survival was 13 months, which is lower than prior studies. This is likely due to several factors. First, median year of diagnosis in our study was 2007. Thus, the majority of men were diagnosed and treated in the docetaxel-only era (i.e. prior to the availability of recent agents which have been shown to extend survival). As such, our median 13-month survival is not far from the TAX 327 study (randomized trial of mitoxantrone vs. docetaxel) wherein median survival was 16.5 months for mitoxantrone and 18.9 for docetaxel. Second, consistent with this, year of mCRPC diagnosis was one of the strongest risk factors for survival. Indeed, the effect for each 1 year of more recent diagnosis (adjusting for baseline characteristics) was a 7% death risk reduction. This effect, compounded over 8 years (from median year of diagnosis of 2007 to 2015) would result in a hazard ratio for death of 0.56 in the year 2015 compared to 2007 (median in the current study) and translate into a median 23-month survival for men diagnosed in 2015. Third, our study was not limited to clinical trial patients, but represents data from clinical practice outside a clinical trial environment. It has been shown that patients enrolled onto clinical trials outperform nontrial patients even if randomized to standard of care.18 As such, survival estimates based upon clinical trial data may overestimate survival and give false hope to patients. Fourth, consistent with this, the age of patients in our study was older than in clinical trials of mCRPC patients. Our sample’s median age was 74 compared to 68 in TAX 32717, 19, 71 in Cougar 302 (pre-chemotherapy trial of abiraterone + prednisone vs. prednisone),20 and 72 in PREVAIL (prechemotherapy trial of enzalutamide vs. placebo).8 For each 1 year older, the risk of death was 4% higher in our study. Thus, adjusting a 74-year-old median age down to 72 as in PREVAIL, would improve our median survival by 8% to 25 months, which is only slightly worse than the 30 month survival for placebo-treated patients. Thus, while 13-month median survival for our whole cohort is quite low for modern mCRPC, accounting for year of mCRPC diagnosis and age at mCRPC diagnosis, our data would predict a survival that is in line, albeit somewhat lower than, the most recent clinical trial data. As such, we believe our findings are robust and present data for non-trial patients. Obviously, other observational datasets will be required to validate our findings.
While median survival for a man diagnosed with mCRPC today may be 2 to 3 years, there is tremendous heterogeneity of prognosis among mCRPC patients. As such, several studies evaluated the predictors of mortality in mCRPC. For example, Halabi et al2 found worse performance status, visceral metastasis, higher lactate dehydrogenase, more opioid analgesic use, lower serum albumin, lower hemoglobin, higher PSA levels, and higher alkaline phosphatase to be associated with worse overall survival. Similarly, Pond et al, 21 evaluating 1006 men treated with docetaxel in a clinical trial, found men with lymph node-only metastatic disease had the most favorable prognosis among mCRPC patients. However, those with liver metastases had the worst overall survival. Likewise, Armstrong et al 4 showed the presence of liver metastases, greater number of metastatic sites, clinically significant pain, worse performance status, shorter PSADT, higher PSA levels, higher tumor grade, higher alkaline phosphatase lower hemoglobin to be independent factors associated with increased mortality. Similar to these previous findings, in our study, we found older age at mCRPC, more remote year of mCRPC diagnosis, greater number of bone metastasis, higher PSA levels and shorter PSADT at mCRPC were independently associated with shorter overall survival. Thus, given the factors associated with prognosis in our study were similar to the ones reported before, our findings using a clinical dataset validate the variable selection used in previous predictive models that used data from clinical trials. However, as discussed above, the models from clinical trials, given they may overestimate survival, need to be validated in clinical data sets. The same applies to our model. Although our nomogram had an acceptable concordance and good calibration, external validation of our findings are still required to evaluate its performance in a sample other than the development cohort.
Although our overall number of patients was small, we had a sizeable percentage of black men. We found that black men had similar (multivariable) or better (univariable) outcomes compared to nonblack men. This is important in that black men generally have more aggressive PC. However, our findings are in line with a prior study from Cancer and Leukemia Group B, which showed across multiple trials that black men had similar, if not better, outcomes.22 The reasons for this are not clear. Whether this reflects improved responsiveness to second and third-line treatments is unknown. Unfortunately, as most clinical trials include a dearth of black men, answering this question using clinical trial data will be virtually impossible.
The present study is limited by the retrospective nature of our cohort. As such, we had no control over how patients were treated before or after the diagnosis of mCRPC. Consequently, it is likely that with the development of newer agents to treat mCRPC in recent years (such as abiraterone and enzalutamide), the prognosis of mCRPC patients has changed over time. Although we adjusted our multivariable model for year of mCRPC diagnosis, we were unable to adjust for mCRPC treatment received, as the data were not available for all patients. Moreover, it is likely that the prognosis of mCRPC patients will continue to improve, which may cause the current nomogram to overestimate the risk of death. Furthermore, important variables that have been previously correlated with survival, such as performance status, comorbidities and various laboratory tests, were not available and thus not included in our models. Based upon this, we were unable to validate these external models or compare performance of our model to theirs. Missing data was an issue given we had to exclude 20% of our initial sample due to missing data. As a result of the limited number of patients with follow-up greater than 3 years, 3- and 5-year predictions may be less precise. Finally, we were unable to evaluate PC-specific mortality as cause of death was not available for all patients.
In conclusion, men with mCRPC have a poor prognosis with most men dying within 13 months, which adjusted to 2015 still amounted to a median survival of only 23 months. Age at mCRPC, more remote year of mCRPC diagnosis, greater number of bone metastasis, higher PSA levels and shorter PSADT at mCRPC were significantly associated with shorter overall survival. Using these variables, we created a nomogram to predict the overall survival probability at 1, 2, 3, and 5 years. After external validation, the nomogram may help physicians and researches stratify mCRPC patients according to their risk of death.
Supplementary Material
Clinical Practice Points.
mCRPC patients generally have an unfavorable prognosis, but not all patients have an identical clinical course. While some patients quickly progress to widespread metastatic disease and die of cancer, others have a much more indolent disease progression. Previously, several predictive models have been proposed to estimate the survival of mCRPC patients. However, most of these studies used data from clinical trials evaluating patients after chemotherapy.
We found that age, more remote year of mCRPC, greater number of bone metastasis, higher PSA levels and shorter PSADT at mCRPC diagnosis remained significantly associated with shorter overall survival. On the basis of these variables, a nomogram was generated yielding a concordance index of 0.67 and good calibration.
After external validation, our nomogram may help physicians and researches stratify mCRPC patients according to their risk of death.
Acknowledgments
Supported in part by the Department of Veterans Affairs, National Institute of Health (NIH) R01CA100938 (W.J.A.), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (W.J.A.), the Georgia Cancer Coalition (M.K.T.), NIH K24 CA160653 (S.J.F.), and Janssen.
Footnotes
Disclaimer: Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Disclosure
The authors have stated that they have no conflict of interest. The views and opinions of, and endorsements by, the authors do not reflect those of the US Army or the Department of Defense.
A supplemental equation and figure as well as supplemental tables accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clgc.2016.08.018
References
- 1.de Liano AG, Reig O, Mellado B, Martin C, Rull EU, Maroto JP. Prognostic and predictive value of plasma testosterone levels in patients receiving first-line chemotherapy for metastatic castrate-resistant prostate cancer. British journal of cancer. 2014;110:2201–2208. doi: 10.1038/bjc.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halabi S, Lin CY, Small EJ, et al. Prognostic model predicting metastatic castration-resistant prostate cancer survival in men treated with second-line chemotherapy. J Natl Cancer Inst. 2013;105:1729–1737. doi: 10.1093/jnci/djt280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong AJ, Garrett-Mayer ES, Yang YC, de Wit R, Tannock IF, Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AJ, Tannock IF, de Wit R, George DJ, Eisenberger M, Halabi S. The development of risk groups in men with metastatic castration-resistant prostate cancer based on risk factors for PSA decline and survival. European journal of cancer. 2010;46:517–525. doi: 10.1016/j.ejca.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. The lancet oncology. 2013;14:1193–1199. doi: 10.1016/S1470-2045(13)70424-8. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 10.Omlin A, Pezaro C, Mukherji D, et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. European urology. 2013;64:300–306. doi: 10.1016/j.eururo.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:536–542. doi: 10.1200/JCO.2012.45.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira DM, Cooperberg MR, Howard LE, et al. Predicting bone scan positivity after biochemical recurrence following radical prostatectomy in both hormone-naive men and patients receiving androgen-deprivation therapy: results from the SEARCH database. Prostate cancer and prostatic diseases. 2014 doi: 10.1038/pcan.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira DM, Howard LE, Sourbeer KN, et al. Predicting bone scan positivity in non-metastatic castration-resistant prostate cancer. Prostate cancer and prostatic diseases. 2015 doi: 10.1038/pcan.2015.25. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:12. [Google Scholar]
- 16.Akaike H. Fitting autoregressive models for prediction. Ann Inst Statist Math. 1969;21:243–247. [Google Scholar]
- 17.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 18.Selby P, Autier P. The impact of the process of clinical research on health service outcomes. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22(Suppl 7):vii5–vii9. doi: 10.1093/annonc/mdr419. [DOI] [PubMed] [Google Scholar]
- 19.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. The New England journal of medicine. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. European urology. 2014;65:3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Halabi S, Small EJ, Vogelzang NJ, Barrier RC, Jr, George SL, Gilligan TD. Impact of race on survival in men with metastatic hormone-refractory prostate cancer. Urology. 2004;64:212–217. doi: 10.1016/j.urology.2004.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.