CD36 is an 88-kDA plasma membrane glycoprotein found on platlets, monocytes, and endothelial cells. It is a type B scavenger receptor and, as is typical for scavenger receptors, it has a broad binding specificity and participates in a wide range of biological processes (reviewed in Platt and Gordon 1998; Yamada et al. 1998; Silverstein and Febbraio 2000). Malariologists have long been interested in CD36 because of its central role in cytoadhesion of Plasmodium falciparum-infected erythrocytes (Barnwell et al. 1989; Oquendo et al. 1989; reviewed in Newbold et al. 1999). Many investigators have hypothesized that another stage of Plasmodium, the sporozoite, also interacts with CD36 (Rich et al. 1990; Asch et al. 1992; Maeno et al. 1994). This hypothesis is based on sequence identity between the circumsporozoite protein (CS; Dame et al. 1984) and the thrombospondin-related anonymous protein (TRAP; Robson et al. 1988), two abundant sporozoite proteins that function during attachment to and invasion of cells (reviewed in Chitnis et al. 1999), and the CD36-ligand, thrombospondin (TSP; Lawler and Hynes 1986).
An alignment of the orthologous motifs from these three proteins is shown in Table I. In TSP, this sequence is found within the type I thrombospondin repeats (TSR; Lawler and Hynes 1986). Although both CS and TRAP have sequences with some similarity to the entire TSR, the region of greatest identity is shown in Table I. In CS this sequence is called region II-plus (Sinnis et al. 1994) and, for simplicity, we will use this name when referring to this sequence in TRAP and TSP also. As shown in Table I, it contains an upstream conserved tryptophan residue, followed by the amino acids CSVTCG and a string of basic and hydrophobic residues.
TABLE I.
Alignment of Region II-plus Motifs from TSP, CS, and TRAP
| Protein | Sequencea |
|---|---|
| Thrombospondin | PWSSCSVTCGDGVITRIR |
| P. falciparum CS Protein | EWSPCSVTCGNGIQVRIK |
| P. yoelii CS Protein | EWSQCSVTCGSGVRVRKR |
| P. falciparum TRAP | EWSPCSVTCGKGTRSRKR |
The conserved tryptophan is underlined, CSVTCG is shown in boldface, and the downstream basic and hydrophobic residues are in italics. The amino acid sequences derive from the following sources: TSP (Lawler and Hynes 1986), P. falciparum CS (Dame et al. 1984), P. yoelii CS (Lal et al. 1987), and P. falciparum TRAP (Robson et al. 1988).
The region II-plus motif is found in many proteins involved in cell–cell and cell–matrix interactions (Goundis and Reid 1988). In CS, TRAP, and TSP, it mediates binding to heparin and cell surface heparan sulfate proteoglycans (Prater et al. 1991; Frevert et al. 1993; Muller et al. 1993; Gantt et al. 1997). In TSP, this sequence also binds to CD36 (Catimel et al. 1992; Leung et al. 1992; Li et al. 1993; Pearce et al. 1995). Experiments with peptides have demonstrated that TSP binds to CD36 via the CSVTCG portion of this motif (Asch et al. 1992; Catimel et al. 1992; Dawson et al. 1997). In CS, peptide experiments have shown that the downstream basic residues are required for binding to heparan sulfate proteoglycans (Sinnis et al. 1994), and the function of the CSVTCG portion of the motif is not yet known (Gantt et al. 1997).
Sporozoites enter a vertebrate host during the bloodmeal of an infected anopheline mosquito. They are injected into the skin, far from their target organ, and must go through several cell barriers before they reach their destination, a hepatocyte (Sidjanski and Vanderberg 1997; Mota et al. 2001; Pradel and Frevert 2001). First they must cross an endothelial cell barrier to enter the blood circulation. From here they travel to the liver sinusoids where they then cross the continuous layer of sinusoidal cells and enter hepatocytes. There is evidence that sporozoite arrest in the liver is mediated by the interaction between CS and hepatic HSPGs (Pinzon-Ortiz et al. 2001 and reviewed in Sinnis and Sim 1997). After their arrest, the parasite must go through either Kupffer cells, the professional phagocytes of the liver sinusoids, or endothelial cells. CD36 is expressed on both Kupffer cells and sinusoidal endothelial cells (Volpes et al. 1990; Maeno et al. 1994). The sequence identity between the cell-adhesive regions of CS and TRAP and the CD36-binding region of TSP has led us and others to postulate that sporozoites may interact with CD36 as they traverse the sinusoids. The goal of this study was to investigate whether CD36 is required for sporozoite infectivity in the vertebrate host.
Initially we tested a monoclonal antibody against CD36, mAb 8A6 (Barnwell et al. 1989), in an in vitro sporozoite invasion assay with HepG2 cells, a hepatoma cell line that expresses CD36 (data not shown; Maeno et al. 1994) and supports sporozoite development in vitro (Hollingdale et al. 1983). It has been previously demonstrated that mAb 8A6 inhibits the interaction between CD36 and TSP (Pearce et al. 1994, 1995) and we hypothesized that it might also inhibit sporozoite invasion of HepG2 cells. As shown in Table II, the antibody had no effect on sporozoite invasion, suggesting that CD36 is not involved in this process. In this assay, however, sporozoites are added directly to a monlayer of target cells. While this is not a problem if one is looking for a receptor required for hepatocyte entry, it does not reflect conditions in vivo, where sporozoites are released at a distance from their target cell. It could be argued, therefore, that a negative result does not rule out the possibility that CD36 is used by sporozoites as they traverse the sinusoid. In fact studies show that in the liver, CD36 is found predominantly on endothelial cells and Kupffer cells and not on hepatocytes (Volpes et al. 1990). The localization of CD36 in vivo, therefore, is more consistant with a role in sporozoite migration to, and not invasion of, hepatocytes.
TABLE II.
Monoclonal Antibody to CD36 Does Not Inhibit Plasmodium Sporozoite Invasion of HepG2 Cells
| Expt. a | Treatment | Number of EEFsb |
|---|---|---|
| 1 | Control | 249 ± 4 |
| MAb 8A6 | 238 ± 13 | |
| 2 | Control | 714 ± 53 |
| Mab 8A6 | 620 ± 22 |
Experiments 1 and 2 were performed with HepG2 cells (ATCC HB8065; Rockville, MD), plated in DMEM with 10% fetal calf serum in labtek chamber slides, and allowed to grow overnight. Plasmodium berghei sporozoites in medium with or without 100 μg/ml mAb 8A6 were added to HepG2 cells. After 2 h at 37°C, unattached sporozoites were removed, fresh medium was added, and 2 days later cells were fixed and exoerythrocytic forms (EEFs) were visualized with mAb 2E6 (directed against the liver stage of P. berghei) and goat anti-mouse Ig conjugated to horseradish peroxidase.
Number of EEFs per 20 fields. Each point was plated in triplicate and shown are the means with standard deviations.
To investigate the role of CD36 in vivo, we tested sporozoite infectivity in CD36-deficient mice. These mice were generated by homologous recombination in 129 Sv embryonic stem cells as previously described (Febbraio et al. 1999, 2000). No CD36 protein is present in these mice in any tissue. In addition, they have been backcrossed four times to C57Bl/6j to achieve a background homozygosity of greater than 90%. Control mice were C57Bl/6j of the same sex and age, raised in the same facility. Mice were injected with Plasmodium yoelii sporozoites, and 40 h later their livers were harvested, total RNA was extracted, and a competitive RT-PCR assay was used to quantify Plasmodium rRNA. In this assay, a known concentration of competitor template is added to each PCR so that simultaneous amplification of parasite and competitor templates occurs. The amplification products of the competitor and parasite DNA differ in size and can be separated by gel electrophoresis and analyzed by densitometry. Results of this experiment are shown in Fig. 1. The PCRs in Figs. 1A and 1B were performed with 1 and 5 pg of competitor, respectively. When 5 pg of competitor was used the intensities of the competitor and target bands were equivalent and there were no significant differences between wild-type and CD36 null mice. Densitometry analysis of these results is plotted in Fig. 1C.
FIG. 1.
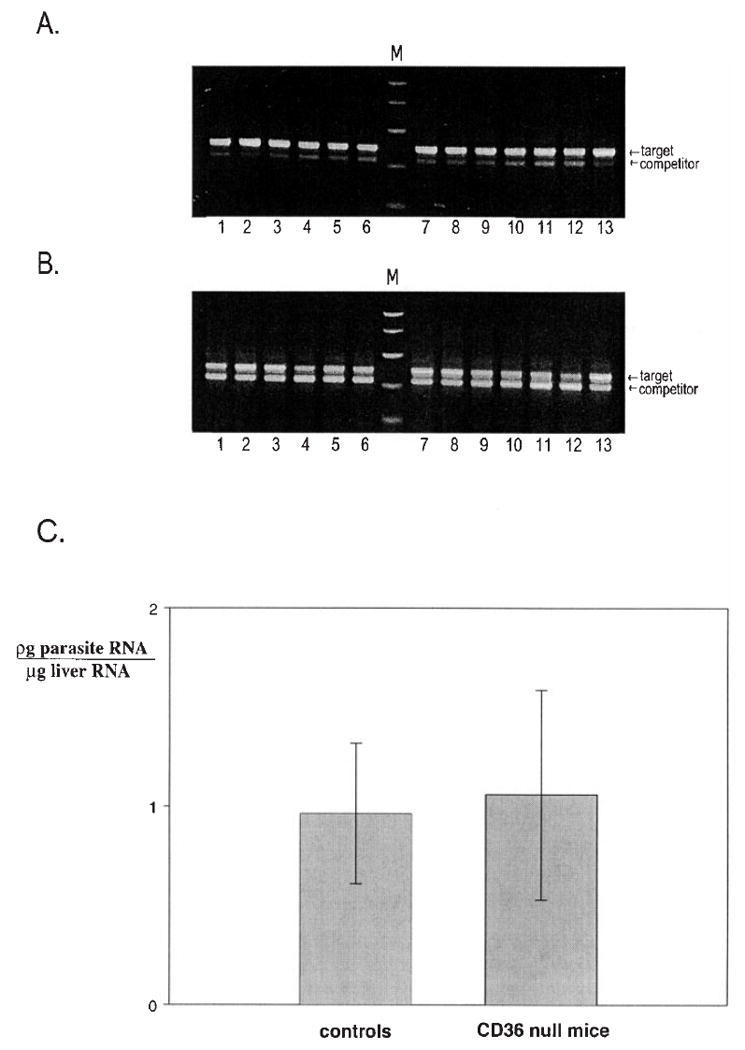
Plasmodium sporozoites are infective in CD36-deficient mice. CD36 null mice and controls were injected intravenously with 4 × 104 Plasmodium yoelii sporozoites and 40 h later their livers were harvested and total liver RNA was isolated using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH). A competitive RT-PCR assay was used to measure parasite rRNA in the livers (Briones et al. 1996). RTs were performed with 1 μg of total RNA and random hexamers (PE Biosystems, Branchburg, NJ). PCRs of this cDNA were performed using primers that recognize P. yoelii-specific sequences within the 18S rRNA, in the presence of a competitor template as previously described (Briones et al. 1996). The reactions were run on an agarose gel, stained with ethidium bromide, photographed, and analyzed by densitometry. Shown are PCR products of reactions using parasite rRNA primers and (A) 1 pg or (B) 5 pg of parasite rRNA competitor. The competitor is 333 bp and the target DNA is 393 bp. Molecular size markers (M); bp: 1000, 750, 500, 300, 150, and 50. (C) The photograph in B was scanned and analyzed by densitometry using the Gel plotting macro of the program NIH IMAGE (http://rsb.info.nih.gov/nih-image). For each amplification reaction a target/competitor ratio was calculated and this ratio was used to determine the amount of parasite RNA per microgram of liver RNA. The mean for each group of mice is plotted with error bars showing the range of values calculated. This experiment was repeated with seven mice per group with identical results.
It is important to note that only sporozoites which invade and undergo many cycles of replication are detected in this assay since the small amount of ribosomal RNA present in the injected sporozoites is below the sensitivity of the assay (Briones et al. 1996; P. Sinnis, unpublished results). Since we saw no difference between the experimental and the control groups, our data indicate that sporozoite localization to invasion of and development in hepatocytes occur with equal efficiency in both groups.
In conclusion, CD36 is not required for infection of hepatocytes by P. yoelii sporozoites. Although our data suggest that CD36 is not utilized by sporozoites during their journey to the hepatocyte, we have not excluded the possibility that CD36 is part of a redundant receptor system and, when absent, the sporozoite uses other molecules to enter or traverse cells.
Acknowledgments
The authors thank Jean Noonan and Ivette Caro for their superb technical assistance in the rearing and infection of mosquitoes, Consuelo Pinzon-Ortiz for dissection of sporozoites, and Dr. John Barnwell for his kind gift of mAb 8A6. This work was supported by National Institutes of Health Grant RO1 AI44470 and The Irma T. Hirschl Trust.
References
- Asch AS, Silbiger S, Heimer E, Nachman RL. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochemical and Biophysical Research Communications. 1992;182:1208–1217. doi: 10.1016/0006-291x(92)91860-s. [DOI] [PubMed] [Google Scholar]
- Barnwell JW, Asch AS, Nachman RL, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. Journal of Clinical Investigation. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones MRS, Tsuji M, Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Molecular and Biochemical Parasitology. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- Catimel B, Leung L, Ghissasi H, Mercier N, McGregor J. Human platelet glycoprotein IIIb binds to thrombospondin fragments bearing the C-terminal region, and/or the type I repeats (CSVTCG motif), but not to the N-terminal heparin-binding region. Biochemical Journal. 1992;284:231–236. doi: 10.1042/bj2840231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis C, Sinnis P, Miller LM. The sporozoite, the merozoite and the infected red cell: Receptors and host cells. In: Wahlgren M, Perlmann P, editors. Malaria: Molecular and Clinical Aspects. Academic Publishers; Amsterdam: 1999. pp. 249–285. [Google Scholar]
- Dame JB, Williams JL, McCutchan TF, Weber JL, Wirtz RA, Hockmeyer WT, Maloy WL, Haynes JD, Schneider I, Roberts DD, Sanders GS, Reddy EP, Diggs CL, Miller LH. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite. Plasmodium falciparum Science. 1984;225:593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Pearce SFA, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. Journal of Cell Biology. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. Journal of Biological Chemistry. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. Journal of Clinical Investigation. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. Journal of Experimental Medicine. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt SM, Clavijo P, Bai X, Esko JD, Sinnis P. Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. Journal of Biological Chemistry. 1997;272:19205–19213. doi: 10.1074/jbc.272.31.19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goundis D, Reid KBM. Properdin, the terminal complement components, thrombospondin and the circumsporozoite protein of malaria parasites contain similar sequence motifs. Nature. 1988;335:82–85. doi: 10.1038/335082a0. [DOI] [PubMed] [Google Scholar]
- Hollingdale MR, Leleand P, Leef JL, Schwartz AL. Entry of Plasmodium berghei sporozoites into cultured cells and their transformation into trophozoites. American Journal of Tropical Medicine and Hygiene. 1983;32:685–690. doi: 10.4269/ajtmh.1983.32.685. [DOI] [PubMed] [Google Scholar]
- Lal AA, De La Cruz VF, Welsh JA, Charoenvit Y, Maloy WL, McCutchan TF. Structure of the gene encoding the circumsporozoite protein of Plasmodium yoelii: A rodent model for examining antimalarial sporozoite vaccines. Journal of Biological Chemistry. 1987;262:2937–2940. [PubMed] [Google Scholar]
- Lawler J, Hynes RO. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. Journal of Cell Biology. 1986;103:1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LLK, Li WX, McGregor JL, Albrecht G, Howard RJ. CD36 peptides enhance or inhibit CD36-thrombospondin binding. Journal of Biological Chemistry. 1992;267:18244–18250. [PubMed] [Google Scholar]
- Li WX, Howard RJ, Leung LLK. Identification of SVTCG in thrombospondin as the conformation-dependent, high affinity binding site for its receptor, CD36. Journal of Biological Chemistry. 1993;268:16179–16184. [PubMed] [Google Scholar]
- Maeno Y, Fujioka H, Hollingdale MR, Ockenhouse CF, Nakazawa S, Aikawa M. Ultrastructural localization of CD36 in human hepatic sinusoidal lining cells, hepatocytes, human hepatoma (HepG2-A16) cells, and C32 amelanotic melanoma cells. Experimental Parasitology. 1994;79:383–390. doi: 10.1006/expr.1994.1100. [DOI] [PubMed] [Google Scholar]
- Mota M, Pradel G, Vanderberg JP, Hafalla JCR, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- Muller HM, Reckman I, Hollingdale MR, Bujard H, Robson KJH, Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO Journal. 1993;12:2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold C, Craig A, Kyes S, Rowe A, Fernandez-Reyes D, Fagan T. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. International Journal of Parasitology. 1999;29:927–937. doi: 10.1016/s0020-7519(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherance of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- Pearce SF, Wu J, Silverstein RL. A carboxyl terminal truncation mutant of CD36 is secreted and binds thrombospondin: Evidence for a single transmembrane domain. Blood. 1994;84:384–389. [PubMed] [Google Scholar]
- Pearce SFA, Wu J, Silverstein RL. Recombinant GST/CD36 fusion proteins define a thrombospondin binding domain. Journal of Biological Chemistry. 1995;270:2981–2986. doi: 10.1074/jbc.270.7.2981. [DOI] [PubMed] [Google Scholar]
- Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to cells. Journal of Biological Chemistry. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt N, Gordon S. Scavenger receptors: Diverse activities and promiscuous binding of polyanionic ligands. Chemistry & Biology. 1998;5:R193–R203. doi: 10.1016/s1074-5521(98)90156-9. [DOI] [PubMed] [Google Scholar]
- Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocytes invasion. Hepatology. 2001;33:1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- Prater CA, Plotkin J, Jaye D, Frazier WA. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. Journal of Cell Biology. 1991;112:1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich KA, George FWI, Law JL, Martin WJ. Cell-adhesive motif in region II of malarial circumsporozoite protein. Science. 1990;249:1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- Robson KJH, Hall JRS, Jennings MW, Harris TJR, Marsh K, Newbold CI, Tate VE, Weatherall DJ. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Sidjanski S, Vanderberg JP. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. American Journal of Tropical Medicine and Hygiene. 1997;57:426–429. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36 and atherosclerosis. Current Opinion in Lipidology. 2000;11:483–491. doi: 10.1097/00041433-200010000-00006. [DOI] [PubMed] [Google Scholar]
- Sinnis P, Sim BKL. Cell invasion by the vertebrate stages of. Plasmodium Trends in Microbiology. 1997;5:52–58. doi: 10.1016/s0966-842x(97)84657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Clavijo P, Fenyo D, Chait B, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. Journal of Experimental Medicine. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpes R, van den Oord JJ, Desmet VJ. Adhesive molecules in liver disease: Immunohistochemical distribution of thrombospondin receptors in chronic HBV infection. Journal of Hepatology. 1990;10:297–304. doi: 10.1016/0168-8278(90)90136-f. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: Roles for atherosclerosis, host defense and disorders of the central nervous system. Cellular and Molecular Life Sciences. 1998;54:628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]


