Abstract
Background
Opioid use disorders (OUDs) are reaching epidemic proportions in the United States, and many geographic areas struggle with a persistent shortage in availability of opioid agonist treatment. Over the past 5 years, Vermont addiction medicine physicians and public health leaders have responded to these challenges by developing an integrated hub-and-spoke opioid treatment network.
Methods
In the present report, we review the development, implementation, and impact of this novel hub-and-spoke model for expanding OUD treatment in Vermont.
Results
Vermont’s hub-and-spoke system has been implemented state-wide and well-received by providers and patients alike. Adoption of this model has been associated with substantial increases in the state’s OUD treatment capacity, with Vermont now having the highest capacity for treating OUD in the United States with 10.56 people in treatment per 1000. There has been a 64% increase in physicians waivered to prescribe buprenorphine, a 50% increase in patients served per waivered physician, and a robust bidirectional transfer of patients between hubs and spokes based upon clinical need. Challenges to system implementation and important future directions are discussed.
Conclusions
Development and implementation of a hub-and-spoke system of care has contributed substantially to improvements in opioid agonist treatment capacity in Vermont. This system may serve as a model for other states grappling with the current opioid use epidemic.
Keywords: buprenorphine, hub-and-spoke system, opioid dependence, opioid use disorder, treatment
Opioid use disorders (OUDs) are reaching epidemic proportions in the United States, and opioid-related consequences (eg, overdoses, premature death, infectious disease, criminality) have resulted in economic costs of $56 billion annually (Becker et al., 2008; Wisniewski et al., 2008; Clausen et al., 2009; Birnbaum et al., 2011; Paulozzi, 2012; Jones et al., 2015b; Substance Abuse and Mental Health Services Administration [SAMHSA], 2016c). Whereas many geographic areas have experienced a persistent shortage in opioid treatment availability, this problem is particularly urgent in rural areas struggling with high rates of opioid dependence and few treatment options (Office of National Drug Control Policy, 2008; Rosenblum et al., 2011; Sigmon, 2014, 2015; Sigmon et al., 2016).
In 2000, Vermont was 1 of 8 states in the United States without opioid agonist treatment (OAT) for OUD. Opioid-dependent Vermonters traveled to neighboring states for methadone (MTD) treatment. After passage of Vermont Senate bill 303 in May 2000 allowing MTD treatment in Vermont, the first opioid treatment program (OTP) opened in October 2002 with a 100-patient capacity. A waiting list quickly developed, indicating continued unmet need for OAT in Vermont. Passage of the Drug Abuse Treatment Act (DATA) in 2000 and the introduction of buprenorphine in 2003 provided a new OAT option in Vermont. Use of buprenorphine expanded throughout Vermont with support from favorable Medicaid coverage, American Society of Addiction Medicine-sponsored waiver trainings, and incentives to support adoption of office-based opioid treatment (OBOT). Vermont emerged as a US leader in per capita number of OBOT providers (SAMHSA, 2006a, 2006b; Department of Justice, 2012). However, most OBOT physicians were treating only a small handful of patients, due in part to concerns about induction logistics, reimbursement challenges, potential for medication diversion, lack of support for providers with managing complex patients, and lack of psychosocial services for patients (Becker and Fiellin, 2006; Kissin et al., 2006; Barry et al., 2008; Netherland et al., 2009). As a result, it remained difficult for opioid-dependent Vermonters to find an available OBOT provider, and the waitlist delay at our state’s OTP grew to almost 2 years (Department of Vermont Health Access, 2012; Sigmon, 2014, 2015).
This prompted recognition of the need for a specialized clinic which could induct patients onto buprenorphine before transfer to OBOT, retain complex patients, and also receive returning patients who destabilize during OBOT. Similar to networks used in the management of other chronic diseases such as cardiology, infectious disease, and endocrinology (Lee et al., 2003; Nobilio and Ugolini, 2003), it was proposed that OAT could benefit from a system which integrates a center of addiction expertise (ie, the OTP as hub) with a network of providers in regional catchment areas (ie, OBOT providers as spokes). With the creation of Vermont’s Blueprint for Health, OUD was designated as a chronic condition, and addiction medicine physicians and public health leaders developed the Care Alliance for Opioid Addictions Initiative, which became known as the hub-and-spoke system (Simpatico, 2015; Casper and Folland, 2016). In this report, we review the development, implementation, and impact of this novel hub-and-spoke model for expanding OUD treatment in Vermont.
METHODS
Hubs
Vermont was divided into 5 geographic regions, each with a “hub clinic” organized around an existing OTP that was given prescriptive authority to dispense buprenorphine along with MTD under its existing OTP licensure (Government Publishing Office, 2012). A gradual, staggered deployment of hubs began in January 2013 across all 5 regions. Briefly, hub staff assess patients’ medical and psychiatric needs at intake and determine the most appropriate treatment placement (eg, in the OTP with MTD or buprenorphine, with spoke providers for OBOT). Entry points into the hubs also include hospitals and emergency rooms (especially after an overdose reversal or medical treatment for injection-related diseases), residential programs, Department of Corrections, and community mental health programs (Fig. 1).
FIGURE 1.
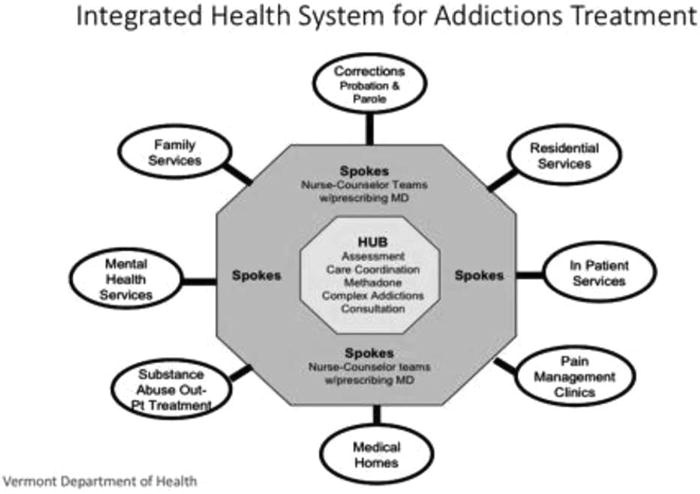
Components and referral sources for an integrated hub-and-spoke system for addiction treatment.
Patient transfers between hubs and spokes are bidirectional. First, hub-to-spoke transfers are a primary aim of the system. Once patients are determined to be stable on buprenorphine, they are assessed for potential referral to a spoke provider (described more below). If the patients have no primary care provider, they are linked with a medical home for ongoing health care and buprenorphine. In geographic areas without OBOT providers, a Medication-Assisted Treatment (MAT) team in each region calls on physicians and encourages them to become certified through trainings offered by the Care Alliance. Second, patients can be transferred from a spoke back to the hub if they destabilize in the OBOT setting. As all hubs are staffed by a board-certified addiction specialist, spoke physicians can receive ongoing consultation on any questions regarding patients in their care. Return transfers of patients from the spoke physician to the hub are prioritized to ensure that providers feel supported and patients receive continuity of care.
Funding for the hub-and-spoke system was tied to Section 2703 of the Affordable Care Act, which allows for Home Health Services as Community Health Teams and provides a bundled, monthly rate (subsidized by Medicaid or a state grant) for 1 standard clinical service and 1 medical service per month. An enhanced rate is available for 1 monthly additional health home encounter (eg, comprehensive care management, care coordination, individual and family support, referral to community services). Buprenorphine cost is carved out from the OTP cost structure, which is based on MTD. Suboxone film is preferred by Vermont Medicaid to a generic combo or mono buprenorphine product, with prior authorization required for doses over 16 mg. To reduce diversion risk, all dosing is observed and dissolving for 5 minutes is required before patients leave. The use of less-than-daily dosing of buprenorphine (Amass et al., 1998; Bickel et al., 1999) is often used to give patients more flexibility before they earn take homes.
Spokes
In each of Vermont’s 5 regions, waivered physicians were paired with that region’s hub and designated as spoke providers. Spokes have direct access to hubs for consultation on referrals, screenings, and induction logistics. Any waivered physician is eligible to become a spoke provider, with the hope that with the extra support of the hub-and-spoke system, they will expand their patient capacity. Spoke providers include family practitioners, internists, psychiatrists, obstetricians, and pediatricians in Federally Qualified Health Centers, private group practices, hospital-owned practices, and solo practices.
Each spoke is supported by a MAT team consisting of 1 full-time equivalent registered nurse and a master’s-level licensed behavioral health provider per 100 Medicaid OBOT patients. The MAT team is provided by the Vermont Chronic Care Initiative at no cost to the practice through a 90/10 funding split leveraged from the Affordable Care Act and Centers for Medicare and Medicaid (Casper and Folland, 2016). MAT team full time equivalents are split based on number of patients per practice, with the team traveling to multiple sites for physicians with few patients and a team embedded in practices with a large number of OBOT patients.
The MAT team nurse meets with new patients, reviews contracts and consents, arranges for insurance authorization, arranges for urine drug testing, authorizes buprenorphine refills to pharmacies, and oversees diversion control through random call-backs and monitoring of Vermont’s Prescription Monitoring System. The behavioral health provider coordinates counseling services, manages acute crises, provides brief supportive counseling or check-ins, helps with practical issues (eg, housing, insurance, transportation issues), and coordinates referrals between the spoke practice and hub. Consistent with Vermont’s MAT guidelines, all patients receive a behavioral health assessment and counseling services provided by a counselor either in the practice or outside of it. Whereas the MAT team counselor can provide brief counseling or case management, this was not intended as a substitute for needed psychosocial services in OUD treatment. The MAT team meets regularly with the spoke physician(s) to discuss cases, protocols, and coordination among staff. When a patient has a positive drug screen, rather than reflexively discharging the patient or transferring to the hub, the MAT team works to evaluate the patient’s needs and provide additional clinical support.
To support state-wide dissemination and implementation of the hub-and-spoke model and establish consistency in all practices, a learning collaborative was developed (Nordstrom et al., 2016). It offers in-person and web-based lectures to spoke physicians and MAT staff covering safe prescribing, use of evaluation tools, treatment plan development, responses to relapse, patient noncompliance, and diversion control. The learning collaborative includes a focus on evidenced-based practices so that hubs and spokes share a common set of practices, which have since been published as the Vermont MAT Practice Guidelines (Vermont Department of Health Access, 2015).
Finally, we identified the need for a brief assessment that would offer an efficient evaluation of patient severity at treatment intake and help physicians to pair patients with the most appropriate care. We developed the Treatment Needs Questionnaire (TNQ) to identify the treatment setting (ie, OTP vs OBOT) and not necessarily the type of agonist therapy best suited to each patient. The lead author (J.B.) sketched together a set of variables that were important in determining the severity of need, loosely based on the Addiction Severity Index (ASI; McLellan et al., 2006). Further refinements based on clinical expertise and reviews of the scientific literature (eg, Sullivan et al., 2010; Bukten and Skurtveit, 2014; Fareed et al., 2014; Perrault et al., 2015) led to a brief 21-item screener. It assesses areas of psychosocial functioning (eg, legal, drug and alcohol use, transportation, chronic pain, social support) with individual items summed for a possible maximum score possible of 26 (Fig. 2, available online in digital appendix). Higher scores indicated greater severity and thus the patient’s potential need for a more intensive treatment approach (eg, hub rather than spoke). The TNQ has been incorporated into the intake assessment at all hubs and is often used as a triage tool by spoke providers.
FIGURE 2.
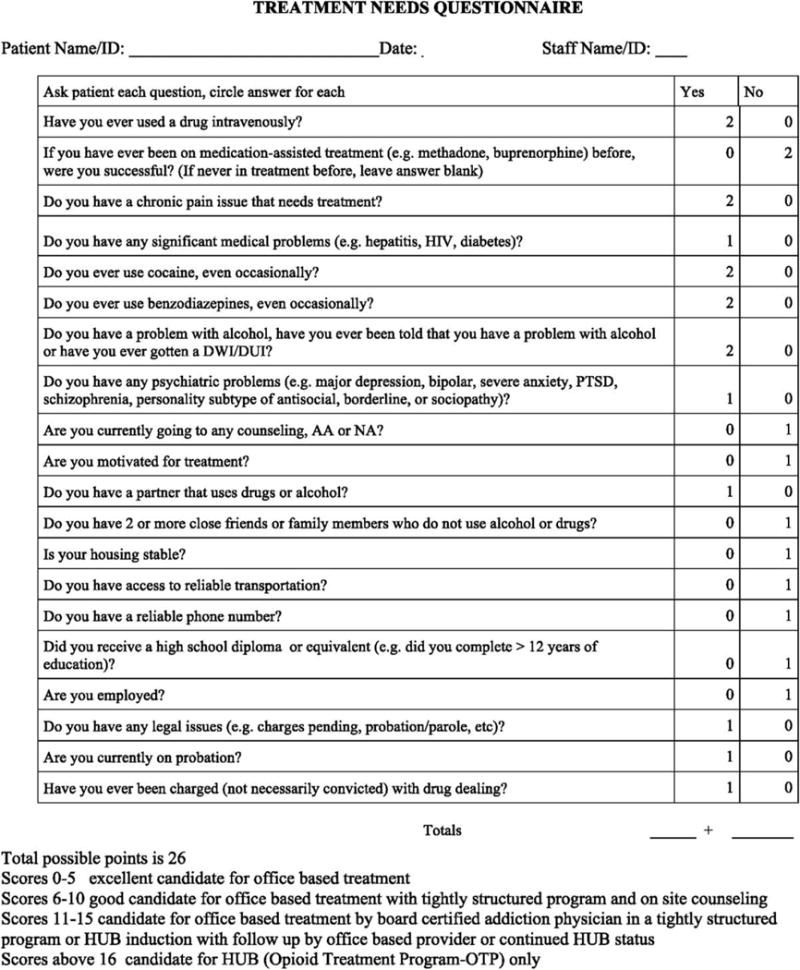
Treatment Needs Questionnaire (for online/digital appendix). @2015 JR Brooklyn & SC Sigmon, Licensed under CC BY-NC-ND 4.0 version 1/21/16.
RESULTS
The hub-and-spoke model supported a substantial increase in Vermont’s OUD treatment capacity. According to Vermont Medicaid data, in April 2012, 650 people were on MTD in OTPs and 1700 were on buprenorphine in OBOTs for a 30:70 split. During its initial 3 years of implementation, approximately 900 patients were served in hubs in January 2013 to over 2800 by September 2015 (Fig. 3). There was a reduction in wait lists, but as more people sought treatment, these waiting lists remained unchanged in certain regions. The Southeast and Southwestern Vermont hubs nearly eliminated their waiting list, and the Central and Northeast Vermont hubs eliminated their waiting lists by March 2016. The Northwestern hub had reduced the waiting time for entry from 6 months to 2 months by March 2016 (Fig. 4).
FIGURE 3.
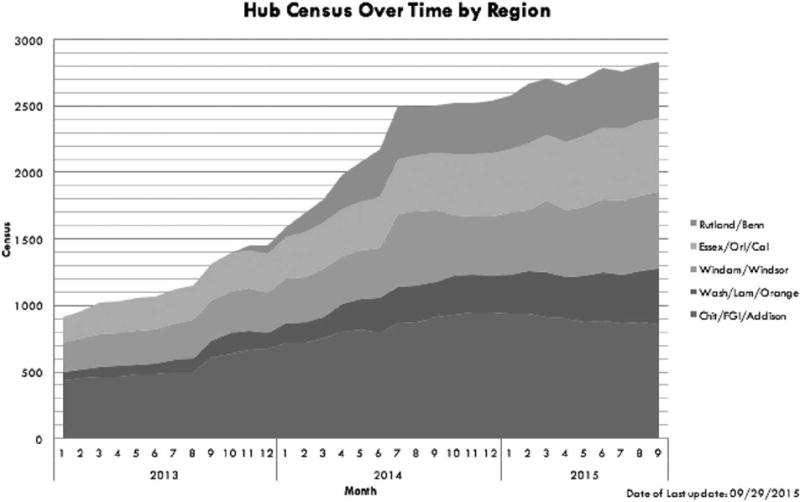
Patient census data presented for Vermont’s 5 regional hubs over time (January 2013–September 2015).
FIGURE 4.
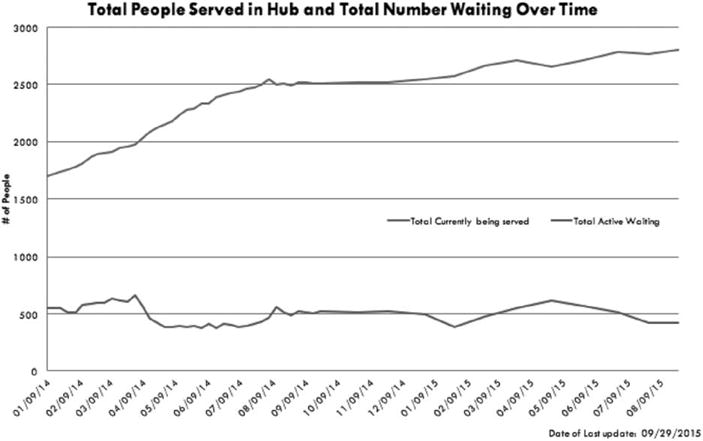
Total number of individuals served in hubs and number of individuals waiting for treatment over time (January 2014–August 2015).
By December 2014, of the 7212 people with a diagnosis of OUD, 5298 were getting OAT for a 73% rate of penetration (Casper and Folland, 2016). By September 2016, there were 3147 people in the hubs (2172 on MTD and 975 on buprenorphine) and 3457 (2621 Medicaid recipients) on buprenorphine in the spokes or 48:52 split in OTP to OBOT (hub to spoke). With 6604 patients on OAT and Vermont’s total population of 625,000, this represents 1.05% of all Vermonters on OAT or 10.56 people treated per 1000 people up from 2012 when it was 3.76 people per 1000.
With regard to other aspects of the hub-and-spoke system, between 2012 and 2016, the number of waivered physicians in Vermont increased from 173 to 283, reflecting a 64% increase. Density of buprenorphine patients per provider improved, with a 50% increase in those prescribing for more than 10 patients. By September 2015, 23% of spoke providers had >30 patients and 10% had >50 patients. From January 2014 to December 2015, 225 “stable” patients had transferred from hubs to spokes. In addition, the safety net component of the hub-and-spoke system is most evident in the transfer of “unstable” patients from the spokes to the hubs. The most recent data show 73 patients moving from spokes to hubs from July 2015 to January 2016. In addition, over 250 patients from various OBOT providers that went out of business or moved were absorbed by hubs or spokes.
Of all Vermonters, 86% are enrolled in a Primary Care Medical Home (PCMH) as defined by the National Committee for Quality Assurance (NCQA). Once the multidisciplinary MAT teams were added to these PCMHs, creating spokes, full coverage existed for all opioid users receiving MAT (National Academy of State Health Policy, 2014). The first hub (Chittenden Center) completed its baseline NCQA data assessment and became the first OTP to receive Medical Home status in the United States. The VT Blueprint for Health provides a project manager and embedded staff to monitor the quality of care for each hub. All other hubs are working on NCQA status, so if an opioid user is not in a spoke, they can receive basic medical services in the hubs.
Cost impacts have been assessed by the Vermont Child Health Improvement Program (VCHIP). Before expansion of OAT in Vermont in 2012, individuals with at least 2 claims for OUD or opioid dependence in a calendar year had healthcare costs derived from Medicaid Claims Data that were higher than those without claims (Mohlman et al., 2016). Since 2013, these overall healthcare costs, including the cost of OAT, have dropped by 7% to 10% (Krantz, 2014). The Department of Vermont Health Access projected in testimony to the Vermont legislature in March 2014 that for the 2164 patients estimated to be served state-wide, the savings will be $6.7 million (Chen and VanDonsel, 2015). A recent article by Tkacz and Volpicelli (2014) reinforces the cost savings of OAT.
DISCUSSION
We believe that the Vermont model is unique in the United States as a state-wide system to integrate OUD disorder and OAT into mainstream medicine, eliminating the “silo effect.” From an integrated care perspective, as described by (Heath et al., 2013), the hub-and-spoke model follows a continuum from collaboration to integration. There is a similar program in Baltimore, MD—the COop model—which allows unstable OAT patients from local physicians to come into a more structured program for a time, and stabilize and return to their home program (Stoller, 2015), and in Rhode Island, a program now exits to link buprenorphine prescribing in Medical Health Homes (Storti, 2016). Our program combines elements of both and is unique in linking all OBOT and OTP programs in a seamless way, state-wide, and linking patients with medical homes. The model has benefitted from a fully funded program through Medicaid expansion and political and governmental support that is also unique in the United States.
Vermont now has the highest capacity for treating OUD in the United States, with 13.8 patients potentially treated per 1000 people (Jones et al., 2015a, 2015b), and currently are at 10.56 per 1000 as noted above. If current physicians prescribed to the limit of their waivers, the state would have close to 150% capacity in its spoke system. From the data, there is an overall increase in access to OAT, an increase in physicians waivered to prescribe BPN, an increase in patients served per waivered physician, a robust transfer of patients to and from each component, and a cost of care for OUD that remains at least neutral. Increased education on OAT and OUD through the Learning Collaborative, the use of the TNQ to triage more effectively, and Vermont policies that promote the treatment of OUD has increased the patients under treatment. The provision of MAT team services to assist with clinical, logistical, and administrative tasks reduces the resistance of providers to prescribe buprenorphine (Netherland et al., 2009).
The primary challenges encountered during implementation of the Vermont hub-and-spoke system include staffing shortages, particularly among nurses and clinicians, and difficulty ensuring accurate data collection across a network of treatment sites. There are also still areas in the state with few buprenorphine prescribers. Efforts are underway to change this, with the University of Vermont Medical Center supporting buprenorphine education with medical students and residents. Additional efforts are also needed to evaluate the drug abstinence and cost outcomes associated with the hub-and-spoke model, and also to establish the validity of the TNQ for assessing patients’ treatment needs.
In summary, the landscape of OAT capacity in Vermont has dramatically improved. The development and implementation of a hub-and-spoke system of care has contributed substantially to this improvement and may serve as a helpful model for other states grappling with the current opioid use epidemic.
Acknowledgments
We thank Richard Rawson and Anthony Folland for consultation on this manuscript and the hub-and-spoke project more generally. We also thank Kris Langabeer for providing editorial support for the manuscript.
Funding: Supported in part by National Institutes of Health research grant (R34DA037385), and also a National Institute of General Medical Sciences center grant (P20GM103644).
Footnotes
Author contributions: J.R.B. conceptualized and developed the manuscript. S.C.S. contributed content and made revisions to the manuscript. Both authors have reviewed and contributed to the study manuscript and approve of the final article.
Conflicts of interest: There are no financial disclosures relevant to this study.
References
- Amass L, Bickel WK, Crean JP, et al. Alternate-day buprenorphine dosing is preferred to daily dosing by opioid-dependent humans. Psychopharmacol. 1998;136:217–225. doi: 10.1007/s002130050559. [DOI] [PubMed] [Google Scholar]
- Barry DT, Bernard MJ, Beitel M, et al. Counselors’ experiences treating methadone-maintained patients with chronic pain: a needs assessment study. J Addict Med. 2008;2:108–111. doi: 10.1097/ADM.0b013e31815ec240. [DOI] [PubMed] [Google Scholar]
- Becker WC, Fiellin DA. Provider satisfaction with office-based treatment of opioid dependence: a systematic review. Substance Abuse. 2006;26:15–22. doi: 10.1300/j465v26n01_02. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, et al. Buprenorphine dosing every 1, 2 or 3 days in opioid-dependent patients. Psychopharmacol. 1999;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Bukten A, Skurtveit S. Factors associated with dropout among patients in opioid maintenance treatment (OMT) and predictors of re-entry. A national registry-based study. Addict Behav. 2014;39:1504–1509. doi: 10.1016/j.addbeh.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Casper K, Folland A. Essential Elements of Vermont’s “Hub and Spoke” Health Homes Model. American Association for the Treatment of Opioid Dependence White Paper. 2016:2–28. [Google Scholar]
- Chen H, VanDonsel A. The Effectiveness of Vermont’s System of Opioid Addiction Treatment. 2015 Available at: http://legislature.vermont.gov/assets/Legislative-Reports/Opioid-system-effectiveness-1.14.15.pdf. Accessed August 13, 2016.
- Clausen T, Waal H, Thoresen M, et al. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Department of Justice, Drug Enforcement Administration, Office of Diversion Control. Arcos 3: Report 4. Cumulative distribution by state in grams per 100,000 population. 2014. p. 15. (Reporting period: 01/01/2012 to 12/31/2012). Accessed March 23, 2016. [Google Scholar]
- Department of Vermont Health Access. Vermont hub and spoke health homes: program and payment overview [web site] State of Vermont Department of Health; 2012. Available at: http://dvha.vermont.gov/administration/1hub-spoke-health-home-framework-payment-12-10-12.pdf. Accessed March 23, 2016. [Google Scholar]
- Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8:345–350. doi: 10.1097/ADM.0000000000000057. [DOI] [PubMed] [Google Scholar]
- Government Publishing Office. Department of Health and Human Services 42 CFR Part 8. [web site] Federal Register. 2012;77 Available at: https://www.gpo.gov/fdsys/pkg/FR-2012-12-06/html/2012-29417.htm. Accessed March 23, 2016. [Google Scholar]
- Heath B, Wise Romero P, Reynolds KA. Standard Framework For Levels of Integrated Healthcare. Washington, DC: SAMHSA-HRSA Center for Integrated Health Solutions; 2013. [Google Scholar]
- Jones CM, Campopiano M, et al. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105:e55–e63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, et al. Vital signs: demographic and substance use trends among heroin users: United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64:719–725. [PMC free article] [PubMed] [Google Scholar]
- Kissin W, McLeod C, Sonnefeld J, et al. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Krantz L. Opiate addiction treatment hubs save money, state says. VT Digger [web site] March 20, 2014 Available at: http://vtdigger.org/2014/03/20/addiction-treatment-hubs-save-money-state-says/. Accessed March 31, 2016.
- Lee TSW, Renaud EF, Hills OF. Emergency psychiatry: an emergency treatment hub-and-spoke model for psychiatric emergency services. Psychiatric Services. 2003;54:1590–1594. doi: 10.1176/appi.ps.54.12.1590. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, et al. The Addiction Severity Index at 25: origins, contributions and transitions. Am J Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- Mohlman MK, Tanzman B, Finison K, et al. Impact of medication-assisted treatment for opioid addiction on Medicaid expenditures and health services utilization rates in Vermont. J Subst Abuse Treat. 2016;5:9–14. doi: 10.1016/j.jsat.2016.05.002. [DOI] [PubMed] [Google Scholar]
- National Academy of State Health Policy. Vermont-Medical Homes [web site] June 1, 2014. Available at: http://www.nashp.org/vermont-695/. Accessed March 31, 2016.
- Netherland J, Botsko M, Egan JE, et al. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobilio L, Ugolini C. Selective referrals in a ‘hub and spoke’ institutional setting: the case of coronary angioplasty procedures. Health Policy. 2003;63:95–107. doi: 10.1016/s0168-8510(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Saunders EC, McLeman B, et al. Using a learning collaborative strategy with office-based practices to increase access and improve quality of care for patients with opioid use disorders. J Addict Med. 2016;10:115–121. doi: 10.1097/ADM.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National Drug Control Policy (ONDCP) Vermont drug control update [web site] 2008 Available at: https://www.whitehouse.gov/sites/default/files/docs/state_profile_-_vermont.pdf. Accessed March 23, 2016.
- Paulozzi LJ. Prescription drug overdoses: a review. J Safety Res. 2012;43:283–289. doi: 10.1016/j.jsr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Perrault M, Julien D, White ND, et al. Psychological predictors of retention in a low-threshold methadone maintenance treatment for opioid addicts: a 1-year follow-up study. Subst Use Misuse. 2015;50:24–31. doi: 10.3109/10826084.2014.957769. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011 doi: 10.1155/2011/948789. [Article ID 948789 (10 pages)]. Available at: http://dx.doi.org/10.1155/2011/948789. Accessed March 23, 2016. [DOI] [PMC free article] [PubMed]
- Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71:359–360. doi: 10.1001/jamapsychiatry.2013.4450. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. The untapped potential of office-based buprenorphine treatment. JAMA Psychiatry. 2015;72:395–396. doi: 10.1001/jamapsychiatry.2014.2421. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Ochalek T, Meyer A, et al. Interim buprenorphine vs waiting list for opioid dependence. N Engl J Med. 2016;375:2504–2505. doi: 10.1056/NEJMc1610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpatico TA. Vermontresponds toits opioidcrisis. Prev Med. 2015;80:10–11. doi: 10.1016/j.ypmed.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Stoller KB. A collaborative opioid prescribing (CoOP) model linking opioid treatment programs with office-based buprenorphine providers. Addict Sci Clin Pract. 2015;10:A63. [Google Scholar]
- Storti S. Integration of Health Homes in Rhode Island OTPs. American Association for the Treatment of Opioid Dependence White Paper. 2016:29–43. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) (Final report [web site]).Diversion and abuse of buprenorphine: a brief assessment of emerging indicators. 2006a Available at: http://buprenorphine.samhsa.gov/Buprenorphine_FinalReport_12.6.06.pdf. Accessed March 23, 2016.
- Substance Abuse and Mental Health Services Administration (SAMHSA) (Results of the Vermont Case Study [web site]).Diversion and abuse of buprenorphine: a brief assessment of emerging indicators. 2006b Available at: http://buprenorphine.samhsa.gov/Vermont.Case.Study_12.5.06.pdf. Accessed March 23, 2016.
- Substance Abuse and Mental Health Services Administration (SAMHSA) Opioids. 2016c Available at: http://www.samhsa.gov/atod/opioids. Accessed March 23, 2014.
- Sullivan LE, Moore BA, O’Connor PG, et al. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. Am J Addict. 2010;19:53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J, Volpicelli J. Relationship between BPN adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat. 2014;46:456–462. doi: 10.1016/j.jsat.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Vermont Department of Health Access. Managed care entity: Vermont burprenorphine clinical practice guidelines [web site] 2015 Available at: http://dvha.vermont.gov/for-providers/buprenorphine-practice-guide-lines-revised-final-10-15.pdf. Accessed March 23, 2016.
- Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis. 2008;27:1–11. doi: 10.1300/J069v27n01_01. [DOI] [PubMed] [Google Scholar]


