Abstract
Mast cells are resident immune cells in the thalamus that can degranulate and release hundreds of signaling molecules (i.e., monoamines, growth factors, and cytokines) both basally and in response to environmental stimuli. Interestingly, mast cell numbers in the brain show immense individual variation in both rodents and humans. We used a Pavlovian conditioned approach (PCA) procedure to examine whether mast cells are associated with individual variation in the attribution of incentive-motivational value to reward-related cues. During the PCA procedure, a lever response-independently predicts the delivery of a food pellet into a magazine, and over training sessions three conditioned responses (CRs) develop: sign-tracking (lever-directed CRs), goal-tracking (magazine-directed CRs), and an intermediate response (both CRs). In Experiment 1, we measured thalamic mast cell number/activation using toluidine blue and demonstrated that sign-trackers have increased degranulated (activated) but not granulated (inactive) mast cells. In Experiment 2, we infused the mast cell inhibitor, cromolyn (200 µg/rat; i.c.v.), immediately before five daily PCA training sessions and demonstrated that mast cell inhibition selectively impairs the acquisition of sign-tracking behavior. Taken together, these results demonstrate that thalamic mast cells contribute to the attribution of incentive-motivational value to reward-related cues and suggest that mast cell inhibition may be a novel target for addiction treatment.
Keywords: Pavlovian conditioned approach, reward, goal-tracking, incentive salience, individual differences, histamine, cromolyn
1. Introduction
Mast cells are a heterogeneous population of immune cells that are generated as precursor cells in the periphery within bone marrow and can rapidly penetrate the blood vessels of the blood-brain barrier where they mature within the brain (Silverman et al., 2000). Mast cells have been identified in the brains of approximately two dozen mammalian species, including both rats and humans (Dropp, 1976, 1979). Within the brains of rats, they reside primarily within the thalamus and epithalamus (Goldschmidt et al., 1984) where they degranulate (Persinger, 1983) and release hundreds of signaling molecules (i.e., monoamines, growth factors, and cytokines) in a radius up to 50 µm (Nautiyal et al., 2008). Degranulation can amplify signaling by affecting the activity of a wide variety of surrounding cells, including neurons (Koszegi et al., 2006; Kovacs et al., 2006), astrocytes (Zeng et al., 2013), and microglia (Yuan et al., 2010; Zhang et al., 2012). Moreover, mast cells are poised to dynamically influence behavior, because their number and activity within the thalamus is highly variable under basal conditions (Florenzano and Bentivoglio, 2000, 2001), fluctuate in response to physiological states and environmental stimuli, and show behaviorally relevant patterns of regional activation (Asarian et al., 2002; Kovacs and Larson, 2006). In addition, mast cells contribute approximately 90% of thalamic histamine in rodents (Goldschmidt et al., 1985), and histamine has been implicated in appetitive behaviors and motivational processes (Torrealba et al., 2012). Although mast cells in the brain have been previously linked to depression- and anxiety-like behaviors (Chikahisa et al., 2013; Nautiyal et al., 2008), it is currently unknown whether mast cells play a role in reward-related behaviors.
Interestingly, there is substantial individual variation in the number of brain mast cells. For example, in male Sprague Dawley rats, brain mast cell numbers can vary from 1,490 to 19,103 per whole brain (Goldschmidt et al., 1984), and human mast cells can vary from 0 to 49,000 depending on the brain region (Dropp, 1979). Given this high variability in mast cell numbers, mast cells could contribute substantially to individual variation in reward-related behaviors that are relevant to vulnerability and resilience to addiction. The attribution of incentive-motivational salience to reward-related cues is believed to underlie addiction-like behaviors (Flagel et al., 2009) and can be studied using a Pavlovian conditioned approach (PCA) procedure. During PCA training, rats are presented with a conditioned stimulus (CS; e.g., a lever) followed by the response-independent delivery of an unconditioned stimulus (US; e.g., a food pellet) into a pellet magazine. Over the course of training, three patterns of conditioned responses (CRs) develop: sign-tracking (CS-directed CRs), goal-tracking (US-directed CRs), and an intermediate response (both CRs). Whereas goal-trackers (GTs) utilize the CS as a predictor of impending food pellet delivery, sign-trackers (STs) attribute incentive-motivational value to the CS whereby it becomes a powerful motivator of behavior (Robinson and Flagel, 2009) even in the absence of the US (Ahrens et al., 2016). Previously, it has been shown that STs, compared to intermediate responders (IRs) or GTs, are more vulnerable to cue-induced reinstatement of drug-seeking behavior (Saunders and Robinson, 2010) and seek drug despite adverse consequences (Saunders et al., 2013), two hallmarks of addiction. STs compared to GTs also have increased neural activity (i.e., induction of c-Fos transcription or translation) following food- and drug-related cue presentation in the paraventricular and intermediodorsal nuclei of the thalamus as well as the lateral habenula (Flagel et al., 2011; Yager et al., 2015). Because mast cells are capable of regulating neuronal activity in the thalamus (Koszegi et al., 2006; Kovacs et al., 2006), it is plausible that individual variation in the number and activity of thalamic mast cells between phenotypes may underlie differences in PCA behavior.
The present study investigated whether thalamic mast cell number and activity is different between PCA phenotypes and influences PCA behavior. In Experiment 1, mast cell number and activity was quantified seven days after five daily PCA training sessions using toluidine blue staining. In Experiment 2, rats were implanted with intracerebroventricular (i.c.v.) cannulas and infused with cromolyn (200 µg/rat), a mast cell inhibitor, immediately before five daily PCA training sessions in order to determine whether mast cell inhibition alters the acquisition of sign- or goal-tracking behaviors.
2. Material and Methods
2.1. Animals
Adult male Sprague Dawley rats (275–300 g) were purchased from Charles River Laboratories. Rats were maintained on a 12:12-hr light/dark cycle, and food and water were available ad libitum for the duration of experimentation. All procedures were approved by the University Committee on the Use and Care of Animals (University of Michigan; Ann Arbor, MI).
2.2. Drugs
Toluidine blue (#T3260), cromolyn (#C0399), and pontamine sky blue (#C8679) were used (Sigma-Aldrich, Inc.; St. Louis, MO).
2.3. Pavlovian Conditioned Approach: Apparatus
Sixteen modular conditioning chambers (24.1 cm width × 20.5 cm depth × 29.2 cm height; MED Associates, Inc.; St. Albans, VT) were used for Pavlovian conditioning. Each chamber was located in a sound-attenuating cubicle equipped with a ventilation fan to provide ambient background noise. During PCA training sessions, each chamber was equipped with a food magazine, a retractable lever (counterbalanced on the left or right side of the magazine), and a red house light on the wall opposite to the magazine. The magazine contained an infrared sensor to detect magazine entries, and the lever was calibrated to detect lever deflections in response to 10 g of applied weight. Whenever the lever was extended into the chamber, an LED mounted inside the lever mechanism illuminated the slot through which the lever protruded.
2.4. Pavlovian Conditioned Approach: Procedure
For two days prior to the start of training rats were familiarized with banana-flavored pellets (45 mg; Bioserv; Frenchtown, NJ) in their home cages. Rats were then placed into the test chambers for one pretraining session during which the red house-light remained on but the lever was retracted. Fifty food pellets were delivered on a variable time (VT) 30-s schedule (i.e., one pellet was delivered on average every 30 s, but varied 0–60 s). Rats were not food deprived at any point during experimentation. Each trial during a PCA training session consisted of presentation of the illuminated lever (the CS) into the chamber for 8 s on a VT 90-s schedule (i.e., time randomly varied 30–150 s between CS presentations). Retraction of the lever was immediately followed by the response-independent delivery of one food pellet (the US) into the magazine. The beginning of the next inter-trial interval commenced immediately after pellet delivery. Each test session consisted of 25 trials of a CS-US pairing. If rats did not consume all the pellets that were delivered, they were excluded from further behavioral testing.
2.5. Experiment 1: Toluidine Blue Staining Procedure
One week following the last session of PCA training, rats were anesthetized with a solution of ketamine (90 mg/kg) and xylazine (10 mg/kg), then transcardially perfused with a 4% paraformaldehyde solution in 0.1 M phosphate buffered saline (pH 7.32–7.36). Next, brains were post-fixed in a 4% paraformaldehyde solution for 24 hours then placed in a 20% sucrose solution containing 0.01% sodium azide. After sucrose saturation, brains were flash frozen in isopentane over dry ice. Then, brains were sectioned on a cryostat (40 µM; Leica CM1850; Leica Microsystems, Inc.; Buffalo Grove, IL) through the thalamus (anterior-posterior [AP]: −1.8 to −4.56 mm measured from bregma; Paxinos and Watson, 2007) and finally processed using toluidine blue staining. Every third section was processed for mast cells, resulting in 24 sections per rat.
When toluidine blue is acidified, it metachromatically stains highly anionic, sulfated proteoglycans within mast cell secretory granules (Ronnberg et al., 2012b). As a result, granulated mast cells are stained dark purple, and degranulated mast cells are stained lighter shades of purple depending on the degree of degranulation. Toluidine blue staining was adapted from Florenzano and Bentivolgio (2000). First, a 2% stock solution of toluidine blue was made. Brain sections were placed in a 0.01% toluidine blue solution containing acidified ddH2O (pH = 2.5) for 30 min. Second, brain sections were dipped briefly in ddH2O, then progressively dehydrated through a series of washes: 50% ethanol in ddH2O (15 s), 70% ethanol (45 s), 95% ethanol (60 s), 100% ethanol (60 s), and 100% ethanol (60 s). Brain sections were kept in the final ethanol solution, individually dipped in xylene, then coverslipped using Permount® (#SP15; Thermo Fisher Scientific, Inc.; Pittsburgh, PA). Following mounting, mast cells were quantified using a light microscope.
2.6. Experiment 2: Cannulation Surgery
Rats were anesthetized with a solution of ketamine (90 mg/kg) and xylazine (10 mg/kg) and placed into a stereotaxic frame (David Kopf Instruments; Tujunga, CA). Rats were implanted with a stainless-steel guide cannula (26-gauge, cut 3 mm below the pedestal; Plastics One, Inc.; Roanoke, VA) with a dummy cannula extending 1.5 mm beyond the cannula tip aimed at the right lateral ventricle (−0.8 mm from bregma, +1.3 mm from the midline, and −3.6 mm from dura; Paxinos and Watson, 2007).
2.7. Experiment 2: Procedure
After surgery, rats underwent a one-week post-surgical recovery period. Following the post-surgical recovery period, rats were familiarized with banana-flavored pellets in their home cages for two days followed by a pretraining session. Next, rats underwent five daily PCA training sessions. Rats were infused with either cromolyn (200 µg/rat) or saline (3 µL total volume; 1 µL/min; 1-min post-infusion period) using an infusion cannula (33-gauge, 1.5 mm projection; Plastics One, Inc.) connected to a Hamilton syringe (#65460-02; 5 µL; Hamilton Company; Reno, NV) in a syringe pump (Harvard Apparatus; Holliston, MA) by PE-50 polyethylene tubing (Instech Laboratories; Plymouth Meeting, PA). This dose was selected because it was previously shown to inhibit the central, behavioral effects of lipopolysaccharide, a well-established mast cell activator (Nava and Caputi, 1999). Following the post-infusion period, rats were immediately placed in conditioning chambers for behavioral testing. During training one rat was excluded for not consuming all food pellets. Following the final session of behavioral testing, rats were anesthetized with a solution of ketamine (90 mg/kg) and xylazine (10 mg/kg), and a 2% solution of pontamine sky blue in ddH2O was infused through the guide cannula to verify placements. Rats (n = 4) that did not have dye within the lateral ventricle were excluded from further analysis.
2.8. Statistical Analysis
SPSS (Version 22; IBM, Inc.) was used for statistical analysis. PCA behavior was scored using an index that incorporates the number, latency, and probability of lever presses (sign-tracking CR) and magazine entries (goal-tracking CR) during CS presentations within a session (Meyer et al., 2012). The PCA index scores behavior from +1.0 (absolute sign-tracking) to −1.0 (absolute goal-tracking), and we classified rats using the following cut-offs: GTs (x ≤ −0.5), IRs (−0.5 < x < 0.5), and STs (x ≥ 0.5). In Experiment 1, PCA behavior was analyzed using a linear mixed model with an autoregressive (AR1) covariance structure, selected using Akaike’s information criterion with Phenotype (ST, GT, and IR) and Session as factors. Thalamic mast cells were analyzed using a linear mixed model (AR1 covariance structure) with Phenotype (ST, GT, and IR) and Coordinate as factors. Habenular mast cells were analyzed using a two-way analysis of variance (ANOVA) with Phenotype (GT, IR, and ST) and Activation (Granulated and Degranulated) as factors. When appropriate, multiple comparisons were performed using Fisher’s Least Significant Difference (LSD) post hoc test. In Experiment 2, the number of lever presses and magazine entries across the 75 trials of a Sessions 1–3 (early PCA training) and the 50 trials of Sessions 4–5 (late PCA training) were analyzed using a linear mixed model (AR1 covariance structure) with Drug (Cromolyn and Vehicle) as a factor. This analysis was performed, because differences in conditioned responding between phenotypes generally do not present until later stages of training (i.e., Sessions 4–5) when learned CRs are fully expressed.
3. Results
3.1. Experiment 1: Sign-trackers have increased total and degranulated thalamic mast cells
Rats underwent five daily PCA training sessions and were classified as GTs (n = 11), IRs (n = 6), and STs (n = 7). Figure 1 shows that phenotypes differed in their lever press number (effect of Phenotype; F(2,26.47) = 24.2, p = 1.05 × 10−6; interaction of Session × Phenotype; F(8,79.67) = 3.33, p = 0.002), latency (effect of Phenotype; F(2,27.76) = 26.6, p = 3.50 × 10−7; interaction of Session × Phenotype; F(8,82.03) = 3.61, p = 0.001), and probability (effect of Phenotype; F(2,26.39) = 30.1, p = 1.57 × 10−7; interaction of Session × Phenotype; F(8,80.6) = 3.64, p = 0.001) as well as magazine entry number (effect of Phenotype; F(2,29.76) = 19.9, p = 3.22 × 10−6 ; interaction of Session × Phenotype; F(8,75.03) = 4.62, p = 1.29 × 10−4), latency (effect of Phenotype; F(2,30.83) = 13.9, p = 4.93 × 10−5; interaction of Session × Phenotype; F(8,78.5) = 4.02, p = 4.85 × 10−4), and probability (effect of Phenotype; F(2,27.66) = 5.29, p = 9.42 × 10−5; interaction of Session × Phenotype; F(8,78.33) = 4.69, p = 1.03 × 10−4). Post-hoc comparisons revealed that each phenotype separated in lever press number, latency, and probability during Sessions 3–5 (p < 0.05). For magazine entry number and latency, phenotypes separated during Sessions 4–5 (p < 0.05). In addition, magazine entry probability separated between GTs and STs during Sessions 3–5 (p < 0.05), but did not separate between GTs and IRs. These variables were incorporated into a PCA index score for each session as previously described (Figure 3A; effect of Phenotype; F(2,28.63) = 44.3, p = 1.72 × 10−9; interaction of Session × Phenotype; F(8,78.87) = 5.72, p = 9.67 × 10−6), and the average PCA index scores from Sessions 4 and 5 were used to classify phenotypes. Post-hoc comparisons revealed that the PCA index scores of each phenotype separated during Sessions 4–5 (p < 0.05).
Figure 1.
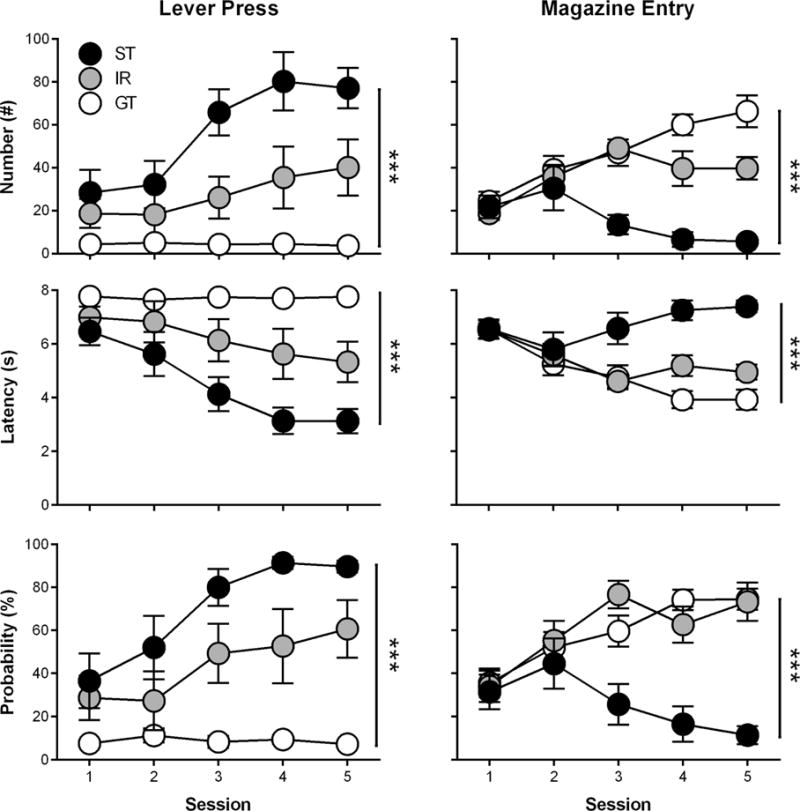
Rats underwent Pavlovian conditioned approach training over five daily sessions and were classified as goal-trackers (GTs; n = 11), intermediate responders (IRs; n = 6), or sign-trackers (STs; n = 7) based on their lever press and magazine entry number, latency, and probability during Sessions 4 and 5. Data are presented as mean and S.E.M. *** - p < 0.001.
Figure 3.
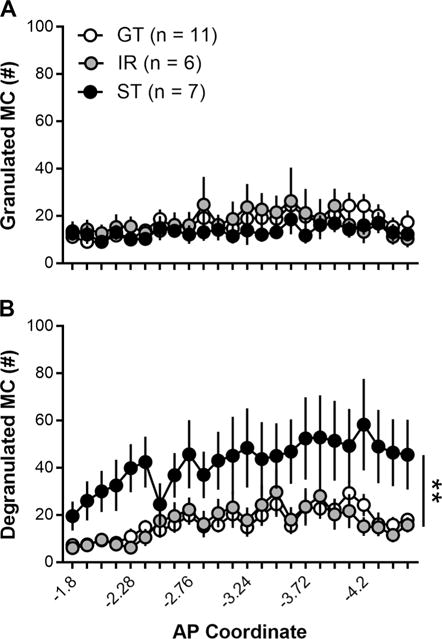
Rats were classified as goal-trackers (GTs; n = 11), intermediate responders (IRs; n = 6), or sign-trackers (STs; n = 7) based on their average Pavlovian conditioned approach index scores during Sessions 4 and 5. (A) GTs, IRs, and STs did not differ in their levels of granulated (inactive) mast cells. (B) Compared with GTs and IRs, however, STs had more degranulated (active) mast cells. Anterior-posterior (AP) coordinates are measured from bregma. Data are presented as mean and S.E.M. ** - p < 0.01.
Figure 2A–C shows the appearance of granulated (inactive) and degranulated (active) mast cells identified using toluidine blue staining in GTs, IRs, and STs. GTs, IRs, and STs did not differ in their number of granulated mast cells throughout the thalamus (Figure 3A; effect of Phenotype; F(2,39.76) = 0.6, p = 0.55; interaction of Phenotype × Coordinate; F(46,356.52) = 0.81, p = 0.81). GT, IRs, and STs did however differ in their number of degranulated mast cells throughout the thalamus (Figure 3B; effect of Phenotype; F(2,33.08) = 7.22, p = 0.003; interaction of Phenotype × Coordinate; F(46,403.35) = 1.56, p = 0.014). Post-hoc comparison revealed that STs had higher degranulated mast cells than both GTs (p = 0.001) and IRs (p = 0.004). In addition, STs had more degranulated mast cells than GTs at AP coordinates (measured in mm from bregma) −2.04, −2.16, −2.28, −2.4, −2.64, −2.76, −2.88, −3.0, −3.12, −3.24, −3.36, −3.6, −3.72, −3.84, −3.96, −4.2, −4.32, −4.4, and −4.56 (p < 0.05). Moreover, STs had more degranulated mast cells than IRs at AP coordinates −2.16, −2.28, −2.4, −3.24, −3.6, −3.72, −3.84, −3.96, −4.08, −4.2, −4.32, −4.44, and −4.56 (p < 0.05).
Figure 2.
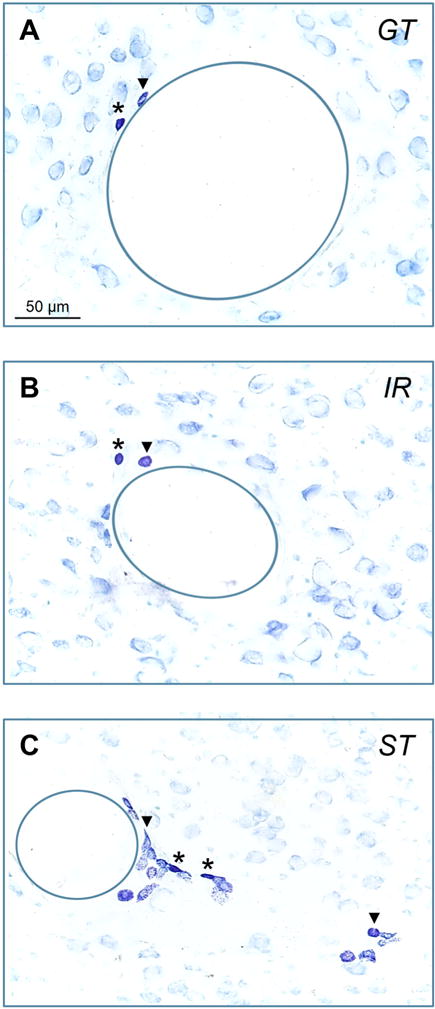
(A) Following PCA training, thalamic sections were stained for mast cells using toluidine blue. Representative photomicrographs are presented showing granulated (dark purple, *) and degranulated (light purple,▼) mast cells for (A) goal-trackers (GTs), (B) intermediate responders (IRs), and (C) sign-trackers (STs). Due to the high number of degranulated mast cells in STs, only two (out of 19) degranulated mast cells are labeled. In addition, blood vessels are outlined (solid circles).
Mast cells were not observed in the cortex or the hippocampus (Supplemental Figure 1A–B). In what remained of the meninges, mast cells were observed in small quantities; however, the brain extraction method used in the present study did not preserve the meningeal layers surrounding the entire brain, and quantification was not possible (Supplemental Figure 3C). Mast cells were present and scored, however, in the habenula, which resides in the epithalamus above the thalamus. Habenular mast cells were few in number and did not differ between phenotypes (Supplemental Figure 2; effect of Phenotype; F(2,42) = 1.85, p = 0.17; interaction of Phenotype and Activation; F(2,42) = 0.19, p = 0.83), although there were higher overall numbers of granulated than degranulated mast cells across all phenotypes (effect of Activation; F(2,42) = 12.4, p = 0.001).
3.2. Experiment 2: Central administration of cromolyn decreases sign-tracking behavior without affecting goal-tracking behavior
Rats underwent five daily PCA training sessions following a one-week postsurgical recovery period. Immediately before the start of each PCA training session, cromolyn (n = 7) or vehicle (saline; n = 6) was administered (i.c.v.). Over the five daily PCA training sessions (data not shown), cromolyn administration did not affect lever press number (effect of Drug; F(1,10.85) = 1.51, p = 0.25; interaction of Session × Drug; F(4,40.61) = 1.78, p = 0.15), latency (effect of Drug; F(1,11.92) = 1.19, p = 0.30; interaction of Session × Drug; F(4,42.27) = 2.04, p = 0.11), or probability (effect of Drug; F(1,12.50) = 1.16, p = 0.30; interaction of Session × Drug; F(4,42.98) = 1.84, p = 0.14) nor magazine number (effect of Drug; F(1,13.37) = 0.13, p = 0.73; interaction of Session × Drug; F(4,41.70) = 0.32, p = 0.86), latency (effect of Drug; F(1,12.94) = 0.27, p = 0.61; interaction of Session × Drug; F(4,41.64) = 0.98, p = 0.43), or probability (effect of Drug; F(1,13.37) = 0.07, p = 0.80; interaction of Session × Drug; F(4,41.27) = 0.67, p = 0.62). Moreover, cromolyn did not affect magazine entries outside the CS-period, a measure of generalized locomotor activity (data not shown; effect of Drug; F(1,13.71) = 0.17, p = 0.69; interaction of Session × Drug; F(4,41.01) = 1.62, p = 0.20). In addition, cromolyn did not affect PCA index scores over the five PCA training sessions (data not shown; effect of Drug; F(1,13.97) = 1.15, p = 0.30; interaction of Session × Drug; F(4,41.64) = 0.98, p = 0.43). Cromolyn did, however, affect the microarchitecture of sign- and goal-tracking behaviors during late PCA training (i.e., the last two sessions). During early PCA training cromolyn, compared to saline, i.c.v. administration did not affect the number of lever presses (effect of Drug; F(1,269.92) = 0.04, p = 0.85) or magazine entries (effect of Drug; F(1,299.78) = 2.21, p = 0.14) between the 25 trials averaged over Sessions 1–3. Figure 4 shows that during late PCA training cromolyn decreased the number of lever presses between the 25 trials averaged over Sessions 4–5 (effect of Drug; F(1,62.72) = 11.27, p = 0.001) without affecting the number of magazine entries (effect of Drug; F(1,122.12) = 3.3, p = 0.072).
Figure 4.
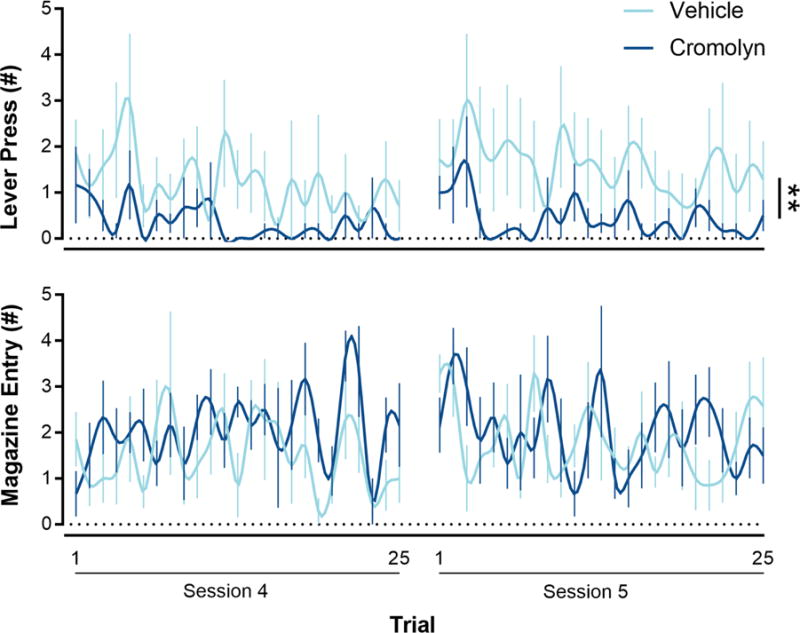
Inhibition of mast cell degranulation decreased the number of lever presses (i.e., sign-tracking behavior), but not the number of magazine entries (i.e., goal-tracking behavior), during late stages of PCA training (Sessions 4–5) when PCA behavior fully expresses and rats are normally characterized. Cromolyn (n = 6), a mast cell inhibitor, or saline vehicle (n = 7) was infused (i.c.v.) immediately before each PCA training session. Data are presented as mean and S.E.M. ** - p < 0.01.
4. Discussion
In Experiment 1, we demonstrated that STs have more degranulated (active) thalamic mast cells than GTs and IRs. The number of granulated (inactive) mast cells, however, did not differ between phenotypes. Moreover, the number of degranulated mast cells in STs were higher compared to GT and IRs throughout much of the thalamus along the anterior-posterior axis. Investigating mast cell number/activity along the anterior-posterior axis is important, because it has previously been demonstrated that mast cell number can differ along this axis in rats (Khalil et al., 2007). Because the thalamus is a heterogenous structure within and throughout this axis that relays cognitive, sensory, and motor signals, it is likely that mast cell activity is increased in STs in a variety of thalamic brain regions along this axis that may promote multiple aspects of sign-tracking behavior. In support of this, sign-tracking behavior in rats is regulated by multiple thalamic nuclei with unique functions including the paraventricular nucleus (emotional/cognitive information; Haight et al., 2015) and the parvicellular region of the ventroposteromedial nucleus (gustatory information; Reilly and Pritchard, 1997).
In addition to mast cells in the thalamus, a small number of mast cells were observed in the meninges and the habenula in the present study. As previously mentioned, our brain extraction method did not allow quantification of meningeal mast cells; however, habenular mast cells were scored, and mast cell number and activity did not differ between phenotypes. Overall, we only observed a significant amount of mast cells in the thalamus, which agrees with previous findings that the thalamus of Sprague Dawley rats contains approximately 98% of total brain mast cells (Goldschmidt et al., 1984).
One challenging aspect of determining the relationship between mast cell activity and PCA behavior is the complexity of mast cell signaling mechanisms and molecules. For example, mast cells typically exocytose all of their granular contents upon activation; however, they can also undergo piecemeal degranulation (i.e., a slow, partial degranulation) or transgranulation (i.e., direct transfer of granular contents into the cytoplasm of neurons; Crivellato et al., 2003; Wilhelm et al., 2005). Through these signaling mechanisms, mast cells can signal using an array of molecules, the most well-studied being monoamines (i.e., histamine, serotonin, and dopamine; Irman-Florjanc and Erjavec, 1983; Lambracht-Hall et al., 1990; Ringvall et al., 2008; Ronnberg et al., 2012a) and cytokines (Metcalfe et al., 1997).
Of these molecules, the current scientific literature suggests that histamine and dopamine may be the most relevant to sign-tracking behavior. Mast cells contribute approximately 90% of thalamic histamine in rodents (Goldschmidt et al., 1985); however, little is known regarding the effect of histamine-mediated neurotransmission in the thalamus on reward-related behaviors. It is known that histamine receptors are densely concentrated in the thalamus (Pillot et al., 2002) and can control presynaptic glutamate release (Garduno-Torres et al., 2007). Moreover, thalamic mast cell degranulation and histamine release coincides with corticosterone secretion (Bugajski et al., 1995), which is reduced by pretreatment with histamine receptor antagonists (Gadek-Michalska et al., 1991). Previously, it has been shown that mast cells can express corticotrophin-releasing factor receptors (Cao et al., 2005) and can even release corticotrophin-releasing factor (Theoharides et al., 2004) and corticosterone (Csaba et al., 1967). STs show higher levels of serum corticosterone than IRs and GTs (Flagel et al., 2009), suggesting that the hypothalamic-pituitary adrenal axis may be involved in the acquisition of sign-tracking behavior, although the precise mechanisms have yet to be identified. It is possible that thalamic mast cells influence sign-tracking behavior either through direct mechanisms (release of histamine and steroid hormones within the thalamic circuits) and/or indirect mechanisms (histaminergic regulation of neural activity in thalamic efferents to the hypothalamic-pituitary adrenal axis). Both the role of histamine and stress hormones in the acquisition of sign-tracking behavior are poorly understood, and future studies are needed in order to determine how these signaling molecules influence PCA behavior.
Mast cells also express tyrosine hydroxylase and store dopamine within their granules (Ronnberg et al., 2012a). Unlike other brain regions, the thalamus integrates a complex network of dopaminergic innervation arising from cell bodies in the hypothalamus, periaqueductal gray, ventral mesencephalon, and lateral parabrachial nucleus (Garcia-Cabezas et al., 2007), and it has been suggested that dopaminergic signaling with the thalamus contributes to PCA behavior (Haight and Flagel, 2014). Therefore, it is possible that mast cells mediate PCA behavior by actively contributing to dopaminergic signaling within the thalamus. In addition, dopamine receptors are present on mast cells and influence the release of cytokines (Laengle et al., 2006; Xue et al., 2015). Although more is known regarding the role of monoamines on reward-related behaviors, particularly sign-tracking behavior, the potential role of cytokines in sign-tracking behavior cannot be discounted. Of particular interest for future investigations are cytokines that are produced selectively by mast cells. For instance, interleukin-4 is produced only by mast cells and a subset of activated T cells (Weiss et al., 1996), and evidence suggests that it has an important role in associative learning (Gadani et al., 2012).
In Experiment 2, we demonstrated that daily administration of cromolyn, a mast cell inhibitor, before PCA training sessions decreases the microarchitecture of sign- but not goal-tracking behavior during late PCA training (Sessions 4–5). On the contrary, cromolyn administration did not affect sign- or goal-tracking behaviors during early PCA training (Sessions 1–3). Because PCA index scores, which represent the combination of both sign- and goal-tracking, separated during PCA Sessions 4–5 during Experiment 1, this distinction was made to differentiate between early and late stages of Pavlovian learning. Previous studies have shown that certain manipulations only affect the acquisition of sign-tracking behavior during later stages of training (Haight et al., 2015; Fitzpatrick et al., 2016). Therefore, it’s possible that mast cells are important for allowing sign-tracking behavior to be fully expressed. Given the fact that mast cells relay and amplify signals (Silver and Curley, 2013), prophylactic inhibition of mast cells may have prevented the initiation of signals early in training that permit maximal expression of sign-tracking behavior. For example, it is possible that thalamic mast cell activity modulates sign-tracking by adjusting excitatory drive or gain of signal transmission to efferent structures (Bickford, 2015). It should be noted that the effects of cromolyn administration were modest, most likely as a result of the fact that studies involving pharmacological manipulation of the acquisition of PCA behavior are not able to differentiate between rats that are GTs, IRs, or STs (i.e., only population averages of sign- and goal-tracking behavior can be measured). Therefore, high variance in sign- and goal-tracking behaviors are observed, because control (and experimental) groups presumably contain a mixture of unscreened GTs, IRs, and STs, which are averaged for their sign- and goal-tracking behaviors.
Mast cells have previously been demonstrated to be highly expressed in mice (B10.PL; Hendrix et al., 2006) within the hippocampus, which regulates spatial learning and anxiety-like behavior (Engin and Treit, 2007; Martin and Clark, 2007). Indeed, mast cell activity in mast cell-deficient mice (C57BL/6 Kit[W-sh/W-sh]) have decreased hippocampal neurogenesis and spatial memory as well as increased anxiety-like behavior (Nautiyal et al., 2008; Nautiyal et al., 2012). In addition, the hippocampus in rats is critical for the acquisition of sign- but not goal-tracking behavior (Fitzpatrick et al., 2016). In the present study, however, mast cells were not observed within the hippocampus, so it is unlikely that our findings are the result of altered hippocampal-dependent modulation of sign-tracking behavior, spatial learning, and/or anxiety-like behavior. It is, however, possible that mast cells exist in other rat lines that are not derived from Sprague-Dawley lineage (i.e., Brown Norway or Long Evans), and mast cell activity in the hippocampus of these strains may influence PCA behavior.
Because mast cell inhibition attenuates the attribution of incentive-motivational value to reward-related cues, cromolyn administration represents a promising strategy for addiction treatment with several beneficial pharmacological properties. First, binding of cromolyn is highly specific to mast cells, and it has been identified that cromolyn inhibits a membrane-bound calcium channel that is necessary for degranulation (Mazurek et al., 1983; Mazurek et al., 1984). In other words, cromolyn prevents mast cell signaling rather than blocking its downstream effects. In addition to its selectivity, it has an excellent safety profile for other indications, such as asthma and rhinitis (Ratner et al., 2002). One shortcoming of cromolyn, though, is that it does not cross the blood-brain barrier and requires central administration to produce its behavioral effects (Nautiyal et al., 2008). However, recent advances in blood-brain barrier assays (Kaisar et al., 2016) and delivery methods (i.e., molecular Trojan horses; Pardridge, 2007) may circumvent this problem in the near future. Taken together, our results show that mast cell activity differs in rats that are vulnerable or resilient to addiction-like behaviors and suggest that inhibiting mast cells may be a viable strategy for addiction treatment.
Supplementary Material
Sign-tracking rats have more thalamic mast cells than goal-tracking rats.
Sign-tracking rats have more degranulated mast cells than goal-tracking rats.
Central blockade of mast cell activity with cromolyn blocks sign-tracking.
Acknowledgments
This work was funded by the Department of Defense (DoD) National Defense Science and Engineering Graduate (NDSEG) Fellowship (CJF) and the National Institute on Drug Abuse (NIDA; K08 DA037912-01 [JDM]; T32 DA007281 [CJF]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res. 2016;296:418–430. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Yousefzadeh E, Silverman AJ, Silver R. Stimuli from conspecifics influence brain mast cell population in male rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME. Thalamic Circuit Diversity: Modulation of the Driver/Modulator Framework. Front Neural Circuits. 2015;9:86. doi: 10.3389/fncir.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugajski AJ, Chlap Z, Gadek-Michalska A, Borycz J, Bugajski J. Degranulation and decrease in histamine levels of thalamic mast cells coincides with corticosterone secretion induced by compound 48/80. Inflamm Res. 1995;44(Suppl 1):S50–51. doi: 10.1007/BF01674391. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotrophin-releasing hormone (CRH) receptors and CRH leaders to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Chikahisa S, Kodama T, Soya A, Sagawa Y, Ishimaru Y, Sei H, Nishino S. Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One. 2013;8:e78434. doi: 10.1371/journal.pone.0078434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Nico B, Mallardi F, Beltrami CA, Ribatti D. Piecemeal degranulation as a general secretory mechanism? Anat Rec A Discov Mol Cell Evol Biol. 2003;274:778–784. doi: 10.1002/ar.a.10095. [DOI] [PubMed] [Google Scholar]
- Csaba G, Olah I, Kiss J, Dunay C. The localization of H3-corticosterone in mast cell granules by electron microscopic autoradiography. Experientia. 1967;23:944. doi: 10.1007/BF02136238. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in mammalian brain. Acta Anat (Basel) 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the human brain. Acta Anat (Basel) 1979;105:505–513. doi: 10.1159/000145157. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of the hippocampus in anxiety: Intracerebral infusion studies. Behav Pharmacol. 2007;18:365–74. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Creeden JF, Perrine SA, Morrow JD. Lesions of the ventral hippocampus attenuate the acquisition but not expression of sign-tracking behavior in rats. Hippocampus. 2016;26:1424–34. doi: 10.1002/hipo.22619. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation of mast cells in the rat thalamus. Ital J Anat Embryol. 2001;106:467–473. [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek-Michalska A, Chlap Z, Turon M, Bugajski J, Fogel WA. The intracerebroventricularly administered mast cells degranulator compound 48/80 increases the pituitary-adrenocortical activity in rats. Agents Actions. 1991;32:203–208. doi: 10.1007/BF01980874. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Garduno-Torres B, Trevino M, Gutierrez R, Arias-Montano JA. Pre-synaptic histamine H3 receptors regulate glutamate, but not GABA release in rat thalamus. Neuropharmacology. 2007;52:527–535. doi: 10.1016/j.neuropharm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J Neurochem. 1985;44:1943–1947. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Fraser KM, Akil H, Flagel SB. Lesions of the paraventricular thalamus of the thalamus differentially affect sign- and goal-tracking conditioned responses. Eur J Neurosci. 2015;42:2478–88. doi: 10.1111/ejn.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix S, Warnke K, Siebenhaar F, Peters EM, Nitsch R, Maurer M. The majority of brain mast cells in B10.PL mice is present in the hippocampal formation. Neurosci Lett. 2006;392:174–7. doi: 10.1016/j.neulet.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Irman-Florjanc T, Erjavec F. Compound 48/80 and substance P induced release of histamine and serotonin from rat peritoneal mast cells. Agents Actions. 1983;13:138–141. doi: 10.1007/BF01967317. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, Cucullo L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin Drug Discov. 2016:1–15. doi: 10.1080/17460441.2017.1253676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszegi Z, Kovacs P, Wilhelm M, Atlasz T, Babai N, Kallai V, Hernadi I. The application of in vivo microiontophoresis for the investigation of mast cell-neuron interactions in the rat brain. J Biochem Biophys Methods. 2006;69:227–231. doi: 10.1016/j.jbbm.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Larson AA. Mast cells accumulate in the anogenital region of somatosensory thalamic nuclei during estrus in female mice. Brain Res. 2006;1114:85–97. doi: 10.1016/j.brainres.2006.07.100. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Hernadi I, Wilhelm M. Mast cells modulate maintained neuronal activity in the thalamus in vivo. J Neuroimmunol. 2006;171:1–7. doi: 10.1016/j.jneuroim.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Laengle UW, Markstein R, Pralet D, Seewald W, Roman D. Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-alpha release in vitro from activated rat mast cells. Exp Eye Res. 2006;83:1335–1339. doi: 10.1016/j.exer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Lambracht-Hall M, Konstantinidou AD, Theoharides TC. Serotonin release from rat brain mast cells in vitro. Neuroscience. 1990;39:199–207. doi: 10.1016/0306-4522(90)90233-t. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Clark RE. The rodent hippocampus and spatial memory: From synapses to systems. Cell Mol Life Sci. 2007;64:401–31. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N, Bashkin P, Loyter A, Pecht I. Restoration of Ca2+ influx and degranulation capacity of variant RBL-2H3 cells upon implantation of isolated cromolyn binding protein. Proc Natl Acad Sci U S A. 1983;80:6014–6018. doi: 10.1073/pnas.80.19.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N, Schindler H, Schurholz T, Pecht I. The cromolyn binding protein constitutes the Ca2+ channel of basophils opening upon immunological stimulus. Proc Natl Acad Sci U S A. 1984;81:6841–6845. doi: 10.1073/pnas.81.21.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Dailey CA, Jahn JL, Rodriquez E, Son NH, Sweedler JV, Silver R. Serotonin of mast cell origin contributes to hippocampal function. Eur J Neurosci. 2012;36:2347–59. doi: 10.1111/j.1460-9568.2012.08138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Caputi AP. Central effects of cromoglycate sodium salt in rats treated with lipopolysaccharide. Eur J Pharmacol. 1999;367:351–359. doi: 10.1016/s0014-2999(98)00986-8. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. sixth. Elsevier/Academic Press; New York: 2007. [Google Scholar]
- Persinger MA. Degranulation of brain mast cells in young albino rats. Behav Neural Biol. 1983;39:299–306. doi: 10.1016/s0163-1047(83)90995-0. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, Arrang JM. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Ratner PH, Ehrlich PM, Fineman SM, Meltzer EO, Skoner DP. Use of intranasal cromolyn sodium for allergic rhinitis. Mayo Clin Proc. 2002;77:350–354. doi: 10.4065/77.4.350. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: III. Simultaneous contrast and autoshaping. Physiol Behav. 1997;62:1355–63. doi: 10.1016/s0031-9384(97)00352-1. [DOI] [PubMed] [Google Scholar]
- Ringvall M, Ronnberg E, Wernersson S, Duelli A, Henningsson F, Abrink M, Garcia-Faroldi G, Fajardo I, Pejler G. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008;121:1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnberg E, Calounova G, Pejler G. Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner. Biol Chem. 2012a;393:107–112. doi: 10.1515/BC-2011-220. [DOI] [PubMed] [Google Scholar]
- Ronnberg E, Melo FR, Pejler G. Mast cell proteoglycans. J Histochem Cytochem. 2012b;60:950–962. doi: 10.1369/0022155412458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36:513–521. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotrophin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Riveros ME, Contreras M, Valdes JL. Histamine and motivation. Front Syst Neurosci. 2012;6:51. doi: 10.3389/fnsys.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DL, Hural J, Tara D, Timmerman LA, Henkel G, Brown MA. Nuclear factor of activated T cells is associated with a mast cell interleukin 4 transcription complex. Mol Cell Biol. 1996;16:228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Silver R, Silverman AJ. Central nervous system neurons acquire mast cell products via transgranulation. Eur J Neurosci. 2005;22:2238–2248. doi: 10.1111/j.1460-9568.2005.04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Li X, Ren HX, Wu F, Li M, Wang B, Chen FY, Cheng WY, Li JP, Chen YJ, Chen T. The dopamine D3 receptor regulates the effects of methamphetamine on LPS-induced cytokine production in murine mast cells. Immunobiology. 2015;220:744–752. doi: 10.1016/j.imbio.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology. 2015;40:1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Zhu X, Zhou S, Chen Q, Zhu X, Ma X, He X, Tian M, Shi X. Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res. 2010;88:1348–1354. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- Zeng X, Zhang S, Xu L, Yang H, He S. Activation of protease-activated receptor 2-mediated signaling by mast cell tryptase modulates cytokine production in primary cultured astrocytes. Mediators Inflamm. 2013;2013:140812. doi: 10.1155/2013/140812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zeng X, Yang H, Hu G, He S. Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem. 2012;29:931–940. doi: 10.1159/000171029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


