Abstract
Objective
Spasmodic dysphonia is a focal dystonia of the larynx with heterogeneous manifestations and association with familial risk factors. There is scarce data to allow precise understanding of etiology and pathophysiology. Screening for dystonia-causing genetic mutations has the potential to allow accurate diagnosis, inform about genotype-phenotype correlations and allow a better understanding of mechanisms of disease.
Study Design
Prospective cohort
Setting
Tertiary academic medical center
Subjects and methods
We enrolled patients presenting with spasmodic dysphonia to the voice clinic of our academic medical center. Data collected included demographic data, clinical features, family history and treatments administered. The following disease-causing mutations previously associated with spasmodic dysphonia were screened: TOR1A (DYT1), TUBB4 (DYT4), and THAP1 (DYT6).
Results
86 patients were recruited comprising 77% females and 23% males. A definite family history of neurological disorder was present in 15% (13/86). Average age of symptom onset was 42.1y (SD±15.7). Most (99%; 85/86) were treated with botulinum toxin and 12% (11/86) received oral medications. Genetic screening was negative in all patients for the GAG deletion in TOR1A (DYT 1) and in the 5 exons currently associated with disease-causing mutations in TUBB4 (DYT4). Two patients tested positive for novel /rare variants in THAP 1 (DYT 6).
Conclusion
Genetic screening targeted at currently known disease-causing mutations in TOR1A, THAP1 and TUBB4 appears to have low diagnostic yield in sporadic spasmodic dysphonia. In our cohort only two patients tested positive for novel/rare variants in THAP 1. Clinicians should make use of genetic testing judiciously and in cost-effective ways.
Keywords: Dysphonia, Dystonia, Genetic testing
Introduction
Spasmodic dysphonia (SD) is a chronic progressive neurological disorder of central motor processing, with action-induced, task-specific focal laryngeal dystonia and no cure available.1 Females are preferentially affected and the average age of symptom onset is 45 years.2 Some cases can be highly disabling with significant psychosocial impact. 3,4 Variants are classified according to the predominant vocal cord muscles affected, including adductor (most prevalent), abductor and mixed types. The condition may co-exist with vocal tremor as well as other non-vocal tremors. 5 Current treatment is largely targeted at symptomatic improvement, including recurrent sessions of chemodenervation of laryngeal muscles with botulinum toxin and denervation-reinnervation surgery on select cases. 6,7
There is little information regarding the pathogenesis of SD. Although functional, neurochemical and neuropathological abnormalities have been found, data is scarce to inform a comprehensive disease model. 8–10 In recent years, a growing interest has emerged in studying genetic contributions to focal dystonias, stemming from evidence that polymorphisms in wild-type forms of generalized dystonia-causing genes can affect the risk of development of focal or segmental dystonia as well as clustering of heterogenous manifestations of dystonia in some families. 11–13
Previous genetic variants that have been studied in association with focal dystonia comprising SD include DYT1/TOR1A 12,14 and DYT6/THAP1 15–17. Results are either conflicting or have not been reproduced. More recently, mutations in the DYT4/TUBB4 gene were identified in an Australian family, with the phenotype of whispering dysphonia originally described by Parker and more recently reappraised by Wilcox et al 18–21. Studies analyzing the prevalence of pathogenic variants in coding regions of TUBB4 in primary dystonia have not identified pathogenic variants in large-scale cohorts. However, patient selection in these studies was not restricted to spasmodic dysphonia phenotypes.22,23 To our knowledge, this is the first report to screen exclusively patients with SD, describing epidemiologic data, family history and performing analysis of genetic abnormalities in coding regions of TOR1A, THAP1 and TUBB4.
Methods
Subjects with SD were recruited from the Voice Center Clinic at Massachusetts Eye and Ear Infirmary. Participants signed informed consent as approved by our Institutional Review Board. A physical examination was followed by speech recordings which were independently scored regarding the presence of at least one voice break in a vowel per 3 sentences of 10 sentences useful for eliciting glottal stops in vowels for identifying adductor SD, prolonged voiceless consonants in sentences for identifying abductor SD, and prolonged vowels for identifying vocal tremor. A speech language pathologist then evaluated each subject. The evaluation, including the flexible digital nasolaryngoscopic exam, was digitally recorded for subsequent review. The larynx was evaluated during quiet breathing, quiet sustained /i/ and loud sustained /i/ vocalization followed by repeating some of the sentences the patient recited earlier for the speech recording. The presence and the degree of symmetry of vocal fold adduction or abduction and ventricular hyperfunction were recorded both during whistling, voiced speech and whispered speech. The history, physical exam and nasolaryngoscopic exam were reviewed at weekly multidisciplinary voice rounds session. During this session a panel of 2 experienced laryngologists (PCS, RAF) and 6 speech language pathologists reviewed the findings. Each panel member rated the following parameters, on a visual analog scale (0 to 100 mm) ranging from normal to asymmetric, for the following qualities: anatomical symmetry while breathing, functional symmetry while breathing, tremor at rest, tremor during voice (prolonged vowel), spasms during vocalizing sentences (the 10 sentences used above) and when repeating specific syllables (si-si-si-, I-I-I, hi-hi-hi). All members of the panel then reached a consensus regarding diagnosis. A final diagnosis was made of adductor SD, abductor SD, vocal tremor, muscular tension dysphonia or ‘other’. Only those subjects with a consensus diagnosis of adductor SD or abductor SD were eligible to participate in this study.24
Subsequently, all subjects were examined by a movement disorders neurologist (NS) to confirm the diagnosis of SD and determine if there was evidence of dystonia elsewhere, according to published criteria and revised according to a recent update. 25,26. Subjects were asked about the onset of any associated events that preceded the onset of SD (viral illness, traumatic events, stressful events such as divorce, marriage, loss of vocation, relocation to a new city). The presence of a family history of dystonia was determined, for first and second-degree relatives, as previously described. 27 Subjects were considered to have a positive family history of dystonia if the first or second-degree relative with dystonia either provided medical records confirming the diagnosis or was examined by one of the clinical investigators who participated in this study (NS). Subjects were considered to have a possible family history if they reported affected first or second-degree members but confirmatory medical records or examination was not obtained. A negative family history was recorded if subjects specifically denied such a history. The local institutional review board approved the study and all participating individuals gave informed consent.
Molecular analysis
DNA was extracted from white blood cells using the Purgene procedure (Gentra Systems, Minneapolis, MN).
The samples were screened for presence of the TOR1A (DYT1) GAG deletion as well as THAP1 (DYT6) and TUBB4A (DYT4) mutations. PCR amplification across the GAG deletion region of the TOR1A gene was performed as previously described.27,28 All exons and flanking regions of the THAP1 gene and the TUBB4A gene were sequenced as previously described.17, 20 Variants were compared to frequencies reported in the Exome Aggregation Consortium (ExAC) database. 28
Results
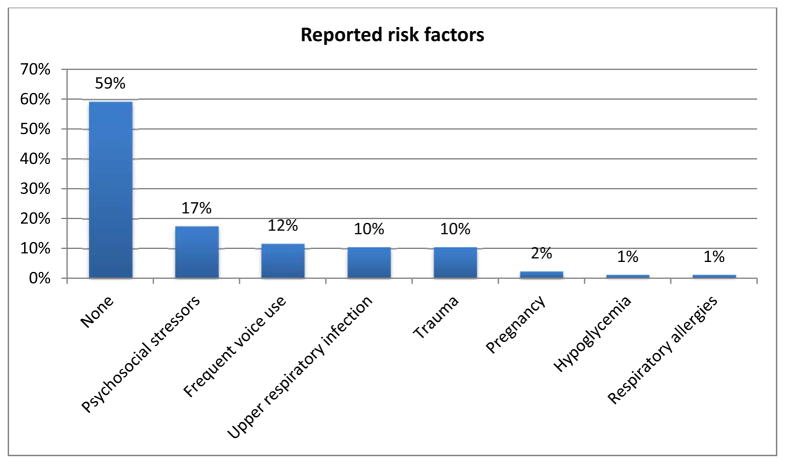
A total of 87 subjects were enrolled in the study. One patient declined genetic screening and was excluded from the final analysis. Clinical and demographical data can be seen on table 1. Average age of symptom onset was 41.1y (SD±16.4), the vast majority with late-onset (defined as >= 27 y; 84%, 72/86), and a female predominance (77%; 66/86). All subjects had onset of dystonia in the larynx and in the majority it remained focally restricted to the vocal folds (84%, 73/86) (Figure 1). There was segmental spread in 10% of subjects and multifocal involvement in 5%. Seven subjects (8%) had upper extremity postural tremor. Forty-one percent (35/86) of subjects associated onset with one or more risk factors, most commonly psychosocial stressors, frequent voice use, upper respiratory infections or trauma (Figure 2).
TABLE 1.
Demographic and clinical data
| Age of onset | Mean: 42.1y( SD± 15.7 ; range 8–87y) Early onset (≤27y): 17% (14/86) Late onset (>27): 83% (72/86) |
| Sex | Female: 77% (67/86) Male: 23% (20/86) |
| Race * | White: 93% (80/86) African American: 2% (2/86) Asian: 1% (1/86) Undisclosed: 4% (3/86) |
| Ethnicity* | French Canadian: 13% (11/86) Latino: 10% (9/86) Ashkenazi Jewish: 3% (3/86) Unnkown/Undisclosed: 74% (64/86) |
| Risk Factors | Endorsed: 40% (35/86) see figure 2 |
| Family History§ | Positive: 15% (13/86) Possible: 18% (16/86) Negative: 67% (58/86) |
| Associated symptoms | Tremor: 8% (7/86) |
| Treatment | Botulinum toxin : 98% (85/86) Benzodiazepines: 9% (8/86) Reported improvement with EtOH: 8% (7/86) |
Self-reported;
Of those with confirmed family history, 5 patients had a history of Parkinson’s disease; 3 had tremors, 2 had SD, 1 had Meige Syndrome, 1 had facial dystonia dystonia, 1 hand dystonia.
Figure 1.
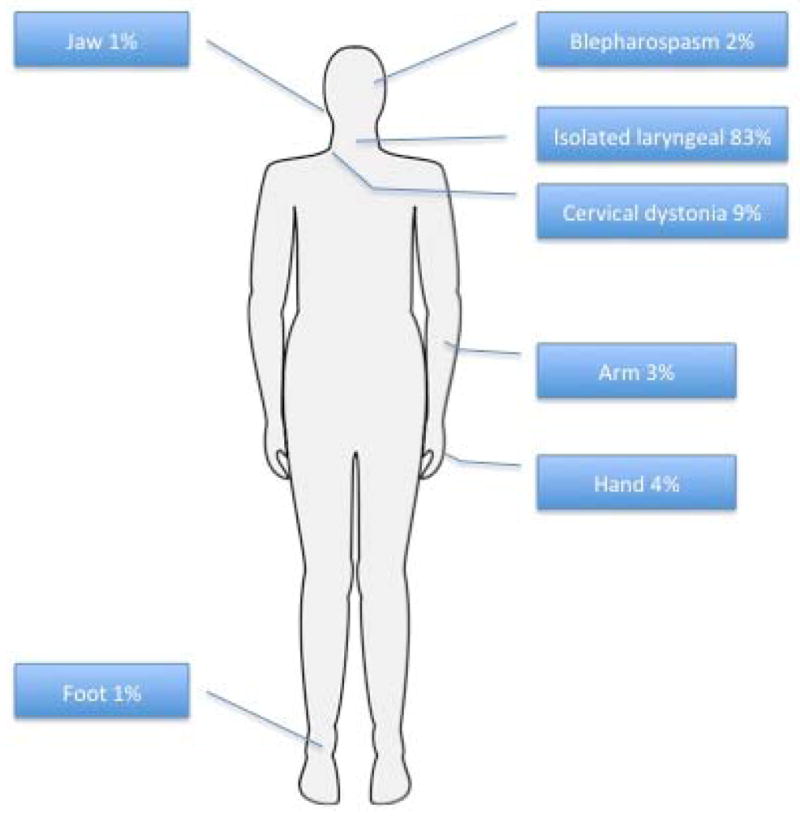
Dystonia distribution in SD subjects
Figure 2.
Thirteen subjects (15%) had a definite family history of neurological disorder, with five specifically having a first-degree family member with dystonia. Other subjects with a positive family history (first or second-degree) included Parkinson’s Disease (5) and Essential Tremor (3).
Most subjects were treated with Botulinum toxin, (98%; 85/86) and 11/86 (13%) received oral medications, most commonly benzodiazepines. Genetic screening was negative in all patients for the GAG deletion in TOR1A (DYT1) and for disease-causing mutations in TUBB4A (DYT4)
Sequence analysis in two patients revealed potential mutations in the THAP 1 gene. In a white male patient of unknown descent with an early onset at age 24 and no family history, a novel c.11C>T (Chr8:42,698,227;hg19) change was identified resulting in a p.Ser4Phe amino acid substitution. This variant was not found in ExAC, dbSNP, ClinVar or any other variant database and was predicted to be deleterious by SIFT and possibly damaging by PolyPhen. This patient enrolled in the study at age 70 and thus had not had any progression in decades, remaining well controlled with botulinum toxin injections. A female patient, with a history of Graves’ disease, had onset of focal spasmodic dysphonia at age 50. She reported a positive family history of Meige Syndrome in her mother. She had Italian, Portuguese and Irish ancestry. In nine years of follow-up she has been stable with periodic botulinum toxin injections and no signs of progression. This patient harbored a c.580T>C (chr8:42,693,167;hg19) missense variant resulting in a p.Ser194Pro substitution. This variant has been reported in a single individual of East Asian Ancestry in the ExAC dataset and is predicted to be tolerated by SIFT and benign by PolyPhen suggesting this may be a rare benign variant or a variant of unknown significance (VUS) in this patient. Unfortunately, a DNA sample from the mother is not available.
Discussion
In this sample of 86 patients with mainly sporadic SD, genetic screening for disease-causing mutations in known dystonia genes previously implicated in SD (TOR1A, THAP1 and TUBB4A) identified one mutation and one VUS in the THAP1 gene. In terms of patient population, age of onset and reported risk factors this cohort is consistent with previous epidemiologic data. 1,29,30
Our findings suggest a low diagnostic yield for indiscriminate genetic screening in a non-selected population of SD patients. The absence of mutations in the TOR1A and TUBB4 is informative. TOR1A is classically described as having onset in a limb during late childhood, with generalization typically sparing the craniofacial muscles, but variations have been reported.31,32. TUBB4 mutations have been associated with isolated SD to generalized dystonia with a characteristic gait and dysphonia. These phenotypes appear to be restricted to the original kindred, although leukoencephalopathies with hypomyelination are increasingly recognized in association with particular mutations in this gene, putatively related to a continuum of phenotypic expressivity. 18,19,22,33,34
The presence of novel/rare variants in THAP1 in two of our patients is of interest, but the conclusions are limited. THAP1 mutations appear to be present but rare in focal and segmental SD patients.17. Previous studies examining genotype-phenotype correlations suggest that computationally-predicted pathogenic mutations or those affecting the DNA-binding domain most often manifest with younger age of onset and more severe disease (generalized dystonia). 35,36 Nevertheless, our patient with the p.Ser4Phe missense mutation in the DNA binding domain had relatively early onset of disease (age 24 yrs) but has remained restricted to the larynx for several decades.
There are limitations to our study. Our sample size is relatively small despite its representativeness of typical SD. Previously published data report similar age of onset, female gender predominance, environmental risk factors and response to treatment.1,2,29,30 In addition, we restricted the evaluation to GAG deletions in the coding region of TOR1A. Other disease-causing mutations in this gene have been described but appear exceedingly rare. As previously mentioned, there may be higher yield in testing those with early-onset speech involvement associated with progression to generalized dystonia. 17 In this cohort, 16% (14/86) of patients had early onset, restricted to the larynx in 78% (11/14). In one patient with early-onset (7%; 1/14) a pathogenic THAP1 mutation was found. This finding is in line with the previous aforementioned studies but unique as the reported patient did not have progression to generalized dystonia.
In summary, our findings suggest that genetic screening for mutations in DYT1/TOR1A, DYT6/THAP1 and DYT4/TUBB4A in patients with sporadic SD has low diagnostic yield and should not be performed routinely. Presently, targeted screening is more likely to have higher yield in those with generalized dystonia, young onset and a positive family history. With widespread use of clinical exome/whole genome sequencing, knowledge of phenotypic expression of monogenic conditions is likely to to expand. In this scenario, candidate gene screening will probably need to be revisited in the near future.
Footnotes
This paper was presented with preliminary data at the American Academy of Neurology Meeting, Washington DC in April 2015.
References
- 1.Tanner K, Roy N, Merrill RM, Sauder C, Houtz DR, Smith ME. Spasmodic dysphonia: onset, course, socioemotional effects, and treatment response. Ann Otol Rhinol Laryngol. 2011 Jul;120(7):465–73. doi: 10.1177/000348941112000708. [DOI] [PubMed] [Google Scholar]
- 2.Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002 Feb;112(2):220–3. doi: 10.1097/00005537-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kaptein AA, Hughes BM, Scharloo M, Hondebrink N, Langeveld TPM. Psychological aspects of adductor spasmodic dysphonia: a prospective population controlled questionnaire study. Clin Otolaryngol. 2010 Feb;35(1):31–8. doi: 10.1111/j.1749-4486.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 4.Gündel H, Busch R, Ceballos-Baumann A, Seifert E. Psychiatric comorbidity in patients with spasmodic dysphonia: a controlled study. J Neurol Neurosurg Psychiatry. 2007 Dec;78(12):1398–400. doi: 10.1136/jnnp.2007.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White LJ, Klein AM, Hapner ER, et al. Coprevalence of tremor with spasmodic dysphonia: a case-control study. Laryngoscope. 2011 Aug;121(8):1752–5. doi: 10.1002/lary.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson DM, Blitzer a, Brashear a, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008 May 6;70(19):1699–706. doi: 10.1212/01.wnl.0000311389.26145.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010 Jul;17(Suppl 1):28–30. doi: 10.1111/j.1468-1331.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 8.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010 Nov;20(11):2749–59. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonyan K, Ludlow CL, Vortmeyer AO. Brainstem pathology in spasmodic dysphonia. Laryngoscope. 2010 Jan;120(1):121–4. doi: 10.1002/lary.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 2013 Sep 11;33(37):14705–14. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma N, Franco RA. Consideration of genetic contributions to the risk for spasmodic dysphonia. Otolaryngol Head Neck Surg. 2011 Sep;145(3):369–70. doi: 10.1177/0194599811411656. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Franco RA, Kuster JK, et al. Genetic evidence for an association of the TOR1A locus with segmental/focal dystonia. Mov Disord. 2010 Oct 15;25(13):2183–7. doi: 10.1002/mds.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groen JL, Kallen MC, van de Warrenburg BPC, et al. Phenotypes and genetic architecture of focal primary torsion dystonia. J Neurol Neurosurg Psychiatry. 2012 Oct;83(10):1006–11. doi: 10.1136/jnnp-2012-302729. [DOI] [PubMed] [Google Scholar]
- 14.Hague S, Klaffke S, Clarimon J, et al. Lack of association with TorsinA haplotype in German patients with sporadic dystonia. Neurology. 2006 Mar 28;66(6):951–2. doi: 10.1212/01.wnl.0000203344.43342.18. [DOI] [PubMed] [Google Scholar]
- 15.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–38. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groen JL, Yildirim E, Ritz K, et al. THAP1 mutations are infrequent in spasmodic dysphonia. Mov Disord. 2011 Aug 15;26(10):1952–4. doi: 10.1002/mds.23682. [DOI] [PubMed] [Google Scholar]
- 17.Djarmati A, Schneider SA, Lohmann K, et al. Mutations in THAP1 (DYT6) and generalised dystonia with prominent spasmodic dysphonia: a genetic screening study. Lancet Neurol. 2009;8:447–52. doi: 10.1016/S1474-4422(09)70083-3. [DOI] [PubMed] [Google Scholar]
- 18.Parker N. Hereditary whispering dysphonia. J Neurol Neurosurg Psychiatry. 1985 Mar;48(3):218–24. doi: 10.1136/jnnp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox Ra, Winkler S, Lohmann K, Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov Disord. 2011 Nov;26(13):2404–8. doi: 10.1002/mds.23866. [DOI] [PubMed] [Google Scholar]
- 20.Lohmann K, Wilcox Ra, Winkler S, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. 2013 Apr;73(4):537–45. doi: 10.1002/ana.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersheson J, Mencacci NE, Davis M, et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann Neurol. 2013 Apr;73(4):546–53. doi: 10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vemula SR, Xiao J, Bastian RW, Momčilović D, Blitzer A, LeDoux MS. Pathogenic variants in TUBB4A are not found in primary dystonia. Neurology. 2014 May 8;82(14):1227–30. doi: 10.1212/WNL.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zech M, Boesch S, Jochim A, et al. Large-scale TUBB4A mutational screening in isolated dystonia and controls. Parkinsonism Relat Disord. 2015 Oct;21(10):1278–81. doi: 10.1016/j.parkreldis.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Daraei P, Villari CR, Rubin AD, et al. The role of laryngoscopy in the diagnosis of spasmodic dysphonia. JAMA Otolaryngol Head Neck Surg. 2014 Mar;140(3):228–32. doi: 10.1001/jamaoto.2013.6450. [DOI] [PubMed] [Google Scholar]
- 25.Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998 Jan;78:1–10. [PubMed] [Google Scholar]
- 26.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013 Jun 15;28(7):863–73. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bressman S, Greene P. Exclusion of the DYT1 locus in familial torticollis. Ann Neurol. 1996;40(4):681–4. doi: 10.1002/ana.410400421. [DOI] [PubMed] [Google Scholar]
- 28.Exome Aggregation Consortium (ExAc) [Internet] Cambridge, MA: [cited 2015]. Available from: http://exac.broadinstitute.org. [Google Scholar]
- 29.Tanner K, Roy N, Merrill RM, Sauder C, Houtz DR, Smith ME. Case-control study of risk factors for spasmodic dysphonia: A comparison with other voice disorders. Laryngoscope. 2012 May;122(5):1082–92. doi: 10.1002/lary.22471. [DOI] [PubMed] [Google Scholar]
- 30.Tanner K, Roy N, Merrill RM, et al. Risk and protective factors for spasmodic dysphonia: a case-control investigation. J Voice. 2011 Jan;25(1):e35–46. doi: 10.1016/j.jvoice.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Kramer PL, Heiman GA, Gasser T, et al. The DYT1 gene on 9q34 is responsible for most cases of early limb-onset idiopathic torsion dystonia in non-Jews. Am J Hum Genet. 1994 Sep;55(3):468–75. [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz U, Yüksel D, Atac FB, Yilmaz D, Verdi H, Senbil N. Atypical phenotypes of DYT1 dystonia in three children. Brain Dev. 2013;35:356–9. doi: 10.1016/j.braindev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Miyatake S, Osaka H, Shiina M, et al. Expanding the phenotypic spectrum of TUBB4A-associated hypomyelinating leukoencephalopathies. Neurology. 2014 May 21;82(24):2230–7. doi: 10.1212/WNL.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 34.Erro R, Hersheson J, Ganos C, et al. H-ABC syndrome and DYT4: Variable expressivity or pleiotropy of TUBB4 mutations? Mov Disord. 2015 May;30(6):828–33. doi: 10.1002/mds.26129. [DOI] [PubMed] [Google Scholar]
- 35.Xiromerisiou G, Houlden H, Scarmeas N, et al. THAP1 mutations and dystonia phenotypes: genotype phenotype correlations. Mov Disord. 2012 Sep 1;27(10):1290–4. doi: 10.1002/mds.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeDoux MS, Xiao J, Rudzińska M, et al. Genotype-phenotype correlations in THAP1 dystonia: molecular foundations and description of new cases. Parkinsonism Relat Disord. 2012 Jun;18(5):414–25. doi: 10.1016/j.parkreldis.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]