Abstract
Research suggests the health consequences of economic hardship can be transmitted across generations. Some of these disparities are thought to be passed to offspring during gestation. But this hypothesis has not been tested in contemporary American samples, and the mechanisms of transmission have not been characterized. Accordingly, this study had two goals: first, to determine if women exposed to economic hardship during childhood showed higher rates of adverse birth outcomes; and second, to evaluate the contribution of inflammation, psychosocial, lifestyle, and obstetric characteristics to this phenomenon. This prospective study enrolled 744 women with singleton pregnancies (59.1% White; 16.3% Black; 18.7% Latina; 5.9% Other). Childhood economic hardship was measured by self-report. Birth outcomes included length of gestation and incidence of preterm birth; birth weight percentile and small for gestational age; length of hospital stay and admission to Special Care Nursery. Analyses revealed that mothers’ childhood economic hardship was independently associated with multiple adverse birth outcomes, even following adjustment for demographics, maternal education, and obstetrical confounders. Women raised in economically disadvantaged conditions had shorter gestation length and higher preterm delivery rates. Their babies had lower birth weights, were more likely to be small for gestational age, stayed in the hospital longer, and had more Special Care Nursery admissions. Mediation analyses suggested these associations arose through multiple pathways, and highlighted roles for inflammation, education, adiposity, and obstetric complications. Collectively, these findings suggest that childhood economic hardship predisposes women to adverse birth outcomes, and highlights likely behavioral and biological mechanisms.
Keywords: Socioeconomic status, pregnancy, inter-generational, health disparities, inflammation
INTRODUCTION
Children from economically disadvantaged families are vulnerable to physical health problems across the lifespan (Miller et al. 2011; Hertzman & Boyce 2010; Chen et al. 2002). Relative to more privileged youth, they show higher rates of and worse outcomes for pediatric disorders like asthma, respiratory infection, and obesity (Braveman & Barclay 2009; Chen et al. 2002). Later in life they continue to have relatively poor outcomes, as reflected in higher rates of chronic illnesses that represent major societal burdens (e.g., diabetes, heart disease, stroke), and more premature mortality (Galobardes et al. 2008; Lynch & Smith 2005). These disparities generally persist after accounting for socioeconomic status in adulthood (Cohen et al. 2010), suggesting that childhood hardship casts a long shadow with implications for health across the lifespan (Shonkoff et al. 2009).
Accumulating evidence suggests the adverse influence of childhood disadvantage may not be restricted to the individual’s own life span, but also transmitted across generations (Kuzawa 2012). Studies in this emerging literature have found that the socioeconomic conditions in which a parent was raised forecast the health of their offspring. For example, to the degree parents were exposed to childhood economic hardship, their adolescent offspring showed more cardiovascular risk, as reflected in higher blood pressure and low-grade inflammation (Schreier & Chen 2010). In youth with asthma, parental economic hardship during childhood predicted worse disease control and larger cytokine responses to allergens (Chen et al. 2016b). In both of these studies, the associations were independent of the family’s current socioeconomic conditions. Findings like this converge with studies reporting trans-generational health effects of inadequate nutrition and cigarette smoking (Bateson et al. 2004; Pembrey et al. 2014). Whether these associations are causal remains unclear. However, experimental studies in animals demonstrate the biological plausibility of a causal effect, showing that toxins and stressors can leave “molecular footprints” detectable in the grand-offspring of exposed organisms (Bale 2015; Hanson & Gluckman 2014; Kundakovic & Champagne 2015).
Pregnancy may represent a particularly sensitive window for mother-to-child transmission of socioeconomic disparities in health. Disadvantage often coincides with exposure to psychological stressors and environmental toxins, and higher rates of cigarette smoking, obesity, and inadequate nutrition (Evans 2004). These exposures can affect characteristics of the gestational milieu (Wadhwa et al. 2011; Burris et al. 2016; Hanson & Gluckman 2014). Consistent with this premise, two recent studies observed that mothers’ socioeconomic conditions during childhood presaged their subsequent pregnancy outcomes. Data from the 1958 British birth cohort showed that childhood hardship was associated with increased risk of preterm delivery and low birth weight (Harville et al. 2010). These associations were partially explained by hardship’s link with lower socioeconomic position in adulthood and cigarette smoking during pregnancy. Similarly, multigenerational data from Aberdeen showed that mothers raised in families of lower social class delivered babies of lower weight, even after correcting for gestation length (Morton et al. 2014).
These provocative findings raise several questions. First, since both studies focused on women raised in mid-century Britain, it is unclear whether similar trends are occurring in contemporary America. In the decades since women in these studies gave birth (1970–1999), smoking during pregnancy has declined by almost 50% (American Lung Association 2011) and access to prenatal care has expanded considerably. Thus, our first goal was to examine whether in modern-day America, women’s childhood socioeconomic conditions forecast their subsequent pregnancy outcomes.
A second question concerns the underlying biological mechanisms. How could a woman’s early-life conditions linger in the body, and influence a pregnancy initiated decades later? One plausible mechanism is excessive inflammation. Research shows that childhood socioeconomic hardship engenders a pro-inflammatory phenotype that persists into adulthood (Miller et al. 2011; Fagundes et al. 2013). The phenotype is characterized by exaggerated cytokine responses to bacterial stimuli, insensitivity to the anti-inflammatory actions of glucocorticoids, and higher circulating levels of inflammatory biomarkers (Miller et al. 2009; Loucks et al. 2010; Levine et al. 2015; Chen et al. 2016a; Phillips et al. 2009). Studies in animals show that high concentrations of inflammatory mediators can restrict fetal growth and speed up parturition (Challis et al. 2009). And in some cohorts of pregnant women, higher circulating inflammatory biomarkers forecast preterm delivery and lower birth weight (Sykes et al. 2012; Gillespie et al. 2015), possibly because they mirror immunologic conditions at the maternal-fetal interface. Based on these observations, our second goal was to determine whether inflammatory biomarkers during gestation link childhood socioeconomic conditions with adverse pregnancy outcomes.
Finally, if the evidence supports a mechanistic role for inflammation, it becomes important to elucidate how childhood conditions trigger this process, and whether steps along the pathway are modifiable. There are several plausible scenarios to consider in this regard. Across the lifespan, individuals exposed to childhood hardship tend to have worse psychosocial (e.g., less education, more distress and isolation) and lifestyle profiles (e.g., more smoking, excessive alcohol use, and adiposity) (Repetti et al. 2002). Both of these clusters of variables are associated with upregulated inflammatory activity and worse pregnancy outcomes (Irwin & Cole 2011; Dunkel Schetter 2011). Similarly, childhood disadvantage might increase women’s risks for obstetric conditions, like hypertension and preeclampsia, whose pathophysiology involves excessive inflammation. Hence, our third goal was to identify what role psychosocial, lifestyle, and obstetric factors have along the pathway from childhood disadvantage to pregnancy outcomes.
METHODS
Between June 2013 and May 2015, 744 women enrolled in the Measurement of Maternal Stress (MOMS) Study. Enrollment occurred at four sites: Northwestern University, University of Texas Health Science Center at San Antonio, University of Pittsburgh, and Schuylkill County, Pennsylvania, a rural site led by Children’s Hospital of Philadelphia.
Recruitment and Assessments
To be eligible, women had to be 18 or older and English speaking, with a singleton pregnancy at less than 21 weeks’ gestation. Exclusion criteria included major fetal congenital anomalies or chromosomal abnormalities, progesterone treatment after 14 weeks, and chronic corticosteroid treatment (not including topical use or inhalers). Institutional Review Boards at each site approved the protocol, and all women gave written informed consent. Women completed study visits in the second (12–20’6 weeks’ gestation) and third (32–35’6 weeks’ gestation) trimesters.
Mothers’ Childhood Disadvantage
Drawing on previous studies of childhood disadvantage (Cohen et al. 2013), we asked women a series of questions about their family’s conditions during childhood. The questions focused on whether the family owned a home, obtained medical treatment when necessary, received public assistance, purchased new clothes on special occasions, and owned a car, television, and washer and dryer. One point was assigned for each response indicative of hardship (e.g., receiving public assistance, lack of needed medical care) and a total score was formed by summing the number of disadvantages. 49% of the sample scored 0, and the rest had values of 1 (21%), 2 (17%), 3 (9%), or 4–7 (4%). To simplify presentation, we combined scores of 3 or more into a single category, and report findings based on that categorization. However, analyses with the full spectrum of scores yielded identical patterns.
Pregnancy Outcomes
Two coders independently abstracted outcomes from maternal and neonatal charts; discrepancies were resolved by consensus. We considered pregnancy outcomes related to the following processes: i) length of gestation (characterized as a continuous variable in completed weeks gestation at birth, and a dichotomous variable - preterm birth (PTB) defined as < 37 weeks gestation), ii) fetal growth (characterized as a continuous variable - birth weight - adjusted for sex and gestational age, and as categorical variable reflecting small for gestational age (SGA), defined as birth weight < 10th percentile, specific for sex and gestational age). Additionally, to glean insights about birth complications and associated medical expenses, we considered length of the baby’s hospitalization and admission to a special care nursery (SCN).
Inflammatory Biomarkers
Antecubital blood was drawn at both study visits, and plasma was used to measure a panel of inflammatory biomarkers implicated in adverse pregnancy outcomes (Sykes et al. 2012; Gillespie et al. 2015). The panel consisted of interferon-γ (IFN-γ), interleukins (IL-) 6, 8, 10, 13, and tumor necrosis factor-α (TNF-α). Cytokines were measured in duplicate with a custom multiplex immunoassay (MesoScale Disovery V-Plex) on a SECTOR Imager 2400A (MesoScale Discovery) (Fu et al. 2010). Across runs, the mean intra-assay coefficients of variation ranged from 3.1% (IL-8) to 7.1 (IL-13) percent. Logged cytokine values were averaged across blood draws. (See Ross et al. 2016 and online supplement for additional details.)
Explanatory Pathways
We examined three pathways through which childhood disadvantage might relate to inflammatory biomarkers and pregnancy outcomes. The Psychosocial pathway included educational attainment, depressive symptoms, perceived stress, and social support. Given the distribution of values, education was converted into a three-level variable, with categories of high-school education or less, some college or Associate’s degree, and Bachelor’s degree or more. Depressive symptoms, perceived stress, and social support were measured at both assessments by self-report. The instruments were the Center for Epidemiologic Studies Depression Scale (Radloff 1977), Perceived Stress Scale (Cohen et al. 1983), and Social Support Survey (Sherbourne & Stewart 1991). Cronbach’s β values for all scales and visits exceeded .75. Given the stability of women’s values on these instruments across time (Spearman r’s = .56, .68, .63, respectively), we averaged responses across assessments.
The Lifestyle pathway included pre-pregnancy body mass index (BMI; from medical charts), and self-reports of cigarette use before and during pregnancy, and alcohol consumed during pregnancy. Because responses to lifestyle questions were highly skewed, we converted them into ordinal variables. Cigarette use before and during pregnancy was coded as none, < 10, 11–19, or > 20 per day. Women reported minimal alcohol use during pregnancy, so this variable was coded as none, less than once a month, and more than once a month.
Models depicting the Obstetric pathway included history of preterm birth, and current pregnancy diagnoses of pre-eclampsia and gestational hypertension, all coded from medical charts. Because pathway analyses cannot accommodate binary mediator variables (Hayes 2013), we developed a composite indicator of obstetric risk, which assigned women one point each for pre-eclampsia, gestational hypertension, and history of PTB.
Missing Data
Of the 744 women, 14 were missing data on childhood disadvantage (1.9%), 58 on birth outcomes (7.8%), 23 on cytokines (3.1%), and 3–40 on variables reflecting explanatory pathways (< 5.4%). Thus, analytic N’s ranged from 665–673.
Statistical Analyses
Initial analyses used a hierarchical regression strategy, where covariates were entered on the first step, followed by childhood disadvantage. Continuous outcomes were modeled in linear regressions and categorical outcomes in logistic regressions. The second wave of analyses used the PROCESS macro in SPSS to examine the mediating role of inflammatory biomarkers (Hayes 2013). PROCESS uses regression-based path-analysis to simultaneously estimate direct (mothers’ childhood disadvantage to adverse pregnancy outcomes) and indirect pathways (mothers’ childhood disadvantage to inflammatory biomarkers to adverse pregnancy outcomes). The final models also used PROCESS to examine psychosocial, lifestyle, and obstetric mediators.
RESULTS
Mothers’ Childhood Disadvantage and Adverse Pregnancy Outcomes
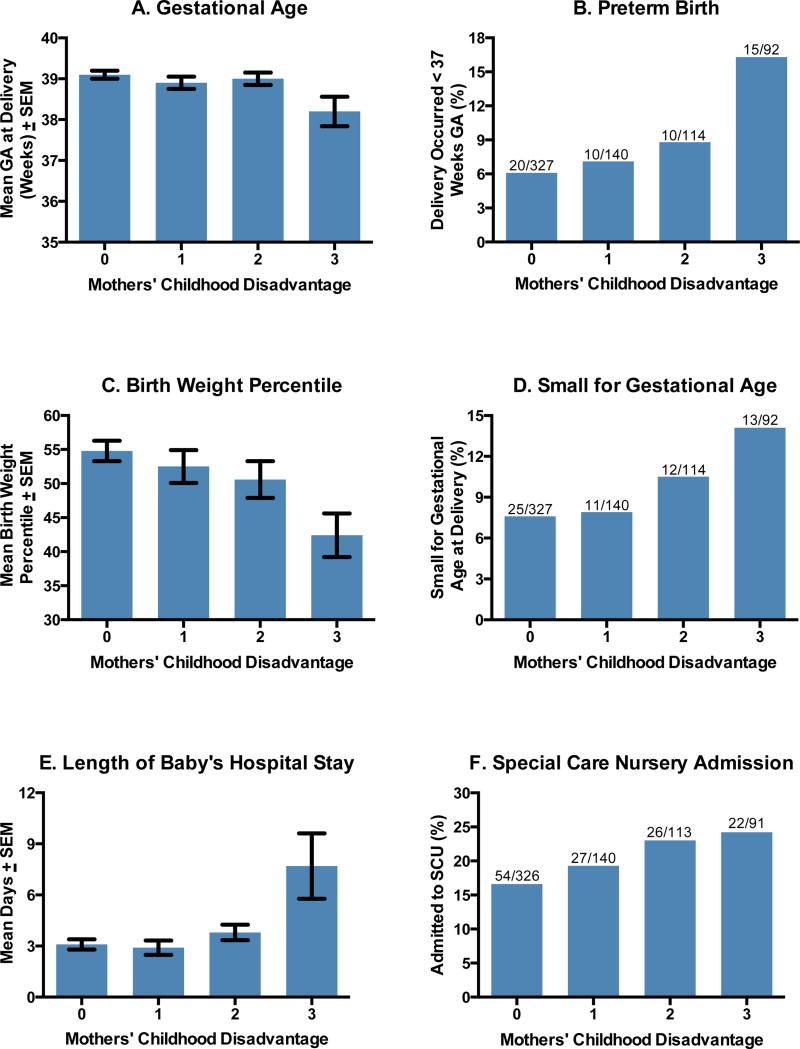
Table 1 displays the sample characteristics. Preliminary regression analyses revealed a pattern of significant bivariate associations between mothers’ childhood disadvantage and adverse pregnancy outcomes (unadjusted p’s from .001 – .05). As Figure 1 shows, for some outcomes these associations were graded (birth weight, SGA, SCN admission), whereas for others the bulk of adverse outcomes occurred in mothers with the greatest childhood hardship (gestation length, PTB, length of hospital stay).
Table 1.
Characteristics of Study Population (N = 673).
| Mean (Standard Deviation) or Number (Percentage) |
|
|---|---|
| Age, years | 29.3 (5.5) |
| Race/ethnicity | |
| Non-Hispanic White | 398 (59.1%) |
| Non-Hispanic Black | 110 (16.3%) |
| Hispanic | 126 (18.7%) |
| Maternal education | |
| High school diploma or less | 175 (26.0%) |
| Bachelor’s degree or more | 266 (39.5%) |
| Parity | |
| Nulliparous | 293 (43.5%) |
| Obstetric Risk Conditions | |
| Previous preterm birth | 47 (7.0%) |
| Pre-eclampsia | 36 (5.3%) |
| Gestational hypertension | 75 (11.1%) |
| Pregnancy and Birth Outcomes | |
| Gestational age at delivery, weeks | 38.9(2.1) |
| Preterm birth (< 37 weeks GA) | 55 (8.2%) |
| Birth weight, grams | 3317(585) |
| Small for gestational age, < 10th percentile | 61 (9.1%) |
| Special care nursery admission | 129 (19.3%) |
| Length of baby hospital stay, days | 3.8 (8.4) |
Figure 1.
Mothers' childhood disadvantage increases risk for adverse pregnancy outcomes.
Contribution of Demographic and Obstetrical Confounders
In the next wave of analyses, we examined whether these associations were independent of demographic and obstetrical factors commonly associated with adverse pregnancy outcomes. As Table 2 indicates, mothers’ childhood disadvantage continued to be significantly associated with adverse pregnancy outcomes when covariates reflecting age, racial, and ethnic background were included in models. These associations also remained significant when obstetrical covariates were included in the models; specifically, nulliparity, gestational hypertension, pre-eclampsia, and PTB history (see Table 3). In the covariate-adjusted models, odds ratios for PTB, SGA, and SCN admission ranged from 1.20–1.40; these values indicate that for each additional type of disadvantage, a woman’s odds of adverse pregnancy outcomes increased by 20–40%. (Complete results of these models appear in Tables S1–S6.)
Table 2.
Summary of regression analyses relating mothers’ childhood disadvantage to birth outcomes, with inclusion of demographic covariates
| Outcome | b for trend | SE | ββ for trend |
Exp (B) | p |
|---|---|---|---|---|---|
| Length of gestation a | −0.16 | .08 | −.08 | -- | .042 |
| Preterm birth b | 0.26 | .13 | -- | 1.29 | .050 |
| Birth weight percentile a | −0.02 | .01 | −.08 | -- | .047 |
| Small for gestational age b | 0.26 | .12 | -- | 1.30 | .045 |
| Length of hospital stay a | 1.11 | .32 | .15 | -- | .001 |
| Special care nursery b | 0.20 | .10 | -- | 1.22 | .036 |
Note. Values reflect association between mothers’ childhood disadvantage and specified pregnancy outcome, adjusted for maternal age, and dummy variables reflecting Black race and Hispanic ethnicity.
Continuous outcome modeled in linear regression, where b and β denote the unstandardized and standardized coefficients, respectively.
Binary outcome modeled in logistic regression, where b and Exp (B) denote the unstandardized coefficient and odds ratio, respectively. P values are two-tailed. N’s = 666–671.
Table 3.
Summary of regression analyses relating mothers’ childhood disadvantage to birth outcomes, with inclusion of obstetric covariates.
| Outcome | b for trend | SE | ββ for trend |
Exp (B) | p |
|---|---|---|---|---|---|
| Length of gestation a | −0.16 | .07 | −.08 | -- | .029 |
| Preterm birth b | 0.34 | .13 | -- | 1.40 | .011 |
| Birth weight percentile a | −0.03 | .01 | −.13 | -- | .001 |
| Small for gestational age b | 0.24 | .12 | -- | 1.28 | .045 |
| Length of hospital stay a | 1.07 | .30 | .14 | -- | <.001 |
| Special care nursery b | 0.18 | .09 | -- | 1.20 | .047 |
Note. Values reflect association between mothers’ childhood disadvantage and specified pregnancy outcome, adjusted for history of preterm birth, nulliparity, gestational hypertension, and pre-eclampsia.
Continuous outcome modeled in linear regression, where b and β denote the unstandardized and standardized coefficients, respectively.
Binary outcome modeled in logistic regression, where b and Exp (B) denote the unstandardized coefficient and odds ratio, respectively. P values are two-tailed. N’s = 666–671.
Mediating Role of Inflammatory Biomarkers?
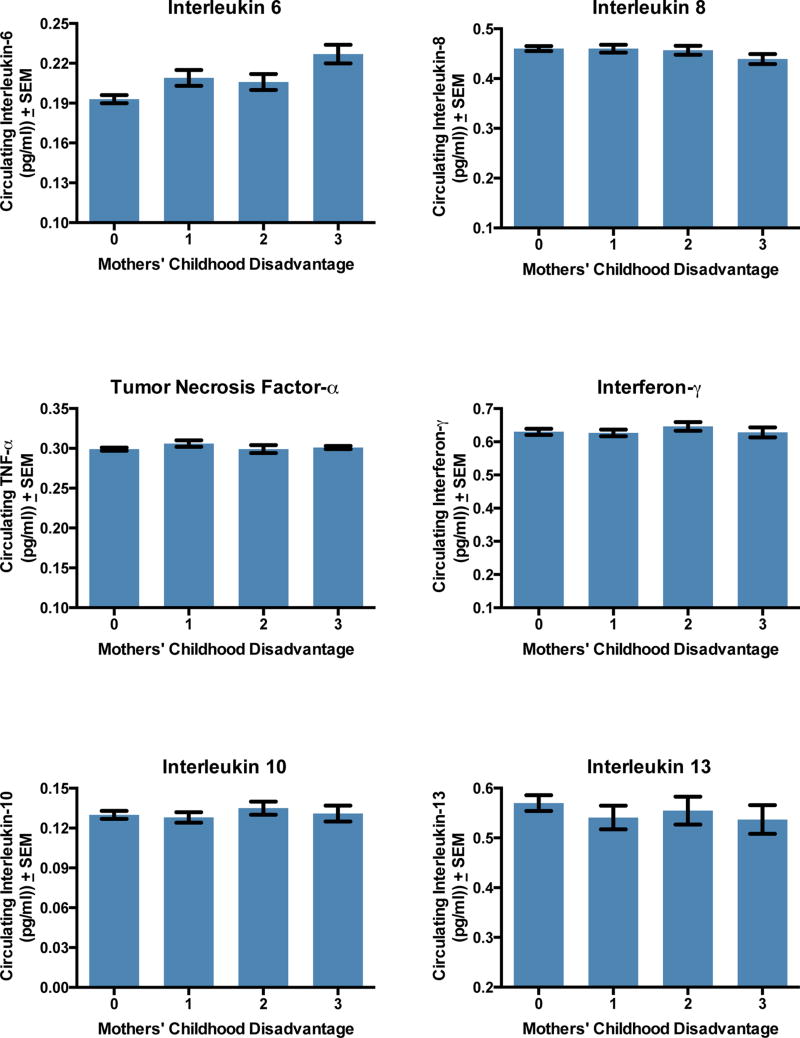
Next, we examined whether patterns were consistent with inflammatory biomarkers operating as mediating pathways between childhood disadvantage and pregnancy outcomes. Bivariate regressions indicated that mothers’ childhood disadvantage was associated with higher circulating levels of IL-6 (b = .01, SE = .002, p < .001; Figure 2), and this relationship persisted after the demographic and obstetrical confounders listed above were included (adjusted b = .007, SE = .003, p = .01). None of the other cytokines was associated with mothers’ childhood disadvantage (Table S7, p’s > .09). Accordingly, we restricted further cytokine-related analyses to IL-6.
Figure 2.
Mothers’ childhood disadvantage and levels of circulating inflammatory biomarkers.
As noted, PROCESS is a tool for testing hypotheses about mediation, and it estimates the strength of both direct (mothers’ childhood disadvantage to adverse pregnancy outcomes) and indirect pathways (mothers’ childhood disadvantage to inflammatory biomarkers to adverse pregnancy outcomes). As Table 4 shows, PROCESS results indicated significant indirect pathways connecting mothers’ childhood disadvantage with shorter gestation length and higher PTB rates through higher IL-6. To gauge the strength of these indirect pathways, we computed the ratio of the indirect effect to total effect for these outcomes. Results indicated that IL-6 explained 17 and 11 percent of childhood disadvantage’s total association with gestation length and PTB rates, respectively. These values, and the significant direct pathways, indicate other mechanisms besides IL-6 were involved in these associations. The non-significant indirect pathways for birth weight, SGA, SCN admission, and stay length indicate that IL-6 did not connect mothers’ childhood disadvantage with these outcomes.
Table 4.
Results of mediational analyses relating mothers’ childhood disadvantage to birth outcomes through circulating maternal IL-6 concentrations.
| Outcome | Indirect effect |
Bootstrap SE |
Bootstrap 95% CI |
Direct effect |
SE | 95% CI | Ratio of Indirect to Total Effect |
|---|---|---|---|---|---|---|---|
| Length of gestation * | −.034 | .017 | −.081, −.009 | −.164 | .075 | −.316, −.017 | .172 |
| Preterm birth * | .039 | .023 | .004, .097 | .302 | .127 | .053, .551 | .114 |
| Birth weight percentile | −.001 | .004 | −.008, .009 | −.358 | .102 | −.559, −.157 | n/a |
| Small for gestational age | −.026 | .022 | −.075, .010 | .265 | .122 | .027, .503 | n/a |
| Length of hospital stay | .027 | .030 | −.027, .097 | 1.067 | .301 | .476, 1.66 | n/a |
| Special care nursery | .018 | .016 | −.007, .059 | .143 | .090 | −.034, .319 | n/a |
Note. Mediational analyses were conducted with the PROCESS macro, which uses a path-analytic framework to estimate direct (mothers’ childhood disadvantage to adverse pregnancy outcomes) and indirect pathways (mothers’ childhood disadvantage to interleukin-6 to adverse pregnancy outcomes). PROCESS uses 1000 bootstrapped samples to estimate bias-corrected confidence intervals for the indirect effect.
indicates a statistically significant indirect pathway through IL-6. N’s = 643–649.
Psychosocial, Lifestyle, and Obstetric Explanatory Pathways
The final models explored the contribution of psychosocial, lifestyle, and obstetrical pathways to the patterns reported above. Specifically, we asked whether variables along these pathways could explain how mothers’ childhood disadvantage came to be associated with higher IL-6 concentrations and adverse pregnancy outcomes. As Tables S8–S9 indicate, simple bivariate analyses showed that women endorsing childhood disadvantage tended to have risker profiles on each of these pathways. They were more likely to be young, racial/ethnic minorities, multiparous, and with a PTB history (p’s < .001). They also had less education, lower social support, more adiposity and smoking before pregnancy, and higher depressive symptoms and perceived stress (p’s < .003).
Psychosocial Pathway
Using PROCESS, we asked whether variables along the psychosocial pathway (educational attainment, depressive symptoms, perceived stress, or social support) could explain how mothers’ childhood disadvantage related to higher IL-6 concentrations and adverse pregnancy outcomes. Results identified lower educational attainment as a significant indirect pathway connecting mothers’ childhood disadvantage with higher IL-6 (coefficient = .004, SE = .001, 37.2% total effect) and SCN admission (coefficient = .126, SE = .049, 69.4% total effect). The direct effect of childhood disadvantage remained significant for IL-6 (coefficient = .006, SE = .002), indicating that mechanisms besides education were involved. The direct effect for SCN admission was non-significant (coefficient = .055, SE = .099). Results did not support a role for educational attainment in other pregnancy outcomes, or mediating roles for depressive symptoms, social support, or perceived stress (all 95% CI’s for indirect pathways included 0).
Lifestyle Pathway
Again, using PROCESS we considered the role of lifestyle variables, including pre-pregnancy BMI, and drug use before and during pregnancy. Analyses identified higher pre-pregnancy BMI as a significant indirect pathway connecting mothers’ childhood disadvantage with multiple outcomes, including higher IL-6 (coefficient = .006, SE = .001, 56.8% total effect), shorter gestation (coefficient = −.038, SE = .022, 167% total effect), and lower birth weight (coefficient = .008, SE = .003, 14.8% total effect). In all cases, the direct effects of childhood disadvantage remained at least marginally significant, suggesting that it operated via mechanisms besides adiposity (p’s from .001 to .053). Analyses did not yield evidence in support of mediator roles for either smoking or alcohol (95% CI’s for indirect pathways included 0).
Obstetrical Pathway
Finally, we considered the role of obstetrical pathways, including pre-eclampsia, gestational hypertension, and PTB history. As noted earlier, mothers’ childhood disadvantage was associated with adverse pregnancy outcomes even once these variables were statistically controlled (see Table 3). Nevertheless, we wondered if obstetrical factors could help explain how these associations came about, so we conducted PROCESS analyses with a composite obstetric risk indicator (see Explanatory Pathways section for details). Results revealed significant indirect pathways linking mothers’ childhood disadvantage with all outcomes considered. They included higher IL-6 (coefficient = .0006, SE = .0004, 6.3% total effect), shorter gestation (coefficient = −.054, SE = .024, 24.9% total effect), PTB (coefficient = .052, SE = .024, 15.6% total effect), lower birth weight (coefficient = −.004, SE = .002, 11.5% total effect), SGA (coefficient =.025, SE = .013, 11.3% total effet), longer hospital stays (coefficient = .096, SE = .059, 15.2% total effect), and SCN admission (coefficient =.026, SE = .013, 8.2% total effect). Importantly, significant direct effects of childhood disadvantage remained for all outcomes (p’s from .0001 to .027), suggesting other non-obstetrical mediators were involved in this phenomenon, too. The only exception was for SCN admissions, where the direct effect was non-significant (p = .107).
DISCUSSION
Mirroring evidence of cross-generational transmission of health disparities (Pembrey et al. 2014; Schreier & Chen 2010; Chen et al. 2016b), we observed a consistent relationship between mothers’ childhood hardship and adverse pregnancy outcomes. Women who experienced disadvantaged childhoods were more likely to deliver preterm, SGA babies. Those babies had lengthier hospital stays and more SCN admissions. These associations were independent of demographic variables, maternal education, and obstetrical factors typically used to identify vulnerable pregnancies. Except for in the case of SCN admissions, these associations were also independent of mothers’ educational attainment, suggesting that childhood hardships leave an imprint that is not simply explained by continued exposure to disadvantage.
These observations converge with earlier reports from British cohorts (Harville et al. 2010; Morton et al. 2014), and extend those findings in several respects. First, our data illustrate an association between childhood disadvantage and pregnancy outcomes in a sample of contemporary American women, who had low rates of smoking (<4%), a key pathway underlying the patterns in earlier research (Harville et al. 2010). Second, we observed that maternal disadvantage presaged a broader array of adverse outcomes than previously reported. Besides PTB and SGA, they included continuous indicators of gestation length and birth weight, as well as care-use outcomes with economic implications. Compared to women with no hardship, those with 3 or more forms of disadvantage were almost 50% more likely to have babies admitted to the SCN (24.2% vs. 16.6%). Those babies spent an average of 4.6 additional days in the hospital (7.7 vs. 3.1). The costs of this additional care are difficult to estimate, because charges vary across institutions (Hsia et al. 2014). Nonetheless, the March of Dimes estimates that four days of SCN care cost approximately $30,000 (March of Dimes 2011). In cases where a child was born prematurely (16.3% vs. 6.1%), expenses related to follow-up care would increase the size of this gap further.
Finally, our analyses extend previous research by identifying behavioral and biological mechanisms along the pathway from childhood hardship to pregnancy outcomes. Though mediation analyses cannot yield causal inferences in an observational study like this, the results are consistent with a scenario wherein childhood disadvantage predisposes women to express higher IL-6 during pregnancy, which accelerates the gestation and/or instigates labor prematurely. This scenario converges with research showing that childhood hardship instantiates a durable pro-inflammatory phenotype (Miller et al. 2009; Loucks et al. 2010; Levine et al. 2015; Chen et al. 2016a; Phillips et al. 2009), and with evidence from animal (Romero et al. 2014) and human (Gillespie et al. 2015) studies linking excessive cytokine activity with PTB. Interestingly, IL-6 is a risk factor for diabetes, metabolic syndrome, coronary heart disease, and premature mortality (Ridker 2016; Wang et al. 2013; Alley et al. 2007). Childhood disadvantage increases risks for all these conditions (Miller et al. 2011; Galobardes et al. 2008), suggesting that IL-6, or upstream inflammatory processes it reflects, may explain multiple long-term health problems linked to early disadvantage.
With that said, it is important to bear in mind that IL-6 was only a partial mediator of the observed associations, and that additional mechanisms were involved. Indeed, analyses highlighted a consistent role for obstetrical conditions. These patterns suggest that pre-eclampsia and hypertension are part of a multistep pathway linking childhood disadvantage with higher IL-6 and adverse pregnancy outcomes. But the significant direct effects for all outcomes (except SCN admits) suggest that other mechanistic routes are involved too, a conclusion echoed in the other mediation analyses. Key in this regard is pre-pregnancy adiposity, which emerged as a mediator linking childhood disadvantage with subsequent IL-6, and pregnancy outcomes including shorter gestation, PTB, and lower birth weight. These patterns probably reflect the tendency of expanded adipose tissue to express large quantities of inflammatory cytokines (Hotamisligil 2006), which may restrict fetal growth and speed up parturition (Challis et al. 2009). More generally, these findings parallel the literature on early-life stress, which highlights adiposity as a route to later cardiometabolic disease (Matthews et al. 2014; Riley et al. 2010).
Mirroring earlier British research (Harville et al. 2010), we found evidence to support a mediating role for education, suggesting childhood disadvantage constrains women’s ability to complete schooling, which in turn relates to higher IL-6 and SCN admissions. The contribution of education was not as large as we anticipated, possibly due to the limited socioeconomic mobility in this sample. Similarly, lifestyle did not figure prominently into the findings - perhaps because of low rates of cigarette and alcohol use - and neither did any of the cytokines measured besides IL-6. Other studies have reported a similar pattern of cytokine findings (Ferguson et al. 2014), suggesting the possibility that gestational IL-6 levels are uniquely informative with regard to subsequent preterm birth. This pattern could reflect IL-6’s role as a transitional cytokine, which regulates the progression from acute, neutrophil-mediated to chronic, macrophage-driven, inflammation (Kaplanski et al. 2003). The latter process appears to be especially important in the pathogenesis of adverse pregnancy outcomes (Nagamatsu & Schust 2010; Ning et al. 2016).
These observations must be considered in light of several limitations. First, mothers completed the childhood disadvantage items retrospectively. Although this approach raises concerns about biased responding, people generally recall salient aspects of their childhood with good accuracy (Hardt & Rutter 2004). Second, the disadvantage items referred to childhood broadly, so cannot reveal whether timing of hardship matters. They also focused narrowly on material hardships, to the exclusion of downstream stressors (family instability, crime, neighborhood violence) and exposures (contaminants, pollutants) that are likely to be involved in the observed associations. Third, the impact of hardship could vary depending on the context in which it is experienced (e.g., urban vs. rural environment; regions with concentrated poverty vs. economic diversity), an issue that needs to be explored in future research. Fourth, maternal education is not a comprehensive indicator of socioeconomic status, raising questions about how effectively our analyses parsed the contributions of early vs. current hardship. Future research should more definitively address this question by including thorough assessments of women’s present conditions (e.g., income, wealth, deprivation). Fifth, our analyses considered six distinct clinical outcomes, but they should not be considered independent of each other. For example, preterm birth is a primary indication for admission to the SCN and for lengthier hospital stays. With that said, the consistency of findings across different types of outcomes (clinical vs. service) and different kinds of variables (categorical vs. continuous) suggests this is a robust phenomenon. Finally, we measured inflammatory biomarkers in maternal circulation. Deeper mechanistic insights would be available from studies in amniotic fluid, cervico-vaginal fluid, or placental tissue.
Despite these limitations, the current study provides a replication and extension of previous research on childhood hardship and pregnancy outcomes (Harville et al. 2010; Morton et al. 2014). In doing so, it draws attention to the incremental costs associated with this phenomenon, and begins to clarify some of the underlying behavioral and biological mechanisms. It also contributes to the growing literature on inter-generational effects (Pembrey et al. 2014; Schreier & Chen 2010; Chen et al. 2016b), and extends knowledge in this domain by highlighting pregnancy as a sensitive period during which health disparities may be transmitted across generations.
Supplementary Material
Highlights.
Women raised in families with economic difficulties had worse pregnancy outcomes They were more likely to deliver preterm babies who were small for gestational age. Those babies had lengthier hospital stays and more admissions to special care nurseries.
These risks were independent of demographic and obstetrical factors typically used to identify vulnerable pregnancies. They also were independent of mothers’ educational attainment, suggesting long-run effects of early hardship.
Mediation analyses suggested these risks arose through multiple pathways, and highlighted roles for inflammation, education, adiposity, and obstetric complications.
Acknowledgments
This project was supported by The National Children’s Study, Vanguard Study, Task Order 5 (HHSN275201200007I- HHSN27500005), and Auxiliary Research Scholar and Research Career Development Awards from NorthShore University HealthSystem to Ann E.B. Borders.
Abbreviations
- IFN
interferon
- IL
interleukin
- PTB
preterm birth
- SGA
small for gestational age
- SCN
special care nursery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-year change in inflammatory markers in elderly people and mortality: the Invecchiare in Chianti study. J. Am. Geriatr. Soc. 2007;55:1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Lung Association. Trends in Tobacco Use. Washington DC: American Lung Association; 2011. [Google Scholar]
- Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015;16:332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Braveman P, Barclay C. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;(124 Suppl 3):S163–S175. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- Burris HH, Baccarelli AA, Wright RO, Wright RJ. Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res. 2016;79:136–140. doi: 10.1038/pr.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Chen E, Shalowitz M, Story RE, Ehrlich KB, Levine CS, Hayen R, Leigh AK, Miller GE. Dimensions of socioeconomic status and childhood asthma outcomes: Evidence for distinct behavioral and biological associations. Psychosom. Med. 2016a;78:1043–1052. doi: 10.1097/PSY.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Shalowitz MU, Story RE, Ehrlich KB, Manczak E, Ham P, Le V, Miller GE. Parents’ childhood socioeconomic circumstances are associated with their children’s asthma osutcomes. J Allergy Clin Immunol. 2016b doi: 10.1016/j.jaci.2016.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Matthews K, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann. N. Y. Acad. Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ. Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain Behav. Immun. 2013;34:31–38. doi: 10.1016/j.bbi.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck TW, Mermelstein R. A global measure of perceived stress. Journal of Health and Social behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu. Rev. Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol. 2014;72:326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Zhu J, Van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010;56:314–318. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Gillespie SL, Christian LM, Neal JL. A proposed bio-panel to predict risk for spontaneous preterm birth among African American women. Med Hypotheses. 2015;85:558–564. doi: 10.1016/j.mehy.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology. Physiol. Rev. 2014;94:1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Harville EW, Boynton-Jarrett R, Power C, Hyppönen E. Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Arch Pediatr Adolesc Med. 2010;164:533–539. doi: 10.1001/archpediatrics.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis. New York: The Guilford Press; 2013. [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hsia RY, Akosa Antwi Y, Weber E. Analysis of variation in charges and prices paid for vaginal and caesarean section births: a cross-sectional study. BMJ Open. 2014;4:e004017. doi: 10.1136/bmjopen-2013-004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40:141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Early environments, developmental plasticity, and chronic degenerative disease. In: Cameron N, Bogin B, editors. Human Growth and Development. Second. London: Academic Press; 2012. pp. 325–341. [Google Scholar]
- Levine ME, Cole SW, Weir DR, Crimmins EM. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med. 2015;130:16–22. doi: 10.1016/j.socscimed.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, Murabito JM. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Soc Sci Med. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- March of Dimes. Special care nursery admissions. White Plains, NY: March of Dimes Perinatal Data Center; 2011. [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav. Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, Cole SW, Kobor MS. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proc. Natl. Acad. Sci. U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, De Stavola BL, Leon DA. Intergenerational determinants of offspring size at birth: a life course and graphical analysis using the Aberdeen Children of the 1950s Study (ACONF) Int. J. Epidemiol. 2014;43:749–759. doi: 10.1093/ije/dyu028. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Ning F, Liu H, Lash GE. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am J Reprod Immunol. 2016;75:298–309. doi: 10.1111/aji.12477. [DOI] [PubMed] [Google Scholar]
- Pembrey M, Saffery R, Bygren LOGR. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain Behav. Immun. 2009;23:677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Ridker PM. From C-Reactive Protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ. Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards J. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health. 2010;64:413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Miller GE, Culhane JF, Grobman W, Simhan HN, Wadhwa P, Williamson DF, McDade T, Buss C, Entringer S, Adam EK, Qadir S, et al. Patterns of peripheral cytokine expression during pregnancy in two cohorts and associations with measures of inflammation in cord blood. Am J Reprod Immunol. 2016;76:406–414. doi: 10.1111/aji.12563. [DOI] [PubMed] [Google Scholar]
- Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav. Immun. 2010;24:1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012;2012:967629. doi: 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.