Abstract
OBJECTIVE
To investigate predictors of time to metastasis among men treated with androgen deprivation therapy for nonmetastatic prostate cancer who developed castration-resistant prostate cancer (CRPC) within the Shared Equal Regional Cancer Hospital cohort.
METHODS
This is a retrospective analysis of 458 nonmetastatic CRPC men. Metastases were detected in routine bone scans or other imaging tests. Predictors of time to metastasis were analyzed using proportional hazards model with CRPC as time zero.
RESULTS
A total of 256 (56%) men were diagnosed with metastatic disease over a median follow-up of 36 months. Metastasis-free survival was 79%, 65%, 52%, 47%, and 41% at 1,2,3,4, and 5 years after CRPC, respectively. In multivariable analysis, Gleason score 8–10 (hazard ration [HR] = 1.61; P = .026), receiving primary localized treatment (HR = 1.38; P = .028), higher prostate-specific antigen (PSA) levels at CRPC diagnosis (logPSA HR = 1.64; P ≤ .001), and PSA doubling time <6 months (HR = 1.42; P = .040) were independently associated with shorter time to metastasis. Race, year of CRPC, age, and time from androgen deprivation therapy to CRPC were not associated with metastasis.
CONCLUSION
Among nonmetastatic CRPC men, nearly 60% developed metastatic disease during the 5 years, with most of the metastasis occurring within the first 3 years. Higher Gleason score, receiving primary treatment, higher PSA, and shorter PSA doubling time were independently associated with shorter time to metastasis. Therefore, these variables can be used to stratify patients according to metastasis risk.
Keywords: Disease-Free Survival, Metastasis, Mortality, Prostate Cancer, Prostatectomy, Prostate Specific Antigen
INTRODUCTION
Metastatic prostate cancer poses a great health and economic burden, given that metastases are frequently associated with skeletal-related events (SREs) such as pain, pathologic fractures, spinal cord compression, and the need for surgery and/or radiotherapy to the bone.1 To date, only a few treatments have been shown to decrease these complications. For example, zolendronic acid,2 denosumab,3 radium-223,4 and abiraterone5 have all been shown to reduce the incidence of SREs and to prolong the time interval to first SRE in patients with metastatic prostate cancer. Given that bone metastases are frequently asymptomatic in the beginning, early diagnosis of metastatic disease by the use of radiologic studies allows for early institution of these treatments, potentially reducing the incidence of SREs and prolonging the time to first SRE.
Men with nonmetastatic castration-resistant prostate cancer (CRPC) have worse prognosis than their hormone-sensitive counterparts and are at a particularly higher risk of developing bone metastasis.6 Thus, nonmetastatic CRPC patients are the ones who theoretically would benefit the most from screening for bone metastasis. Yet the time to progression to metastatic disease varies from patient to patient. Additionally, most scans used to screen bone metastasis in patients with prostate cancer are negative.7 Thus, it seems reasonable to tailor the follow-up of these patients according to their risk of disease progression. However, there are only a few studies examining the natural history of these patients.8,9 In addition, the factors associated with rapid progression to metastatic disease have been evaluated in a small number of studies with limited sample sizes.8–11 Therefore, we sought to investigate the predictors of time to metastasis among men treated with androgen deprivation therapy (ADT) for nonmetastatic prostate cancer who developed CRPC within the Shared Equal Access Regional Cancer Hospital (SEARCH) cohort.
METHODS
Study population
After obtaining Institutional Review Board approval, data from 7,888 prostate cancer patients who received ADT between 1983 and 2013 at 2 Veteran Affairs Medical Center (San Diego, CA and Durham, NC) and had prostate-specific antigen (PSA) levels ≥2ng/mL after initiating ADT were entered in the study database. The database included information on patient age at time of CRPC, race, height, weight, PSA levels, prostate cancer pathology (tumor grade and stage), imaging tests and primary and secondary treatments for prostate cancer. A total of 869 (11%) patients had documented CRPC as defined by PSA progression per the PSA Working Group 2 definition (relative increase of 25% and absolute increase of 2ng/mL or more above the nadir) in patients receiving continuous ADT (luteinizing hormone-releasing hormone agonist, antagonist, or bilateral orchiectomy).12 Of these, 201 (23%) had metastatic disease at or before the time of CRPC diagnosis and were excluded. Of the remaining 668 patients, a total of 458 (69%) had radiologic follow-up with bone scans or other relevant imaging tests (abdomen or pelvic magnetic resonance imaging or computed tomography, spine imaging, and any other imaging tests positive for prostate cancer metastases) available for review and were included in the study. Primary and secondary treatments for prostate cancer were at the discretion of the patient and treating physician. The number, interval and trigger for imaging tests were also at the discretion of the patient and treating physician. Bone metastases were determined based on bone scans or X-rays. Soft tissue metastases were detected by magnetic resonance imaging or computed tomography. Imaging tests were read by radiologists who were not blinded to patient demographics, laboratory, and radiologic or pathologic results. Imaging tests were coded as positive or negative based upon the radiology report as interpreted by trained personnel. Equivocal scans, given they usually do not prompt a change in management, were considered negative unless confirmed positive by a biopsy.
Statistical analysis
PSA doubling time (PSADT) was calculated as the natural log of 2 divided by the slope of the linear regression of the natural log of PSA over time in months. All PSA values from the PSA nadir following ADT and within two years of CRPC diagnosis date were included. Patients were required to have at least 2 PSAs over at least 3 months. Patients with PSADT < 0 or > 120 were assigned to 120 months for ease of analysis. Baseline patient and disease characteristics at the time of CRPC diagnosis are presented as absolute numbers and percentages, and as median and interquartile range (IQR) for categorical and continuous variables, respectively. The association of patient and disease characteristics with time to metastasis was evaluated with Cox proportional hazard model in both univariable and multivariable analysis. Variables analyzed included race (black vs. other), biopsy Gleason score (2–6 vs. 7 vs 8–10 vs unknown), primary localized treatment (none vs. radical prostatectomy and/or radiation therapy), year of CRPC diagnosis, age at CRPC, PSA at CRPC (log-transformed), PSADT at CRPC (>6 months vs. ≤6 months vs. missing), and time from ADT to CRPC. The multivariable analysis was also adjusted for treatment center. The variables found to be significantly associated with time to metastasis were then evaluated with the log-rank test and plotted using the Kaplan-Meier method. In addition, we created a nomogram using stepwise variable selection. We used the Cox proportional hazards to analyze the association between the above variables and time to metastasis. The nomogram was designed to predict the 1-, 2-, 3-, 4- and 5-year metastasis-free probability. We internally validated the nomogram by determining the raw and boostrapped bias-corrected concordance index and plotting a calibration plot depicting the predicted and actuarial metastasis-free probabilities at 4 years. All statistical analyses were two-tailed and performed using Stata 12.0 (StataCorp, College Station, TX) and R 3.2.5 (R Foundation for Statistical Computing, Vienna, Austria). A P< .05 was considered to indicate statistical significance.
RESULTS
The median (and IQR) age at CRPC of our sample was 75 (67–81) years. A total of 143 (31%) patients were black. The median (and IQR) PSA at diagnosis of CRPC was 4.3 (2.9–9.2). There were 183 (40%) patients with PSADT >6 months, 141 (31%) with PSADT ≤ 6 months, and 134 (29%) with PSADT incalculable with available PSA values. Low (2–6), intermediate (7), high-grade (8–10) and unknown Gleason scores from the diagnostic biopsy were present in 76 (17%), 116 (25%), 116 (25%) and 150 (33%) patients, respectively. The median (and IQR) time from prostate cancer diagnosis to ADT, ADT to CPRC were 0.7 (0.2–3.6) and 3.6 (1.8–6.2) years, correspondingly. There were 274 (60%) patients who received primary localized treatment including radical prostatectomy and/or radiation therapy (Supplementary Table S1).
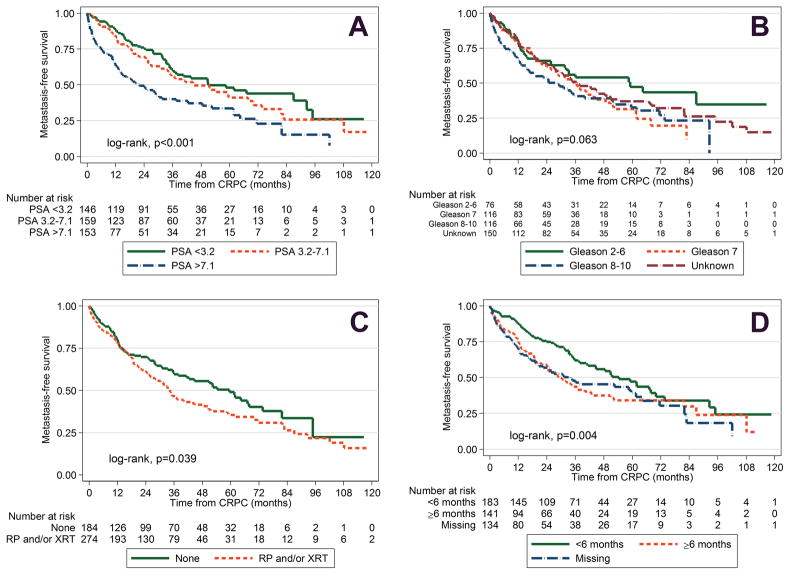
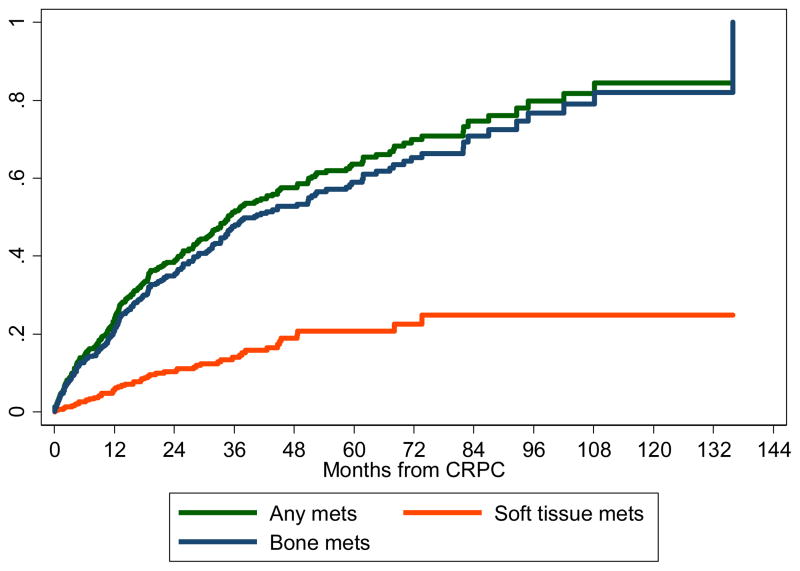
A total of 256 (56%) men developed metastatic disease over a median follow-up of 3 years after CRPC. We detected bone metastases in 89% of men with metastasis, visceral metastasis in 8%, and lymph node metastasis in 17%. Metastasis-free survival was 79%, 65%, 52%, 47% and 41% at 1, 2, 3, 4 and 5 years after CRPC, respectively (Supplementary Figure S1). In univariable analysis, Gleason scores 8–10 (hazard ratio [HR] = 1.69, 95% confidence interval [CI] 1.13–2.54, P= .011), receipt of primary localized treatment (HR = 1.31, 95% CI 1.02–1.69, P= .040), higher PSA at CRPC diagnosis (logPSA HR = 1.57, 95% CI 1.40–1.76, P< .001), shorter PSADT (HR=1.61, 95% CI 1.20–2.17, P= .002), missing PSADT (HR = 1.60, 95% CI 1.18–2.17, P= .003), younger age (HR=0.98, 95% CI 0.97–0.996, P= .010), and shorter time from ADT to CRPC (HR=0.96, 95% CI 0.92–0.996, P= .030) were associated with shorter time from CRPC to metastasis. In multivariable analysis, Gleason score 8–10 (HR = 1.61, 95% CI 1.06–2.43, P= .026), receipt of primary localized treatment (HR=1.38, 95% CI 1.04–1.82, P= .028), higher PSA levels at CRPC diagnosis (logPSA HR=1.64, 95% CI 1.44–1.87, P< .001) and shorter PSADT (HR=1.42, 95% CI 1.02–1.98, P= .040) were independently associated with shorter time from CRPC to metastasis. Race and year of CRPC were not associated with time to metastasis in either uni- or multivariable analyses (Table 1).
Table 1.
Multivariable predictors of metastasis among CRPC patients
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P* | |
| Ethnic group | ||||||
| White | ref | - | - | ref | - | - |
| Other | 1.04 | 0.80–1.36 | 0.742 | 0.90 | 0.67–1.21 | 0.490 |
| Biopsy Gleason score | ||||||
| 2 – 6 | ref | - | - | ref | - | - |
| 7 | 1.43 | 0.95–2.15 | 0.085 | 1.36 | 0.90–2.07 | 0.148 |
| 8 – 10 | 1.69 | 1.13–2.54 | 0.011 | 1.61 | 1.06–2.43 | 0.026 |
| Unknown | 1.26 | 0.85–1.86 | 0.249 | 1.22 | 0.81–1.82 | 0.340 |
| Primary localized treatment | ||||||
| None | ref | - | - | ref | - | - |
| RP and/or XRT | 1.31 | 1.02–1.69 | 0.040 | 1.38 | 1.04–1.82 | 0.028 |
| Year of CRPC diagnosis (years) | 0.99 | 0.95–1.02 | 0.456 | 0.99 | 0.95–1.04 | 0.774 |
| Age at CRPC (years) | 0.98 | 0.97–0.996 | 0.010 | 0.99 | 0.98–1.00 | 0.170 |
| PSA at CRPC (log[ng/mL]) | 1.57 | 1.40–1.76 | <0.001 | 1.64 | 1.44–1.87 | <0.001 |
| PSADT at CRPC (months) | ||||||
| >6 | ref | - | - | ref | - | - |
| ≤6 | 1.61 | 1.20–2.17 | 0.002 | 1.42 | 1.02–1.98 | 0.040 |
| Missing | 1.60 | 1.18–2.17 | 0.003 | 1.06 | 0.73–1.54 | 0.753 |
| Time from ADT to CRPC (years) | 0.96 | 0.92–0.996 | 0.030 | 1.01 | 0.97–1.05 | 0.727 |
Adjusted for Veteran Affairs Medical Center
ADT: androgen deprivation therapy; CI: confidence interval; CRPC: castration-resistant prostate cancer; HR: hazard ratio; RP: radical prostatectomy.
Figure 1A to 1D shows the individual associations between time to metastasis and PSA levels, Gleason score, primary localized treatment, and PSADT divided into groups. Figure 2 shows the cumulative incidence curve of development of soft tissue metastasis, bone metastasis, or any metastasis. Figure 3 shows a nomogram predicting metastasis-free survival. Supplementary Figure S2 depicts the calibration plot comparing the predicted and actuarial metastasis-free probabilities at 4 years. The nomogram’s raw and bias-corrected concordance indexes were, respectively, 0.67 and 0.66.
Figure 1.
a) Metastasis-free survival of CRPC patients by PSA groups, b) metastasis-free survival of CRPC patients by Gleason score, c) metastasis-free survival of CRPC patients by primary localized treatment, d) metastasis-free survival of CRPC patients by PSA doubling time. (Color version available online.)
Figure 2.
Cumulative incidence of all metastases, bone metastases, and soft tissue metastases for patients with castration-resistant prostate cancer. (Color version available online.)
Figure 3.
Nomogram predicting metastasis-free survival
DISCUSSION
The natural history of patients with non-metastatic CRPC is variable.13 Many patients rapidly progress to metastatic disease and eventually die of the disease; while others have an indolent progression. At present, there no treatment options for non-metastatic CRPC that have shown a survival benefit. However, patients with asymptomatic metastatic CRPC have been shown to benefit from specific treatments such as docetaxel,14 sipuleucel-T,15 and abiraterone plus prednisone,5 and enzalutamide.16 Given many patients with documentable metastatic disease to the bone by radiologic studies are asymptomatic, it is reasonable to screen CRPC patients for metastasis to institute life-prolonging treatment as early as possible. As such, it is important to determine the factors associated with rapid progression to metastasis to individualize the screening for metastatic disease among these patients. Therefore, we investigated the incidence of metastasis in CRPC patients and the predictors of time from CRPC to metastatic disease among men treated with ADT who developed CRPC. We found about two- thirds of patients developed metastatic disease in the first 5 years after CRPC. Higher biopsy Gleason score, higher PSA and younger age were independent predictors of shorter time to metastasis. We also generated a nomogram to predict the metastasis-free survival probabilities.
A few previous studies have evaluated the natural history of non-metastatic CRPC patients. For example, Smith et al performed an analysis of 201 patients in the placebo group from an aborted randomized trial to evaluate the effects of zoledronic acid on time to first bone metastasis.9 Later, the same authors used a similar strategy to study 331 subjects in the placebo group of another randomized trial of atrasentan on time to disease progression.8 In both studies, the median time to metastasis was nearly 30 months, compared to approximately 36 months in our study. In both studies, patients had a significantly higher mean PSA level at baseline (31 and 35 ng/dL) compared to our sample (4.2 ng/dL). In addition, patients were considerably younger (mean age 73 and 73 versus 78 years in our cohort) and had a more remote diagnosis of prostate cancer (mean 6.5 and 7.0 versus 4.3 years prior to entering the study in our cohort). Importantly, patients in these trials were not captured at the time of CRPC diagnosis but were afterwards identified after having a negative bone scan. This is similar to our study that followed men from the initial diagnosis of CRPC. Moreover, their studies used data from patients in clinical trials while we used a clinical cohort. These differences can potentially explain why patients in our study had a longer time to metastasis. Nevertheless, despite the differences across studies, the compounded evidence indicates patients with CRPC are at a very high risk for metastasis, with most developing metastases within 36 months.
Patients with CRPC progress to metastatic disease at different rates. Some of the factors associated with rapid progression to metastasis have been previously described. Similar to our study, Smith at al, in their earlier study, found higher PSA levels and faster PSA velocity to be associated with shorter time to metastasis in 2 separate studies.8,9 However, different from our findings, Gleason score was not associated with time to metastasis in either of their studies. More recently, a study by Metwali et al found metastasis to be associated with higher PSA levels (at diagnosis and nadir PSA after ADT), rapid alkaline phosphatase rise, and shorter PSADTs in men with CRPC.10 Thus, the combined evidence in the literature suggests that PSA level and kinetics may be the most important factors to predict progression to metastatic disease. Thus, the use of PSA levels and kinetics in combination with other predictors such as Gleason score may be useful to stratify patients according to their risk of developing metastatic disease. Consequently, follow-up strategies may be tailored to the risk of metastatic disease where patients at higher risk for metastasis may be screened more frequently than those at a lower risk. This strategy can potentially minimize costs and reduce the exposure to radioactive contrast media in the low-risk group and allow for an early diagnosis of metastatic disease among the high-risk group.
The current study has several strengths including the large number of CRPC patients from a racially diverse cohort and a relatively long follow-up. However, it is not devoid of limitations. The main limitation is its retrospective nature. Consequently, we were not able to decide when and how bone scans were performed. It is plausible that patients with more advanced and aggressive disease at baseline had more frequent and earlier bone scans compared to those with less advanced and aggressive disease for whom the bone scan may have been deferred to a later point in time. If this hypothesis is true, some patients with worse disease were more likely to be diagnosed with metastasis whereas a number of patients with more favorable disease may have been excluded from the study, given they never had a previous bone scan. Similarly, we had no control over when and how patients were treated with ADT or other therapies. Although these limitations add noise and unwanted variability to the study, they reflect the current clinical practice in the CRPC treatment. Moreover, we were unable to evaluate different ADT schemes (such as continuous and intermittent) as the data was not available for all patients. We were unable to determine the reasons why active primary treatment (surgery or radiotherapy) before CRPC would be associated with faster time to metastasis. It is possible that selection bias and/or confounding factors may the reason for such an association. As such, the causal relationship between primary treatment and time from CRPC to metastasis could not be determined. In addition, given only 250 patients had prostate cancer diagnosis date available, we were unable to evaluate time from diagnosis to ADT in multivariable models. Finally, although bone scans are very sensitive to detect metastasis, false positives and negatives do occur, but we were unable to identify them given confirmatory imaging was not available for all patients in our sample.17
In conclusion, among subjects with non-metastatic CRPC, nearly 70% developed metastatic disease during the first 5 years after CRPC, with most of the metastasis occurring within the first 3 years. Higher Gleason score, higher PSA, and shorter PSADT were independently associated with shorter time to metastasis. Therefore, if these results are confirmed in future studies, Gleason score, PSA, and PSADT can be used to stratify patients according to the risk of metastasis.
Supplementary Material
Acknowledgments
Research support: Department of Veterans Affairs, National Institute of Health R01CA100938 (WJA), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (WJA), the Georgia Cancer Coalition (MKT), NIH K24 CA160653 (SJF) and Amgen.
APPENDIX. SUPPLEMENTARY DATA
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2016.06.011.
Footnotes
Disclaimer: Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:23–7. doi: 10.1038/pcan.2012.42. [DOI] [PubMed] [Google Scholar]
- 2.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Autio K, Ryan CJ, Mulders P, Shore N, Kheoh T, Fizazi K, Logothetis CJ, Rathkopf D, Smith MR, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14:1193–9. doi: 10.1016/S1470-2045(13)70424-8. [DOI] [PubMed] [Google Scholar]
- 6.Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G, Akdas AM, Small EJ, Donnelly BJ, Sundram SK, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450–6. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palvolgyi R, Daskivich TJ, Chamie K, Kwan L, Litwin MS. Bone scan overuse in staging of prostate cancer: an analysis of a Veterans Affairs cohort. Urology. 2011;77:1330–6. doi: 10.1016/j.urology.2010.12.083. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Cook R, Lee KA, Nelson JB. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–85. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman C, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 10.Metwalli AR, Rosner IL, Cullen J, Chen Y, Brand T, Brassell SA, Lesperance J, Porter C, Sterbis J, McLeod DG. Elevated alkaline phosphatase velocity strongly predicts overall survival and the risk of bone metastases in castrate-resistant prostate cancer. Urol Oncol. 2014;32:761–8. doi: 10.1016/j.urolonc.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira DM, Howard LE, Sourbeer KN, Amarasekara HS, Chow LC, Cockrell DC, Hanyok BT, Pratson CL, Aronson WJ, Kane CJ, et al. Predicting bone scan positivity in non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2015;18:333–7. doi: 10.1038/pcan.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–11. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 14.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Fizazi K, Scher HI, Miller K, Basch E, Sternberg CN, Cella D, Forer D, Hirmand M, de Bono JS. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15:1147–56. doi: 10.1016/S1470-2045(14)70303-1. [DOI] [PubMed] [Google Scholar]
- 17.Sadik M, Suurkula M, Hoglund P, Jarund A, Edenbrandt L. Quality of planar whole-body bone scan interpretations--a nationwide survey. Eur J Nucl Med Mol Imaging. 2008;35:1464–72. doi: 10.1007/s00259-008-0721-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.