Abstract
Background
Inferior vena cava filters (IVCF) have been associated with improved survival in patients with acute pulmonary embolism (PE) in some studies. However, without randomization, those with early mortality who did not receive an IVCF may have died prior to treatment decision about filter placement, falsely contributing a survival advantage to those receiving IVCF and biasing the results of previous observational studies. The objective of this study is to evaluate the impact of IVCF on in-hospital mortality after adjusting for this survivor treatment selection.
Methods
National Inpatient Sample datasets from 2009–2012 were analyzed to assess the impact of IVCF placement on in-hospital mortality in all patients with acute PE. Subgroup analyses were performed in those with high-risk PE (hemodynamic shock) and also for those with both shock and concomitant thrombolysis. Inverse-propensity-score-weighting was used to balance clinical and comorbid differences between filter and non-filter groups. To account for survivor treatment selection bias, an extended Cox model was fitted with IVCF placement as a time-dependent covariate.
Results
We identified 263,955 patients with acute PE over this period; 36,702 (13.9%) received IVCF. Those receiving IVCF in the unadjusted cohort were older (IVCF: 66.3±15.9 vs. non-IVCF:62.4±17.4;p<0.001) with higher rates of shock (6.8% vs. 3.8%;p<0.001), deep venous thrombosis (32.8% vs. 13.9%;p<0.001), thrombolytic therapy (5.9% vs. 1.6%;p<0.001), and lower crude mortality (6.0% vs. 6.7%;p<0.001). Propensity-weighted extended Cox analysis showed that IVCF placement did not significantly decrease mortality hazard compared to an untreated patient (HR 0.93, 95% CI[0.89–1.01]). Similar results were seen in the combined high-risk and thrombolysis (HR 0.85, 95% CI[0.60–1.21]) subgroup and associated with worse outcomes in the high-risk (HR 1.2, 95% CI[1.11–1.38]) subgroup.
Conclusions
Placement of IVCF in all patients with acute PE, in high-risk patients, or in high-risk patients concurrently treated with thrombolysis is not significantly associated with improvement of in-hospital mortality when accounting for survivor treatment selection bias.
1. INTRODUCTION
Inferior vena cava filters (IVCF) are often utilized as an adjunct in the treatment of venous thromboembolism (VTE) and acute pulmonary embolism (PE). Many published guidelines recommend the use of IVCF in a patient with a contraindication to anticoagulation1–3 in order to prevent morbidity and mortality from a recurrent PE. However, the impact of IVCF in PE patients outside of this setting has not been extensively studied, with current studies reporting conflicting results. Recent observational studies4,5 have suggested that using IVCF as an adjunct to treatment in all patients with acute PE may improve short-term survival, with one study recommending placement of IVCF in any patient with acute PE and hemodynamic instability concomitantly with thrombolytic therapy6. Despite favorable results, these studies suffer from potential bias due to survivor treatment selection, or the “immortal time bias”7, resulting from the usage of a fixed-time analysis with misclassification of untreated time as treated. This misclassification is known to falsely elevate the treatment effect in certain situations, leading to conclusions which may not accurately reflect the true effect of IVCF on mortality in this population. The objective of our study is to evaluate the impact of IVCF placement in patients with acute PE in a large national database while accounting for survivor treatment selection.
2. METHODS
This retrospective study of national database data was approved by the university institutional review board prior to data analysis (#PRO15060452). Data was obtained from the National Inpatient Sample (NIS) from 2009–2012. The NIS is a database compiled by the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project8 containing a sample of discharge-level data from inpatient admissions nationwide. Prior to 2012, all discharges in any given year from a 20% nationwide sample of acute care hospitals were included in the dataset; from 2012 forward, this sampling method was changed to a 20% sample of all discharges irrespective of institution. The sampled NIS data can be used as a standalone study cohort, or sampling weights can be applied to calculate a national population estimate. Our study used the sampled data as a standalone cohort for the comparative analysis and the national population estimates to analyze trends. Diagnoses and procedures in the NIS are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification system (ICD-9-CM).
2.1 Survivor Treatment Selection Bias
The primary objective of this study was to perform a comparative analysis while accounting for the effects of survivor treatment selection, or the “immortal time bias”. Immortal time is defined as the period of time in which, by virtue of the study design, a patient is guaranteed to survive7. In this study all patients enter into the analysis at the time of admission, but for those receiving IVCF the intervention may not be done immediately. The time from admission to intervention is immortal time and may incorrectly attribute survival to the intervention if analysis is performed according to a fixed-time assumption. Because of this, survivor treatment selection can falsely bias results in favor of the treatment group7,9. The effect of survivor treatment selection bias can be accounted for by analyzing the intervention as a time-dependent covariate.
2.2 Study Design
We identified patients with a primary or secondary diagnosis of acute pulmonary embolism (ICD-9-CM code 415.1x), excluding pregnant patients, those carrying a diagnosis of chronic PE, and those under the age of 18. Comorbid conditions were identified using the Elixhauser comorbidity index10; the presence of respiratory failure (ICD-9-CM 518.81, V46.1) and hemodynamic shock (ICD-9-CM 785.5) were also identified.
IVCF placement was identified using ICD-9-CM code 38.7. Because surgical interruption of the vena cava is rarely performed in the modern era of VTE and PE management, this coding has been used almost exclusively to refer to percutaneous IVCF placement4. The time in days from admission to placement of the IVCF was noted. We also identified PE intervention with any thrombolytic therapy (ICD-9-CM 99.10).
The primary comparison groups were those with acute PE with and without IVCF placement. The primary outcome was in-hospital mortality. Propensity methods were used in order to balance clinical and comorbid characteristics between those receiving and not receiving IVCF. A logistic model was fit based on the likelihood of receiving an IVCF over a range of clinically important factors (Appendix A) in order to obtain a propensity score and applied to the cohort as both time-static and time-varying inverse propensity of treatment weights as appropriate. The primary analysis was then performed on the weighted cohort using a logistic regression model in the fixed-time analysis and an extended Cox model with a robust variance estimator and IVCF placement as a time-dependent covariate in the time-varying analysis.
We also investigated the effect of IVCF placement in certain subpopulations that have been suggested to derive a benefit from this intervention in the presence of acute PE. Subgroup analyses in a similar manner to the primary analysis were performed on those classified as high-risk PE (defined as the presence of hemodynamic instability1–3) and those with high-risk PE undergoing thrombolytic therapy of any kind.
2.3 Statistical Analysis
All statistical analyses were performed using Stata SE 13.1 (StataCorp, College Station, TX). Student t, Fisher exact, Chi square, Wilcoxon rank-sum, and Kruskal-Wallis tests were used where appropriate for unadjusted comparison of baseline characteristics. Normality was assessed qualitatively. The Cochran-Armitage test statistic was used to determine significance of proportional trends over time. Standardized differences were used to verify balance in the weighted cohorts. Sampling weights provided by HCUP were used to derive national population estimates and trends.
3. RESULTS
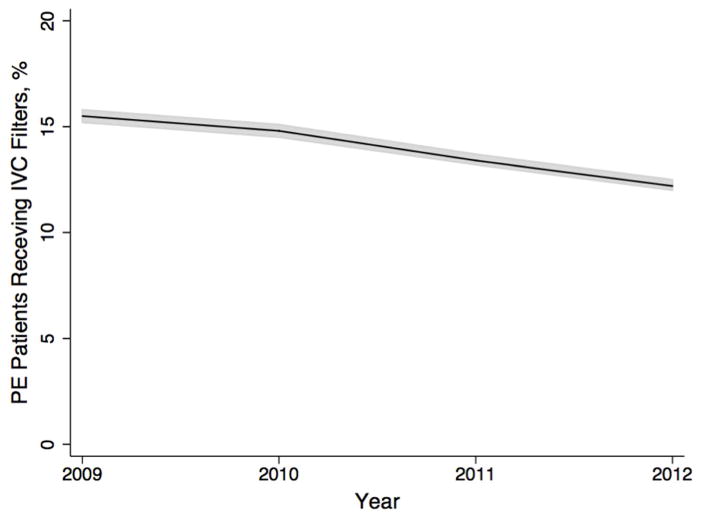
We identified 263,955 patients with acute PE in the NIS dataset from 2009–2012. Of these, 14% (n=36,702) received an IVCF during their hospitalization. Using sampling weights provided by HCUP, we calculated that this represented a national population estimate of 1,264,660 patients with acute PE (95% CI 1,264,195–1,265,125), of which approximately 176,729 received an IVCF (95% CI 175,038–178,421) . Both the raw numbers and proportion of PE patients receiving IVCF declined over the four years of study (P<0.001, Figure 1).
Figure 1.
Trends over time
Trends in IVCF usage in patients with acute PE over time in a national population estimate. Line: point estimate. Shaded area: 95% confidence interval.
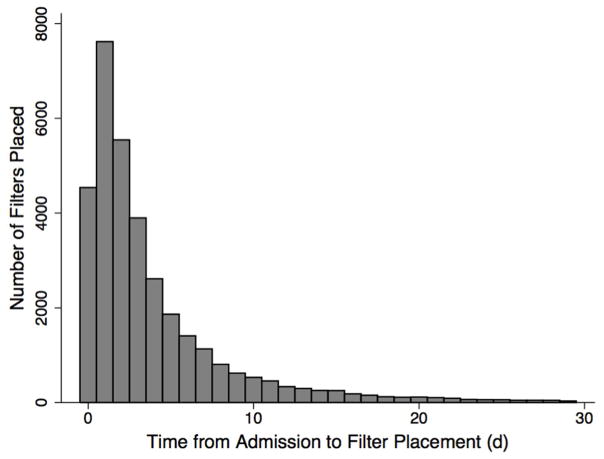
In the unadjusted cohorts, patients with IVCF placement tended to be older with more comorbid conditions, had significantly higher rates of respiratory insufficiency and hemodynamic instability, and were more likely to have a documented VTE. They were also more likely to undergo or have undergone treatment with thrombolytic therapy or operative thrombectomy for acute PE (Table 1). The median time from admission to IVCF placement was 2d (IQR 1–5; Figure 2).
Table 1.
Unadjusted Baseline Characteristics
| Total | IVCF | No IVCF | P | |
|---|---|---|---|---|
| N=263,955 | N=36,702 | N=227,253 | ||
| Age (y) | 62.9 ± 17.2 | 66.3 ± 15.9 | 62.3 ± 17.3 | <0.001 |
| Female Sex | 138,726 (52.6) | 18,580 (50.6) | 120,146 (52.9) | <0.001 |
| Respiratory Failure | 31,938 (12.1) | 6463 (17.6) | 25,475 (11.2) | <0.001 |
| Hemodynamic Instability | 11,218 (4.2) | 2507 (6.8) | 8711 (3.8) | <0.001 |
| Deep Venous Thrombosis | 43,624 (16.5) | 12,028 (32.8) | 31,596 (13.9) | <0.001 |
| Any Cancer | 43,760 (16.6) | 8318 (22.7) | 35,442 (15.6) | <0.001 |
| Congestive Heart Failure | 39,548 (15.0) | 6099 (16.6) | 33,449 (14.7) | <0.001 |
| Peripheral Arterial Disease | 15,069 (5.7) | 2495 (6.8) | 12,574 (5.5) | <0.001 |
| Hypertension | 144,881 (54.9) | 20,794 (56.7) | 124,087 (54.6) | <0.001 |
| Chronic Pulmonary Disease | 63,955 (24.2) | 8756 (23.9) | 55,199 (24.3) | 0.073 |
| Diabetes | 55,522 (21.0) | 7936 (21.6) | 47,586 (20.9) | 0.003 |
| Chronic Renal Failure | 28,917 (11.0) | 4096 (11.2) | 24,821 (10.9) | 0.18 |
| Coagulation Disorder | 18,209 (6.9) | 3787 (10.3) | 14,422 (6.3) | <0.001 |
| Obesity | 42,136 (16.0) | 5384 (14.7) | 36,752 (16.2) | <0.001 |
| Intervention | ||||
| None/Anticoagulation | 257,823 (97.7) | 34,300 (93.5) | 223,523 (98.4) | <0.001 |
| Thrombolytic Therapy | 5704 (2.2) | 2148 (5.9) | 3556 (1.6) | |
| Thrombectomy | 428 (0.2) | 254 (0.7) | 174 (0.1) |
Unadjusted total and baseline cohort characteristics. All values are mean±SD or N(%).
Figure 2.
Distribution of Time to IVCF Placement
Time from admission to IVCF placement
In the unadjusted comparison, patients who received IVCF placement had a lower in-hospital mortality rate (IVCF: 6%, non-IVCF: 6.7%, P<0.001). They were also more likely to be diagnosed with major bleeding (12.7% vs 3.2%, P<0.001), to incur a procedure related hematoma (2.2% vs 0.5%, P<0.001), and had significantly longer length of stay (median 8d [IQR 5–14] vs 5d [IQR 3–8], P<0.001). In addition, those receiving IVCF placement were less likely to be discharged home and more likely to be transferred to a non-acute care nursing or skilled nursing facility (32% vs 17%, P<0.001).
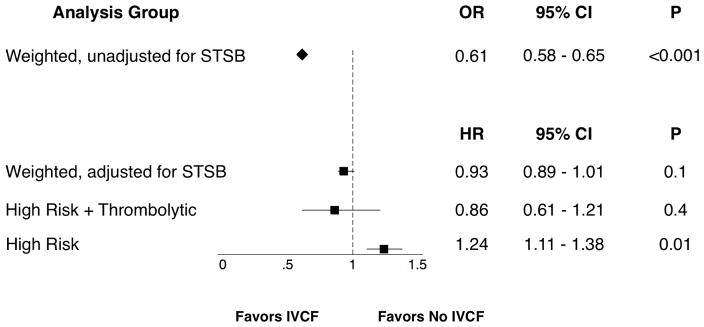
Before adjustment for survivor treatment selection bias, propensity-weighted analysis of in-hospital mortality heavily favored IVCF placement (OR 0.61, 95% CI 0.58–0.65, P<0.001; Figure 3). However, this effect was lost when accounting for survivor treatment selection bias, with no significant difference in mortality between the two groups and a hazard ratio close to one (HR 0.93, 95% CI 0.0.89–1.01, P=0.1). Subgroup analysis similarly demonstrated no significant benefit of IVCF in this analysis for the combined high-risk and thrombolysis group (HR 0.86, 95% CI 0.61–1.21, P=0.4) and suggested an association of increased mortality in the high-risk group (HR 1.24, 95% CI 1.11–1.38, P=0.01).
Figure 3.
Outcomes in Propensity-Weighted Cohorts
Outcomes in propensity-weighted cohorts for overall group and subgroups. STSB: survivor treatment selection bias. OR: odds ratio. HR: hazard ratio.
4. DISCUSSION
Usage of vena cava filters for any indication has increased over time11, but the evidence supporting use for prevention of recurrence and further morbidity or mortality in patients with acute PE is limited. Current guidelines recommend the placement of IVCF in patients with acute PE only in the setting of contraindications to anticoagulation1–3. However, some observational studies have demonstrated favorable results for the reduction of short-term mortality. Consensus remains mixed, with few randomized controlled trials in this population.
Our analysis of national administrative data suggests that both the number and percentage of patients with acute PE receiving an IVCF are decreasing over time from 2009–2012. Trends in this specific population have not been extensively studied, but previous analyses of national discharge data have demonstrated in contrast a gradually increasing volume of IVCF placement over time before 2009. Stein and colleagues11 demonstrated increasing use of IVCF in patients with acute VTE for prevention of PE, with a three-fold increase in IVCF placement from 2001–2006 compared to the previous 5-year period. This discrepancy may be due to recent increasing awareness of the low amount of evidence for efficacy of filters in many areas, as well as recognition that indwelling IVCFs may pose long-term risks related to infection, caval thrombosis, recurrent DVT, or mechanical complications such as strut penetration or migration12. Many researchers13,14 and medical societal guidelines, citing the lack of quality evidence, have been hesitant in recommending liberal vena cava filter usage. This may contribute to the decreasing rate of IVCF placement in patients with acute PE.
Overall, our study showed no benefit for in-hospital mortality with IVCF placement in patients with acute PE after propensity and survivor treatment selection adjustments. This is consistent with the results of the recently published PREPIC-2 trial15, which analyzed 200 patients with acute PE randomly assigned to receive IVCF compared to controls and found no difference in all-cause mortality or recurrent PE at 30 days. This trial followed the original PREPIC trial16, which found a potential benefit for IVCF for new-onset or recurrent PE in a mixed cohort with or without PE at the expense of increased risk of VTE recurrence. However, our results differ from other observational studies that suggest improvement in short-term mortality with IVCF. Goldhaber and colleagues17 found a small decrease in recurrent PE and death in the observational ICOPER registry, while Muriel and colleagues5 showed a 30-day survival benefit in a mixed population of patients with VTE with or without acute PE in a prospective observational trial. Stein and colleagues4 suggested benefit of IVCF for acute PE based on lower case fatality rates in the intervention group based on a retrospective study using NIS data.
None of these studies were randomized, and the study by Stein did not include any adjustment for comorbid factors, contributing to the difficulty of interpretation. However, the potential impact of survivor treatment selection bias in these studies was not evaluated and may have been significant due to the nature of the observational study design. Nevertheless, those studies are often quoted by medical professionals and intensivists to justify the use of filters, especially in high risk patients or patients undergoing thrombolysis. We revisited this issue using the data in our study and found a large bias due to the survivor treatment selection effect. After propensity weighting, analyses were performed with and without a time-varying covariate to account for survivor treatment selection effect. In the non-timevarying analysis, IVCF placement significantly decreased the hazard for in-hospital mortality by a large amount; however, upon introduction of the time-varying covariate, the analysis was no longer significant with a point estimate hazard ratio close to one. The impact of these biases, which have only been recently recognized, has been demonstrated in multiple medical fields in situations where the delay between study onset and nonrandomized treatment assignment has resulted in falsely significant results7,18. The difference between the two outcomes in our study is striking and suggests that this effect should be taken into account in observational studies where immortal time periods are likely.
In a similar manner, we did not find any significant effect of IVCF placement on in-hospital mortality for any of the subgroups in our analyses. In fact, IVCF usage in the high-risk group was associated with a small increase in mortality, an unintuitive finding for which the reason is unclear. This may be potentially related to risks of intervention in an unstable patient; in addition, the possibility of unmeasured confounders based upon physiologic data not present in the NIS in this small subgroup cannot be ruled out. IVCF usage for those at high risk with hemodynamic instability or in conjunction with thrombolytic therapy has also been studied in a limited fashion and suggested to have some beneficial effect4,6. However, based upon the results of our study, we cannot recommend routine concurrent usage of IVCF in these subpopulations due to lack of demonstrated efficacy.
Overall, we find no evidence in our study suggesting benefit for routine placement of IVC filters. . The most recent guidelines from the European Society for Cardiology3 reference possible benefit for IVCF placement based on previous studies, but conclude that there is not enough conclusive evidence for IVCF usage in patients with acute PE and that usage in conjunction with thrombolytic treatment is not recommended. The American Heart Association2 and American College of Chest Physicians1 likewise recommends that IVCF not be routinely utilized in the treatment of patients with acute PE (level IIIC recommendation).
The limitations of using national administrative data for diagnosis and treatment data of VTE and PE have been well documented19,20 and include accuracy of diagnosis coding as well as the lack of disease or procedure specific data. Other limitations of our study, such as missing data regarding time from admission to IVCF placement, though limited, may introduce selection bias. Contraindications for anticoagulation in this population cannot be reliably categorized, and so subanalysis of IVCF effect in those with or without contraindication cannot be performed. In addition, the data within the NIS is limited to the inpatient period; therefore, any examination of longer-term mortality, recurrent PE, or PE-related readmission outcomes related to IVCF placement cannot be undertaken. Regardless of these limitations, we believe that the results of the analyses in this study present a comprehensive picture of the effects of vena cava filter placement in patients with acute PE and accurately adjust for differences between treatment groups and for survivor treatment selection.
5. CONCLUSIONS
Inferior vena cava placement in patients with acute PE for mortality prevention has been advocated by some studies, but evidence is limited and may be biased by survivor treatment selection. Our results do not demonstrate benefit with IVCF placement in all comers with acute PE, those with high-risk PE, or concurrent with thrombolysis those with high-risk PE, in a propensity weighted analysis of national administrative data when adjusting for the effect of survivor treatment selection. We find no evidence to support the routine use of IVCF in patients with acute PE outside of current consensus guideline recommendations.
Acknowledgments
This study was funded in part by grant 2T32HL098036-06 from the National Institutes of Health.
Appendix Table A
Propensity Model Variables
| Age |
| Gender |
| Respiratory Failure |
| Hypotension |
| Chronic Pulmonary |
| Disease |
| Hospital Size |
| Transfer Status |
| Malignancy |
| Fluid/Electrolyte |
| Disorder |
| Coagulopathy |
| Malnutrition |
| Chronic Anemia |
| Heart Failure |
| Chronic Kidney Disease |
| Diabetes |
| Alcohol Dependency |
| Chronic Liver Disease |
| Deep Venous |
| Thrombosis |
Footnotes
Presented at the plenary session of the 2016 annual winter meeting of the Vascular and Endovascular Surgery Society.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. 3069a–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 4.Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of Vena Cava Filters on in-Hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125(5):478–484. doi: 10.1016/j.amjmed.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Muriel A, Jiménez D, Aujesky D, et al. Survival effects of inferior vena cava filter in patients with acute symptomatic venous thromboembolism and a significant bleeding risk. J Am Coll Cardiol. 2014;63(16):1675–1683. doi: 10.1016/j.jacc.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Stein PD, Matta F. Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism: Saves Lives but Underused. Am J Med. 2012;125(5):465–470. doi: 10.1016/j.amjmed.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Lévesque LE, Hanley Ja, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340(april):b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project (HCUP) HCUP National Inpatient Sample (NIS) Rockville M: www.hcup-us.ahrq.gov/nisoverview.jsp. Published 2012. [Google Scholar]
- 9.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 10.Elixhauser a, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Stein PD, Matta F, Hull RD. Increasing Use of Vena Cava Filters for Prevention of Pulmonary Embolism. Am J Med. 2011;124(7):655–661. doi: 10.1016/j.amjmed.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Jia Z, Wu A, Tam M, Spain J, McKinney JM, Wang W. Caval Penetration by Inferior Vena Cava Filters: A Systematic Literature Review of Clinical Significance and Management. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.016468. (Ivc):CIRCULATIONAHA.115.016468. [DOI] [PubMed] [Google Scholar]
- 13.Imberti D, Dentali F, Ageno W, et al. Evidence and clinical judgment: vena cava filters. Thromb Haemost. 2014;111(4):618–624. doi: 10.1160/TH13-11-0938. [DOI] [PubMed] [Google Scholar]
- 14.Girard P, Meyer G, Parent F, Mismetti P. Medical literature, vena cava filters and evidence of efficacy A descriptive review. Thromb Haemost. 2013;111(4):761–769. doi: 10.1160/TH13-07-0601. [DOI] [PubMed] [Google Scholar]
- 15.Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313(16):1627–1635. doi: 10.1001/jama.2015.3780. [DOI] [PubMed] [Google Scholar]
- 16.PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 17.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/S0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 18.Suissa S, Azoulay L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care. 2012;35(12):2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanfilippo KM, Wang T, Gage BF, Liu W, Carson KR. Improving accuracy of International Classification of Diseases codes for venous thromboembolism in administrative data. Thromb Res. 2015;135(4):616–620. doi: 10.1016/j.thromres.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126(1):61–67. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]