Abstract
Aim
To evaluate the association between retinal capillary non-perfusion and diabetic retinopathy (DR) severity using optical coherence tomography-based microangiography (OMAG).
Methods
33 patients (51 eyes) with a history of diabetes underwent imaging with a 68 kHz Cirrus-5000 spectral domain OMAG prototype. Demographic and clinical characteristics were collected. The perfusion index (PI) was defined as per cent coverage of area by retinal vessels with flow, measured within a minimum of 6.8×6.8 mm2 OMAG scan. The PI in each ETDRS zone was analysed using an automated algorithm. Univariate and multivariate analyses were used to determine the degree of association between PI and DR severity.
Results
51 eyes with different DR severities were imaged. More severe DR was significantly associated with lower PI after adjusting for logarithm of the minimum angle of resolution best-corrected visual acuity, hyperlipidaemia, diabetes type and ETDRS ring in a multivariate mixed linear model. Compared with the none–mild non-proliferative diabetic retinopathy (NPDR) group, the moderate–severe NPDR group had 2.7 lower PI (p=0.03) and proliferative DR group had 4.3 lower PI (p=0.003). All ETDRS zones except for the foveal centre showed inverse associations between PI and DR severity (p values<0.001 to 0.862).
Conclusions
A statistically significant inverse association exists between PI and DR severity. Our study suggests that PI may become a useful biomarker in evaluating and following the progression of DR.
INTRODUCTION
Diabetes mellitus causes considerable morbidity and mortality and affects >422 million people. Diabetic retinopathy (DR) is the leading cause of blindness in the working-age population and accounts for 2.8% of blindness worldwide. Patients with diabetes are approximately 25 times more likely to become vision impaired than the general population.1,2 Multiple studies have demonstrated that hyperglycaemia, hyperlipidaemia and hypertension contribute to the pathogenesis of DR.3,4 Proposed pathways include vascular disruptions characterised by abnormal vascular flow, disruptions in structure, permeability and non-perfusion of capillaries.5,6 Thus, evaluating capillary perfusion and measuring areas of non-perfusion are critical in understanding DR.
The current gold standard for evaluating the retinal capillary perfusion is fluorescein angiography (FA). However, FA is invasive, requiring intravenous injection of fluorescein dye with possible side effects including urticaria, hypotension, nausea/emesis and rarely anaphylaxis.7 Additionally, FA can be a time-intensive procedure requiring multiple personnel to complete the imaging process. While FA is effective and accurate in delineating the foveal avascular zone, the evaluation of the adjacent multilaminar networks is limited; therefore, the earliest non-perfusion of capillaries in vaso-occlusive diseases may not be visualised.8 Furthermore, while FA shows the anatomical arrangement of the superficial retinal vessels, it does not correlate with the deeper retinal capillaries.9
With recent advances in optical coherence tomography angiography (OCTA), the ability to assess the microvasculature throughout different retinal layers in DR has transformed. Areas of non-perfusion in both superficial and deep vascular plexuses and loss of normal capillaries are easily detected with OCTA.10–12 OCT-based microangiography (OMAG), a type of OCTA, is an effective non-invasive imaging modality and has been used to evaluate the retinal and choroidal vasculature in various pathologies.13–15 By using both amplitude and phase in the OCT signals, OMAG allows imaging and measurements of both the morphological and functional parameters of blood perfusion in patients with DR.14,15
While multiple studies have evaluated the characteristic features of DR using OCTA,10,11 the association between capillary non-perfusion and the severity of DR has not been clearly established. The purpose of this study is to evaluate the association between the retinal capillary non-perfusion and the severity of DR using OMAG.
METHODS
Patients with a diagnosis of diabetes who were seen in retina clinics at the University of Washington, Seattle, Washington, USA between 1 January 2014 and 1 November 2015 were voluntarily enrolled in the study. Exclusion criteria included age <18 years old, non-English speakers or any other documented retinal vascular pathology. After a written informed consent, bilateral OMAG images were obtained. Images with poor signal strength or severe motion artefact were excluded from the final analysis.
OMAG imaging
A total of 33 patients (51 eyes) underwent imaging with a 68 kHz Cirrus-5000 SD-OCT-based angiography prototype (Carl Zeiss Meditec, USA), which operates at a central wavelength of 840 nm. To achieve OMAG imaging of retinal vasculature, a repeated B-mode scanning protocol was implemented in the prototype. Four repeated B-scans were acquired at one position and used to extract the blood flow signal as previously described13 with the total time for single-volume acquisition being 3.6 s excluding the adjustment time prior to data collection.
In this study, the Cirrus-5000 SD-OCT-based angiography prototype was equipped with motion tracking through an auxiliary real-time line scan ophthalmoscope and allowed montaging of images.16 The minimum array for the grid was 3×3, giving a field of view (FOV) of 6.8×6.8 mm2 (approximately 30–40° FOV) while maximum array was 4×6, providing a coverage of 9.0×13.4 mm2. Previously described OMAG algorithm14,15,17 was applied to all the volumetric data sets, and the large FOV en face OMAG was obtained by stitching the images automatically by use of a software coded with Matlab. Images were then converted into a binary format for data analysis using an automated algorithm. The en face images were created by using a maximum amplitude projection (MAP) method for total retinal flow. Before the MAP, a 3×3×3 pixel-kernel Gaussian filter was applied to enhance the quality of the images.
Perfusion index
Each en face projection of retinal circulation was aligned such that the fovea was the centre of the image and regions were segmented according to the nine ETDRS regions of the macula.18 The foveal region was defined as the central circle 1 mm in diameter. The inner ring was defined as having radii of 1–3 mm. The outer ring was defined as having radii of 3–6 mm. The inner and outer rings were further subdivided into superior, nasal, inferior and temporal regions.18 Each ETDRS region was used to independently sample perfusion. PI was defined as the per cent coverage of the area by retinal vessels with flow.
The following clinical characteristics were collected on each patient: age, gender, best-corrected visual acuity (BCVA) on logarithm of the minimum angle of resolution (logMAR) scale, type of diabetes (type 1 vs type 2), most recent haemoglobin A1c value (HbA1c), use of insulin, clinical severity of DR, history of hypertension and history of hyperlipidaemia on the last recorded date closest to the acquisition of the OMAG image.
STATISTICAL ANALYSES
Patients were divided a priori into three groups (none–mild non-proliferative diabetic retinopathy (NPDR), moderate–severe NPDR and proliferative diabetic retinopathy (PDR)) based on diabetic severity determined by the clinical examination of retina-trained specialists using the ETDRS criteria.18 Analysis of variance and Fisher’s exact test were performed for univariate analysis on continuous and categorical variables, respectively. Clinical variables trending towards significance on univariate analysis (p<0.200) were force entered into multivariate mixed linear regression models, which were used to determine the association of capillary PI with DR severity using R (http://www.r-project.org). All univariate analyses and the multivariate sub-field analyses included adjustment for within-subject correlation using mixed modelling. Multivariate analysis examining the perfusion by annulus ring included adjustment for within-subject and within-eye correlation using mixed modelling.
RESULTS
Representative images of each group of DR are shown in figure 1A–C. The analysis of capillary PI was performed in 51 eyes (17 with none–mild NPDR, 21 with moderate–severe NPDR and 13 with PDR). Demographic data for the 51 eyes are summarised in table 1. The mean±SD of age of the study population was 52.4±10.6 years, 47.6±11.7 years and 46.2±12.9 years for the none–mild NPDR group, moderate–severe NPDR group and PDR group, respectively. The mean±SD of BCVA on logMAR scale was 0.10±0.12 in the none–mild NPDR group, 0.15±0.16 in the moderate–severe NPDR group and 0.25±0.33 in the PDR group. The use of insulin varied from 52% to 77% with the lowest use being in the moderate–severe group and the highest being in the PDR group. The proportion of type 1 diabetics was similar in the moderate–severe NPDR and PDR groups (33% vs 31%) but lower in the none–mild NPDR group (24%). The association between capillary PI and each clinical variable including age, gender, logMAR BCVA, type of diabetes and presence of hypertension or hyperlipidaemia was analysed with univariate models, and those with p values of <0.200 were forced entered into multivariate analysis (table 2).
Figure 1.
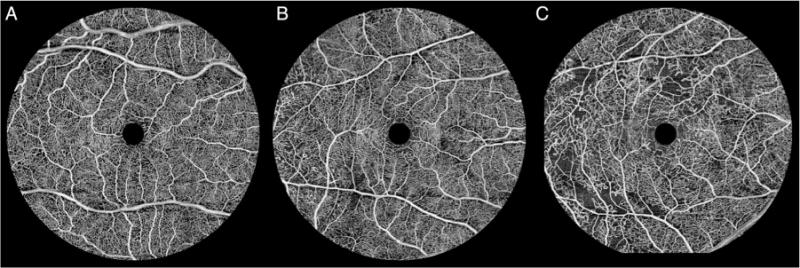
En face ocular coherence tomography-based microangiography images of macular capillary networks for the following diabetic retinopathy groups: none–mild non-proliferative diabetic retinopathy (A), moderate–severe non-proliferative diabetic retinopathy (B) and proliferative diabetic retinopathy (C).
Table 1.
Patient demographic and clinical characteristics
| No–mild NPDR (n=17) |
Moderate–severe NPDR (n=21) |
PDR (n=13) |
p Value | |
|---|---|---|---|---|
| Age, mean (SD) | 52.4 (10.6) | 47.6 (11.7) | 46.2 (12.9) | 0.32 |
| BCVA, mean (SD) | 0.10 (0.12) | 0.15 (0.16) | 0.25 (0.33) | 0.17 |
| HbA1c, mean (SD) | 7.40 (1.41) | 8.27 (1.47) | 8.90 (2.11) | 0.11 |
| Female gender, n (%) | 7 (41%) | 3 (14%) | 6 (46%) | 0.08 |
| HTN, n (%) | 7 (41%) | 12 (57%) | 8 (62%) | 0.56 |
| HLD, n (%) | 11 (65%) | 9 (43%) | 8 (62%) | 0.38 |
| DM type 1, n (%) | 4 (24%) | 7 (33%) | 4 (31%) | 0.86 |
| Insulin use, n (%) | 10 (56%) | 11 (52%) | 10 (77%) | 0.36 |
BCVA, best-corrected visual acuity; DM, diabetes mellitus; HbA1c, haemoglobin A1c; HLD, hyperlipidaemia; HTN, hypertension; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Table 2.
Mixed model univariate analysis of capillary perfusion index
| Factor | Estimates | p Value |
|---|---|---|
| Grade of retinopathy | ||
| None–mild NPDR | Reference | |
| Moderate–severe NPDR | −2.271 | 0.096 |
| PDR | −4.200 | 0.005* |
| Age | −0.063 | 0.206 |
| Male | 0.046 | 0.972 |
| logMAR BCVA | −5.177 | 0.154 |
| HgbA1c | −0.336 | 0.370 |
| Type 2 DM | −1.844 | 0.149 |
| Hypertension | −0.973 | 0.417 |
| Hyperlipidaemia | −1.576 | 0.188 |
| ETDRS zone | ||
| Central foveal | Reference | |
| Inner inferior | 34.725 | <0.001* |
| Inner nasal | 34.484 | <0.001* |
| Inner superior | 34.583 | <0.001* |
| Inner temporal | 34.418 | <0.001* |
| Outer inferior | 38.506 | <0.001* |
| Outer nasal | 37.550 | <0.001* |
| Outer superior | 38.479 | <0.001* |
| Outer temporal | 38.224 | <0.001* |
p Values <0.05.
BCVA, best-corrected visual acuity; DM, diabetes mellitus; HbA1c, haemoglobin A1c; logMAR, logarithm of the minimum angle of resolution; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
The PI was inversely proportional to the severity of DR. The mean capillary perfusion was 40.1 (SD=9.4) in none–mild NPDR, 37.6 (SD=11.0) in moderate–severe NPDR and 35.4 (SD=12.3) in PDR. A statistically significant, inverse relationship was found between the capillary PI and the severity of DR after adjusting for logMAR BCVA, hyperlipidaemia, diabetes type and ETDRS ring on a multivariate mixed model adjusting for within-subject and within-eye correlation (table 3). The mean PI values were nearly identical across subfields within each annulus (inner or outer). Increasing DR severity was significantly associated with lower capillary perfusion indices. In comparison to the none–mild NPDR group, the moderate– severe NPDR group had 2.7 lower PI (p=0.03) and the PDR group had 4.3 lower PI (p=0.003). No significant association was found between capillary perfusion indices and logMAR BCVA (p=0.95), hyperlipidaemia (p=0.35) or diabetes type (p=0.25).
Table 3.
Mixed model multivariate analysis of capillary perfusion index
| Factor | Estimates | p Value |
|---|---|---|
| Grade of retinopathy | ||
| None–mild NPDR | Reference | |
| Moderate–severe NPDR | −2.744 | 0.033* |
| PDR | −4.314 | 0.003* |
| logMAR BCVA | 0.244 | 0.947 |
| Type 2 DM | −1.541 | 0.246 |
| HLD | −1.154 | 0.345 |
| ETDRS ring | ||
| Inner ring | Reference | |
| Outer ring | 10.548 | <0.001* |
p Values <0.05.
BCVA, best-corrected visual acuity; DM, diabetes mellitus; HLD, hyperlipidaemia; logMAR, logarithm of the minimum angle of resolution; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
The negative association between the PI and the severity of DR was found to be significant within each ETDRS zone (figure 2). All ETDRS zones showed a similar pattern of capillary perfusion indices inversely correlating with diabetic severity scale (p value range <0.001 to 0.862) (table 4). The inner ETDRS zones demonstrated a stronger inverse association (p value range <0.001 to 0.818) between DR severity than the outer ETDRS zones.
Figure 2.
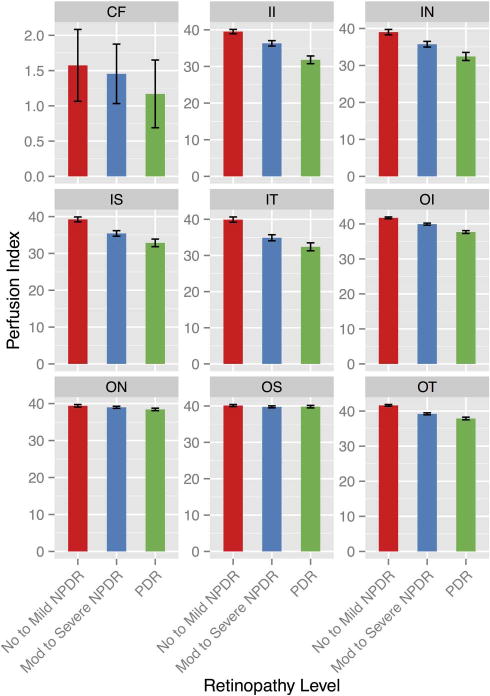
Perfusion index association with diabetic retinopathy severity groups across ETDRS zones. Macular retinal mean perfusion indices with SE bars by diabetic retinopathy severity groups: no to mild non-proliferative diabetic retinopathy (NPDR), moderate to severe non-proliferative diabetic retinopathy, proliferative diabetic retinopathy (PDR), calculated for each individual ETDRS zone. CF, central foveal; II, inner inferior; IN, inner nasal; IS, inner superior; IT, inner temporal; OI, outer inferior; ON, outer nasal, OS, outer superior; OT, outer temporal.
Table 4.
Mixed model multivariate analysis of capillary perfusion index by ETDRS zone adjusted for BCVA, type 2 DM, HLD and with Bonferroni adjusted p values
| None–mild NPDR | Moderate–severe NPDR
|
PDR
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ETDRS field | Crude mean perfusion |
Crude mean perfusion |
Estimates | p Value | Adjusted p value |
Crude mean perfusion |
Estimates | p Value | Adjusted p value |
| Central foveal | 1.57 | 1.45 | −0.058 | 0.818 | 1.000 | 1.17 | −0.323 | 0.258 | 1.000 |
| Inner inferior | 39.52 | 36.31 | −3.849 | 0.038* | 0.343 | 31.81 | −7.802 | <0.001* | 0.004* |
| Inner nasal | 38.99 | 35.78 | −3.523 | 0.070 | 0.629 | 32.32 | −5.978 | 0.007* | 0.066 |
| Inner superior | 39.25 | 35.44 | −4.031 | 0.008* | 0.071 | 32.86 | −6.346 | <0.001* | 0.003* |
| Inner temporal | 39.91 | 34.80 | −5.216 | 0.010* | 0.093 | 32.36 | −6.787 | 0.003* | 0.031* |
| Outer inferior | 41.74 | 39.94 | −1.619 | 0.284 | 1.000 | 37.68 | −3.076 | 0.073 | 0.655 |
| Outer nasal | 39.38 | 38.99 | −1.275 | 0.492 | 1.000 | 38.42 | −0.860 | 0.678 | 6.102 |
| Outer superior | 40.13 | 39.76 | −0.452 | 0.719 | 1.000 | 39.81 | 0.243 | 0.862 | 7.758 |
| Outer temporal | 41.62 | 39.20 | −2.179 | 0.129 | 1.000 | 37.88 | −2.370 | 0.141 | 1.269 |
p Values <0.05.
BCVA, best-corrected visual acuity; DM, diabetes mellitus; HLD, hyperlipidaemia; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
DISCUSSION
This study demonstrates that the capillary PI is inversely proportional to the severity of DR after adjusting for age, logMAR BCVA, hyperlipidaemia, diabetes type and ETDRS subfields. This inverse relationship between increasing DR severity and decreasing capillary perfusion indices was significant both within each DR severity group as a whole, as well as within each ETDRS zone. Additionally, increasing age was significantly associated with decreasing capillary perfusion indices.
Measuring capillary perfusion and areas of non-perfusion are crucial components of DR evaluation.5,6 Currently, the gold standard of assessing perfusion is FA; however, the ETDRS trial has shown that substantial interobserver variation exists when evaluating capillary loss, capillary dilation and narrowing of arteriolar branches in DR (weighted kappa, 0.41–0.60).19 Therefore, a consistently reliable, objective method of evaluating retinal capillary perfusion is needed.
Several studies have shown OCTA as a better imaging modality to examine the capillary perfusion. Spaide et al12 demonstrated that OCTA is superior in delineating the retinal capillary networks compared with FA. The radial peripapillary capillary networks in different layers of the retina were fully mapped on OCTA but only in few superotemporal or inferotemporal regions on FA. OCTA was shown to better image all different layers of the retinal vasculature unlike FA. This finding was consistent with the study by Weinhaus et al,8 which showed that FA was only able to delineate a fraction of the adjacent multilaminar capillary network compared with histology in monkey retinas. Thus, OCTA allows more detailed detection of the retinal microvasculature than FA.
Few studies have used OCTA to quantify the degree of capillary non-perfusion in DR. Ishibazawa et al10 compared the vascular abnormalities detected on FA versus OCTA in 47 eyes of 25 patients with DR. OCTA was superior in detecting the microaneurysms, areas of neovascularisation and areas of capillary non-perfusion. In this study, the areas of perceived non-perfusion were outlined manually by two masked observers, which differed from our study’s automated calculation of non-perfusion. A total of seven eyes underwent quantitative analysis of capillary non-perfusion near the macula, revealing larger areas of non-perfusion in the superficial plexus compared with deeper plexus. The number of eyes that underwent these evaluations were relatively small, and capillary non-perfusion was not used as a means of grading the severity of DR. Agemy et al20 focused on mapping of retinal vascular perfusion density in diabetic patients using OCTA and compared the capillary perfusion densities of 21 control and 56 eyes with DR. Skeletonised OCTA maps were used to examine the different layers of the retinal vasculature both in a 3×3 mm and 6×6 mm scan. A statistically significant difference was found between the capillary perfusion densities of control and diabetic patients. However, the subgroup analysis comparing the capillary perfusion density between eyes of varying stages of DR did not show a statistical significant difference in any group, except when comparing PDR to mild NPDR using 3×3 mm scan data. Our study differed in scanning techniques and analysis in several ways. First, their study used a different imaging system, Avanti RTVue-XR commercial spectral domain OCT system. Second, all of our scans were at least 6.8×6.8 mm2, thus we were able to analyse a larger area in its entirety as well as within each ETDRS zone. Lastly, a multivariate mixed model was used in our study to compare the capillary PI among stages of DR after adjusting for other clinical and demographic variables and for within-subject correlation.
Several reasons may explain an inverse relationship between capillary perfusion and DR severity. Hyperglycaemia results in microvascular damage through oxidant stress and the polyol pathway, specifically affecting the health of pericytes.5,21 Multiple studies have demonstrated that the retinal vascular tone is regulated both by pericytes and smooth muscle cells.5,22 Therefore, damage or loss of pericytes, one of the earliest manifestations in DR, results in disruptions of retinal haemodynamics and decreased capillary perfusion.21,23 Multiple animal models have demonstrated the toxic effects of hyperglycaemia on pericytes, resulting in cell death and loss of pericyte function.24,25 Thus, an increase in DR severity could result in increased microvascular damage, and consequently manifest as decreased capillary perfusion, which was observed in our study.
Not surprisingly, higher HbA1c values were weakly associated with higher degree of DR severity (p=0.37) in our study. This may represent the variable level of systemic diabetes control in our patient population. Interestingly, we found that the inner ETDRS zones had higher inverse associations with increasing diabetic severity than the outer ETDRS zones. We hypothesise that the significant variations in size of the larger retinal arcades and venules may have played a role in capillary perfusion variability and therefore may have limited the ability to detect more significant differences in capillary non-perfusion.
Several limitations exist in our study. We only sampled the macula region; thus, our PI may not reflect fully the degree of perfusion loss that exists in the periphery. Widefield OCTA may provide more accurate information on the PI by including peripheral vasculature. Furthermore, a larger sample size would have allowed for further classification of DR severity. Only one OCTA system was used; therefore, our results may not be generalisable to other algorithms. In addition, our patients had relatively good vision even in the PDR group, which could suggest some selection bias towards people with relatively good vision in the study (ie, patients with severe macular ischaemia may not have been recruited to our study due to poor fixation or vision). However, this bias would have decreased the probability of finding a significant association between the capillary index and DR severity. Lastly, our study did not include any evaluations of neovascularisation, a key aspect of PDR, and a future OCTA analysis on preretinal perfusion would be a useful method of correlating PDR versus NPDR.
In conclusion, this study demonstrates that the capillary PI has significant inverse associations with the severity of DR using OMAG. Capillary PI detected using OCTA may be used as an important clinical end point for follow-up and management of DR. Future studies to determine the temporal sequence of decrease in capillary perfusion and progression of DR may lead to important insights in the pathogenesis and potential preventive measures of DR progression.
Acknowledgments
Funding This work was supported in part by research grants from the Carl Zeiss Meditec (Dublin, California), the National Eye Institute (R01EY024158, K23EY024921), an unrestricted grant from Research to Prevent Blindness and the Department of Bioengineering at the University of Washington.
Competing interests RW received research support from Carl Zeiss Meditec and co-owns a patent that is related to the subject matter discussed in this manuscript with Oregon Health & Science University.
Ethics approval This study was approved by the Institutional Review Board of the University of Washington and was in adherence with the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Footnotes
Contributors ADL and AYL contributed equally and should be considered as co-first authors. Concept and design: QZ, CSL, RKW and AYL. Analysis and interpretation: ADL, CSL, AYL, QZ, RKW, KAR and JK. Writing the article: AYL, CSL, ADL and KAR. Data collection: ADL, CSL, AYL, QZ, RKW, JK and KAR. Literature search: ADL, CSL, AYL and KAR.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the views of the grant-giving bodies. All authors had access to clinical data.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.World Health Organization. The World Health Report. 2015 http://www.who.int/whr/en/ (accessed 5/2016)
- 2.Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–49. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–84. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy: XVII: the 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 5.Ciulla TA, Harris A, Latkany P, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80:468–77. doi: 10.1034/j.1600-0420.2002.800503.x. [DOI] [PubMed] [Google Scholar]
- 6.Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–7. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- 7.Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography. Ophthalmology. 1991;98:1139–42. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- 8.Weinhaus RS, Burke JM, Delori FC, et al. Comparison of fluorescein angiography with microvascular anatomy of macaque retinas. Exp Eye Res. 1995;61:1–16. doi: 10.1016/s0014-4835(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 9.Mendis KR, Balaratnasingam C, Yu P, et al. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010;51:5864–9. doi: 10.1167/iovs.10-5333. [DOI] [PubMed] [Google Scholar]
- 10.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160:35–44. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Hwang TS, Jia Y, Gao SS, et al. Optical coherence tomography angiography features of diabetic retinopathy. Retina (Philadelphia, Pa) 2015;35:2371. doi: 10.1097/IAE.0000000000000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Lee CS, Chao J, et al. Wide-field optical coherence tomography based microangiography for retinal imaging. Sci Rep. 2016;6:22017. doi: 10.1038/srep22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RK, Jacques SL, Ma Z, et al. Three dimensional optical angiography. Opt Express. 2007;15:4083–97. doi: 10.1364/oe.15.004083. [DOI] [PubMed] [Google Scholar]
- 15.Wang RK. Optical microangiography: a label-free 3-D imaging technology to visualize and quantify blood circulations within tissue beds in vivo. IEEE J Sel Top Quantum Electron. 2010;16:545–54. doi: 10.1109/JSTQE.2009.2033609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Huang Y, Zhang T, et al. Wide-field imaging of retinal vasculature using optical coherence tomography-based microangiography provided by motion tracking. J Biomed Opt. 2015;20:066008. doi: 10.1117/1.JBO.20.6.066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein T, Wieser W, Eigenwillig CM, et al. Megahertz OCT for ultrawide-field retinal imaging with a 1050 nm Fourier domain mode-locked laser. Opt Express. 2011;19:3044–62. doi: 10.1364/OE.19.003044. [DOI] [PubMed] [Google Scholar]
- 18.Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification: ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 19.Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:807–22. [PubMed] [Google Scholar]
- 20.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normal and diabetic retinopathy patients. Retina (Philadelphia, Pa) 2015;35:2353–63. doi: 10.1097/IAE.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 21.Paget C, Lecomte M, Ruggiero D, et al. Modification of enzymatic antioxidants in retinal microvascular cells by glucose or advanced glycation end products. Free Radic Biol Med. 1998;25:121–9. doi: 10.1016/s0891-5849(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 22.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–8. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 23.Ansari NH, Zhang W, Fulep E, et al. Prevention of pericyte loss by trolox in diabetic rat retina. J Toxicol Env Heal A. 1998;54:467–75. doi: 10.1080/009841098158755. [DOI] [PubMed] [Google Scholar]
- 24.Miyamura N, Bhutto IA, Amemiya T. Retinal capillary changes in Otsuka Long-Evans Tokushima Fatty rats (spontaneously diabetic strain) Ophthalmic Res. 1999;31:358–66. doi: 10.1159/000055559. [DOI] [PubMed] [Google Scholar]
- 25.Brignardello E, Beltramo E, Molinatti PA, et al. Dehydroepiandrosterone protects bovine retinal capillary pericytes against glucose toxicity. J Endocrinol. 1998;158:21–6. doi: 10.1677/joe.0.1580021. [DOI] [PubMed] [Google Scholar]


