Abstract
Prenatal stress exposure is associated with adverse psychiatric outcomes, including autism and ADHD, as well as locomotor and social inhibition and anxiety-like behaviors in animal offspring. Similarly, maternal immune activation also contributes to psychiatric risk and aberrant offspring behavior. The mechanisms underlying these outcomes are not clear. Offspring microglia and the pro-inflammatory cytokine interleukin-6 (IL-6), known to influence microglia, may serve as common mechanisms between prenatal stress and prenatal immune activation. To evaluate the role of prenatal IL-6 in prenatal stress, microglia morphological analyses were conducted at embryonic days 14 (E14), E15, and in adult mice. Offspring microglia and behavior were evaluated after repetitive maternal restraint stress, repetitive maternal IL-6, or maternal IL-6 blockade during stress from E12 onwards. At E14, novel changes in cortical plate embryonic microglia were documented—a greater density of the mutivacuolated morphology. This resulted from either prenatal stress or IL-6 exposure and was prevented by IL-6 blockade during prenatal stress. Prenatal stress also resulted in increased microglia ramification in adult brain, as has been previously shown. As with embryonic microglia, prenatal IL-6 recapitulated prenatal stress-induced changes in adult microglia. Furthermore, prenatal IL-6 was able to recapitulate the delay in GABAergic progenitor migration caused by prenatal stress. However, IL-6 mechanisms were not necessary for this delay which persisted after prenatal stress despite IL-6 blockade. As we have previously demonstrated, behavioral effects of prenatal stress in offspring, including increased anxiety-like behavior, decreased sociability, and locomotor inhibition, may be related to these GABAergic delays. While adult microglia changes were ameliorated by IL-6 blockade, these behavioral changes were independent of IL-6 mechanisms, similar to GABAergic delays. This and previous work from our laboratory suggest multiple mechanisms, including GABAergic delays, may underlie prenatal stress-linked deficits.
Keywords: il-6, microglia, prenatal stress, gaba, embryonic
Introduction
Prenatal stress is a risk factor for the development of psychopathologies including autism spectrum disorder, schizophrenia, anxiety disorders, and childhood behavioral problems (Kinney et al., 2008; Li et al., 2009; Ronald et al., 2010; Markham and Koenig, 2011; Holloway et al., 2013; Ratajczak et al., 2015; Tearne et al., 2015). In animal models, research has linked prenatal stress to heightened levels of anxiety, decreased sociability, and deficits in cognitive and motor function (Akatsu et al., 2015; Said et al., 2015). Despite evidence for such phenotypes, the mechanisms by which maternal stress alters offspring neural function are largely unknown.
Events in prenatal brain development set the stage for psychopathologies diagnosed in later childhood, adolescence, and adulthood (Stevens et al., 2010). Embryonic neuronal differentiation, migration, and cellular specialization have all been demonstrated to be critical processes determining mature brain function (Beckmann and Jakob, 1991; Martínez-Cerdeño et al., 2014). The development of the GABAergic system is significant for multiple brain functions; maturational disruptions have been linked to psychopathologies including Tourette syndrome, schizophrenia, and autism (Pleasure et al., 2000; Yip et al., 2008; Kataoka et al., 2010; Matrisciano et al., 2013). Prenatal stress delays GABAergic progenitor migration from their birthplace in the medial ganglionic eminence to their destination in the cortical plate (Stevens et al., 2013) and GABAergic migration is critical for cortical function (Volk and Lewis, 2013; Muraki and Tanigaki, 2015). The subsequent maturation of GABAergic cells is also affected by prenatal stress and has been linked to altered social and anxiety-like behaviors after prenatal stress (Stevens et al., 2013; Lussier and Stevens, 2016). Neurobiological changes that result from prenatal experiences, such as maternal stress, are prime pathoetiological candidates in psychiatric disorders.
There are many aspects of maternal gestational physiology that may affect offspring neurodevelopment, but few mechanisms are understood. Interaction between the maternal immune system and the embryonic brain is a potential mechanism for the long-term effects of prenatal stress exposure on neurodevelopment. Maternal inflammatory factors, such as interleukins, may directly or indirectly alter offspring neuronal progenitors and/or microglia, a myeloid-derived class of cells in the brain (McAllister and Patterson, 2012). These maternal factors act as important mediators of embryonic brain changes; elevated maternal interleukins have been demonstrated to alter offspring behavioral and brain outcomes in animal models; specific cytokines during pregnancy have been associated with offspring neuropsychiatric disorders such as autism spectrum disorder and schizophrenia (Petitto et al., 1997; Gilmore et al., 2004; Meyer et al., 2007; Ponzio et al., 2007; AL-Ayadhi and Mostafa, 2012).
Both maternal immune activation (MIA) and prenatal stress exposure result in altered neurodevelopmental and behavioral phenotypes in offspring. Specifically, the pro-inflammatory cytokine, interleukin-6 (IL-6), has been implicated as a potential mediator of prenatal MIA effects on offspring neurodevelopment (Choi et al., 2016; Mouihate and Mehdawi, 2016; Wu et al., 2017). IL-6 may also be similarly critical to the etiology of prenatal stress effects (Smith et al., 2007). In rodent models, both chronic variable and repetitive restraint stress, typical methods of prenatal stress, have also been shown to alter circulating maternal cytokine levels, with prolonged restraint stress resulting in increased levels of circulating IL-6 for as long as 14 days after cessation (Houri-Haddad et al., 2003; Cyr et al., 2007; Moazzam et al., 2013; Voorhees et al., 2013). Further, both MIA and prenatal stress exposure result in microglia changes in offspring, implicating a common, underlying neuroimmune mechanism (Sebire et al., 1993; Diz-Chaves et al., 2012; Diz-Chaves et al., 2013; Pratt et al., 2013). Microglia have critical roles to play in the developing brain—regulating proliferating neural progenitors, synaptic development, and sub-cortical projections—and could thus be significant for multiple neurobiological changes observed in prenatally stressed offspring (Antony et al., 2011; Diz-Chaves et al., 2012; Cunningham et al., 2013; Kettenmann et al., 2013).
In the present study, we investigate the role of IL-6 as a mediator of neurobiological and behavioral prenatal stress effects. To better understand the inflammatory components of these effects, we determined the role of IL-6 in prenatal stress-induced alterations to embryonic and adult microglia. Because of our previous work demonstrating the effects of prenatal stress on GABAergic system development (Stevens et al., 2013; Lussier and Stevens, 2016) and converging evidence of its sensitivity to maternal immune manipulation (Oskvig et al., 2012), we also examined the direct role of IL-6 in prenatal stress effects on GABAergic progenitor migration.
Methods
Mice
CD1 females (The Jackson Laboratory, Bar Harbor, Maine) were bred to CD1 wildtype males (for microglia and behavior analyses) or GAD67-GFP +/− CD1 males (for embryonic, GABAergic analyses) (Tamamaki et al., 2003). Upon detection of a vaginal plug, indicating embryonic day (E) 0, females were singly-housed. Offspring for behavioral testing were left with mothers at birth and then group-housed (three to four per cage) after weaning at three weeks of age. Dams and adult offspring were euthanized by ketamine/xylazine anesthesia followed by intracardiac perfusion or rapid decapitation. Fetal offspring were euthanized by rapid decapitation. All experimental procedures involving animals were performed in accordance with the Yale University and University of Iowa Animal Resources Center/Office of Animal Resources and Institutional Animal Care and Use Committee (IACUC) policies. Additionally, all animal experiments were conducted in compliance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Prenatal Stress and Injections
CD1 wildtype dams were pseudo-randomly assigned to one of seven maternal treatment groups: nonstress alone (NS), prenatal stress alone (PS), nonstress saline-injected (NS/Saline), nonstress IL-6-injected (NS/IL-6), nonstress anti-IL-6-injected (NS/Anti-IL-6), prenatal stress saline-injected (PS/Saline), and prenatal stress anti-IL-6-injected (PS/Anti-IL-6).
From E12 until embryo collection or birth, prenatally stressed (PS) females were exposed to three-times-daily, 45-min restraint sessions under bright light at approximate three to four hr intervals. Mothers in injection conditions were administered intraperitoneal (i.p.) injections three times a day, beginning on E12, of 0.9% sterile NaCl, 100 ng IL-6 dissolved in 0.9% sterile NaCl from 500 ng/ml solution (Biolegend; purified recombinant mouse, carrier free, catalog #575702), or 10 μg anti-IL-6 antibody dissolved in 0.9% sterile NaCl from a 65 μg/ml solution (R&D systems; purified rat monoclonal IGG anti-mouse IL-6; catalog #MAB406). IL-6 dosing was estimated to recapitulate the increases in IL-6 that occur after restraint stress, calculated across mouse volume of distribution (Zhou et al., 1993; Takaki et al., 1994; Nukina et al., 2001). To ensure an adequate blockade, neutralizing anti-IL-6 antibody was administered at a molar dose equivalent to 100 times the estimated whole animal molar quantity of IL-6 (Smith et al., 2007) evoked by a restraint stress session. In those PS mothers receiving saline or anti-IL-6, injections were administered 20 min prior to restraint.
Neurobiological measures were made at E14 and E15. One to two offspring were randomly selected from each embryonic litter for neurobiological assessment. All embryonic groups were balanced for sex (determined with sex genotyping by PCR)(Clapcote and Roder, 2005). Behavioral testing was conducted in adulthood (between eight and eleven weeks of age) and then adult brain was assessed at 11-12 weeks of age. Because of our previous use of male offspring for similar assessments (Lussier and Stevens, 2016), adult measures were all made in males only. Two to four offspring were randomly selected from each litter for behavioral testing. One to two offspring were randomly selected from each litter for adult brain analysis. Microglial morphologies were assessed in both NS and NS/Saline offspring, grouped hereafter as NS as well as in both PS and PS/Saline, grouped hereafter as PS. Experimental design and animal genetic backgrounds, total numbers of litters, and animals used are shown in Figure 1 and Table 1.
Figure 1.
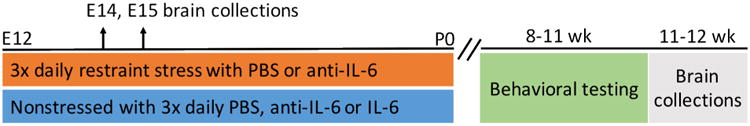
The experimental design included seven experimental groups and multiple time points of assessment.
Table 1.
| Background | Litters | Total Offspring Analyzed | |
|---|---|---|---|
| Embryonic (E14, E15) offspring: neurobiology | |||
| Nonstress controls (NS) | CD1 | E14: 4 | 7 |
| E15: 3 | 6 | ||
| Prenatal Stress (PS) | CD1 | E14: 4 | 6 |
| E15: 3 | 8 | ||
| Saline injected (NS/Saline) | GAD67-GFP +/− | E14: 4 | 7 |
| IL-6 injected (NS/IL-6) | GAD67-GFP +/− | E14: 4 | 7 |
| Nonstress/anti IL-6 injected (NS/Anti-IL-6) | GAD67-GFP +/− | E14: 3 | 8 |
| Prenatal Stress/anti IL-6 injected (PS/Anti-IL-6) | GAD67-GFP +/− | E14: 3 | 7 |
| Adult offspring: behavior and neurobiology | |||
| Nonstress (NS) | CD1 | Behavior: 4 | 14 |
| Neurobiology: 3 | 5 | ||
| Nonstress/saline injected (NS/Saline) | CD1 | Behavior: 3 | 9 |
| Nonstress/anti IL-6 injected (NS/Anti-IL-6) | CD1 | Behavior: 3 | 8 |
| Neurobiology: 3 | 3 | ||
| Prenatal Stress/anti IL-6 injected (PS/Anti-IL-6) | CD1 | Behavior: 3 | 9 |
| Neurobiology: 3 | 3 | ||
| Prenatal Stress (PS) | CD1 | Neurobiology: 3 | 3 |
| Prenatal Stress/saline injected (PS/Saline) | CD1 | Behavior: 3 | 9 |
| IL-6 alone (NS/IL-6) | CD1 | Behavior: 4 | 13 |
| Neurobiology: 3 | 3 | ||
Tissue Processing and Immunocytochemistry
Dams were euthanized and embryonic brains were collected at E14 and E15, post-fixed for at least 15 hrs in 4% paraformaldehyde (PFA)/phosphate buffered saline (PBS), and then dehydrated in 20% sucrose/PBS for a minimum of 15 hrs.
Adult tissues were collected at 11-12 weeks of age. Brains were dissected out after animals were perfused with ice cold PBS and then 4% PFA, post-fixed and dehydrated in 20% sucrose. Prior to immunocytochemistry, both adult and embryonic tissues were embedded in Optimal Cutting Temperature (OCT) compound and coronally cryo-sectioned (Leica, CM1900, Bannockburn, Illinois) at 25 μm (embryonic) or 50 μm (adult).
Sections were incubated in 10% goat serum/PBS ++ blocking solution (with 0.025% TritionX-100, 0.0125% Tween20) for one hr at room temperature (RT) then in 5% goat serum/PBS++ primary antibody containing anti-Iba-1 (WAKO; rabbit polyclonal 1:300-600, #019-19741), and for embryonic tissue, anti-GFP (1:1000; Abcam, chicken AB13970, #660556) overnight. Secondary antibodies were applied in 5% goat serum/PBS ++ using Alexa dye-conjugated secondary antibodies (1:500-1000; Molecular Probes) and incubated for one hr at RT. Slides were cover slipped using DAPI (4′,6-diamidino-2-phenylindole, Vector Laboratories, #H-1200) mounting medium.
Neurobiological Measures
Microglia Analyses
To determine the density of microglia, stereological, unbiased estimates of Iba-1+ cells were determined. A Carl Zeiss Axiocam, equipped with motorized stage and digital camera, coupled to StereoInvestigator software (Microbrightfield, Colchester, Vermont), was used to count and characterize microglia present in three-dimensional 250 × 150 × 10 μm counting frames on a 500 × 300 μm grid for embryonic analyses or 1000 × 600 μm grid for adult analyses, from the cortical plate or neocortex every 20th coronal section. A minimum of four sections containing cortex were assessed. Cells were counted using an optical fractionator approach and unbiased counting rules of stereology (Peterson, 1999) including systematic, randomly placed counting frames, balanced exclusion and inclusion boundaries of counting frames, and unique cell points for counting to determine exclusion/inclusion.
In embryonic brain, we created a four-category classification method to identify multiple morphologies defined as (modified from Cunningham et al., 2013):
multivacuolated as those with multiple, pyknotic nuclei and/or vacuoles present within the cell;
amoeboid as those with a normal nucleus and no cellular processes;
transitional as those with a normal nucleus and one process;
ramified as those with a normal nucleus and two or more processes.
In adult brain, microglia morphologies were defined as (modified from Diz-Chaves et al., 2012):
amoeboid as those with zero to one process;
lowly ramified as those with two or three processes;
moderately ramified as those with four processes or those with multiple thin, spindly processes and a small soma;
highly ramified as those with five or more processes and a large soma, also referred to as bushy microglia.
Density was calculated by dividing cell number by total cortical plate or neocortical volume, as computed by the Cavalieri volume estimation approach.
GABAergic Progenitor Migration Analyses
GAD67GFP+ progenitor migration into the dorsal telencephalon was taken as a percentage of total external cortical plate circumference for each section (both left and right hemisphere), as previously described (Stevens et al., 2013). The distance from the lateral-most extent of the cortical plate, where a clear GAD67GFP+ stream is apparent, to the leading-most edge of the migrating GAD67GFP+ stream immediately deep to the pia was taken to indicate total migration distance. The percent migration of total cortical plate circumference, computed by dividing total migration distance by the distance from the lateral- to medial-most extent of the cortical plate in each coronal section, was then averaged across all sections (three to four) in each offspring brain.
Behavioral Measures
All behavioral measures were taken in 8-11 wk old male mice in the same testing room, under standard overhead room lighting (300 lux) during the animals' light cycle. Tests were conducted one per day in the same order for each animal and a one hr habituation was conducted before all tests. Two cohorts of mice were examined at separate times and results were not collapsed across groups. The first cohort contained the NS/Saline, PS/Saline, NS/anti-IL-6 and PS/Anti-IL-6 groups and the second cohort contained the NS and NS/IL-6 groups.
Open Field
In a 1,500 cm2 rectangular clear plastic chamber, locomotor activity was measured using Anymaze software (Stoelting Co., Wood Dale, Illinois) coupled to a ceiling-mounted digital USB camera. Animals were tested over two, 30-min sessions, approximately 24 hrs apart, and results from the second session were analyzed to avoid stress and field novelty effects present on the first day of testing.
Elevated Plus Maze
For the elevated plus maze (EPM) task, mice were placed at the center of a standard plus-shaped, opaque plastic apparatus (Stoelting Co), elevated approximately 0.6 m above the ground, consisting of two, 35 cm-long closed arms with 20 cm-tall walls and two open arms without walls. Mouse time spent in open arms, closed arms, and center of the maze was tracked using Anymaze software coupled to a USB digital camera for five min. Time, rather than distance or arm entries, was analyzed to avoid any direct locomotor effects on anxiety-like measures.
Social Approach
A three-chambered, clear plastic social approach apparatus (Nadler et al., 2004) was used to assay mice for sociability. The apparatus consisted of a 20 × 40 × 22 cm central chamber with two 5 × 8 cm portals to two, equally-sized 20 × 40 × 22 cm side chambers, each containing a 9 cm diameter, 10 cm tall wire cup. Experimental mice were habituated individually to the center chamber for 10 min. A conspecific of the same age, sex, and strain was then placed in the inverted wire cup in one of the two side chambers (“stranger chamber”) while the experimental animal was allowed to traverse all three chambers for 10 min, analyzed as two, five-min epochs. As with the EPM analysis, time in zones was the main outcome, to avoid effects of locomotor differences. Mouse time spent in the empty, center, and stranger chambers was recorded with an overhead camera coupled to Anymaze software. Mouse regulation of sociability was measured with a “sociability ratio.” This ratio's numerator was the difference in time spent in the chamber with the stranger mouse from the first five minute epoch to the second. The denominator was the total amount of time spent with the stranger during both epochs combined. Positive values indicated increased social interaction over time while negative values indicated decreased. A complimentary measure to assess non-social behavior—an “exploratory ratio”—was calculated for time spent in the chamber with the empty cup instead of the stranger mouse.
Statistical Analyses
For all tests and measures, outliers were defined as deviating by two or more standard deviations from the mean and were excluded from analyses (SPSS v. 22, IBM; IL, USA). Sample size for each group and details of statistical analyses were included in figure captions or in the text. A value of p<0.05 was taken to indicate statistical significance.
For measures for which it was applicable, either one-way or two-way (stress condition, anti-IL-6 treatment) analyses of variance (ANOVA) were performed to detect main effects and/or interactions. Repeated measures ANOVA with a Greenhouse-Geisser correction was used to analyze locomotor activity in the open field test. Significant ANOVA effects were planned to be followed by independent sample, student's two-tailed t-tests. A statistically significant interaction between stress status and anti-IL-6 status was taken to indicate a possible amelioration of prenatal stress-linked behavior and thus a fundamental role for IL-6.
For each test performed, all relevant underlying statistical assumptions were tested. First, the assumption of random assignment was met, as all animals were randomly and in no particular order assigned to each possible condition. A kurtosis and skew of < ±2 were taken to define normally distributed frequency data. All groups met this normality assumption and the homogeneity of variances assumption per Levine's test.
Results
Across all intervention groups, the numbers of offspring per litter were not significantly different (ANOVA, F=1.546, df=6, p=0.185; average pups per litter: NS=9.1±1.9, PS=9.7±3.7, NS/Saline=11.1±2.6, PS/Saline=9.3±5.5, NS/IL-6=8.4±1.8, NS/Anti-IL-6=11.4±2.7, PS/Anti-IL-6=11±2.3).
Both prenatal stress and IL-6 resulted in altered microglia morphology
Myeloid progenitors first begin to migrate into the brain at E9, then populate the embryonic mesenchyme and neuroepithelium at E10 (Ginhoux et al., 2010). To capture changes in microglia, we examined Iba-1 stained microglia at both E14 and E15 in the developing cortical plate of both prenatally stressed (PS) and nonstressed (NS) control animals. Morphologies were classified at both time points for degree of ramification and presence of multiple vacuoles (Fig. 2A). Prenatal stress resulted in significantly increased multivacuolated microglia density at E14 but not at E15 (Fig. 2B, C), a morphology previously identified as relevant to early neurodevelopmental processes (Cunningham et al., 2013). Total density of microglia remained unchanged between PS and NS offspring at both E14 and E15 (E14 NS=3.82 ×10-6 ±6.34×10-7 cells/μm3, PS=5.40 ×10-6 ±1.08 ×10-6 cells/μm3, p=0.22; E15 NS=5.76 ×10-6 ±0.39-6 cells/μm3, PS=6.09 ±0.33×10 -6 cells/μm3, p=0.55). The density of transitional microglia was significantly increased by prenatal stress at E15 only (Fig. 2C). Because of the extent of multivacuolated microglia density change, further results focused on the multivacuolated microglia morphology at E14.
Figure 2.
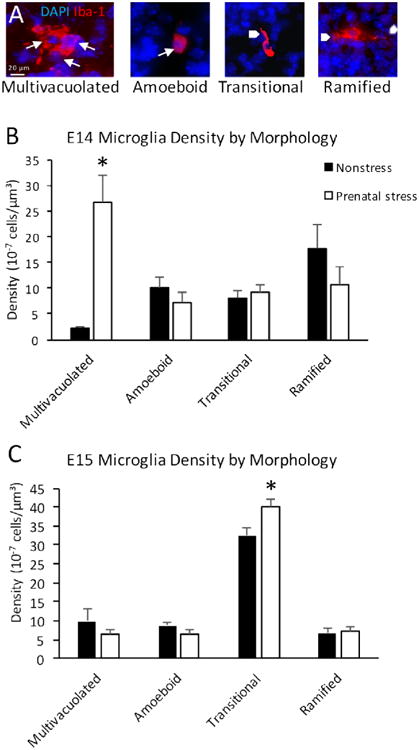
Increased density of multivacuolated microglia after prenatal stress at E14 but not at E15. A, Embryonic day 14 (E14) embryonic microglia morphologies of four major subtypes: 1) multivacuolated, with multiple, pyknotic nuclei and/or vacuoles, 2) amoeboid, with normal nucleus and no processes, 3) transitional with normal nucleus and one process, and 4) ramified with normal nucleus and two or more processes. B, Prenatal stress resulted in increased multivacuolated cells at E14 (p=0.00095), C, which was not present at E15 (p=0.45). The density of transitional microglia is increased at E15 only (p=0.00049). Means ± SEMs are shown. n= 6-8 animals per group. (* p<0.0005 by two-tailed student's t-test; Arrows indicate cell nuclei. Arrowheads indicate cell processes.)
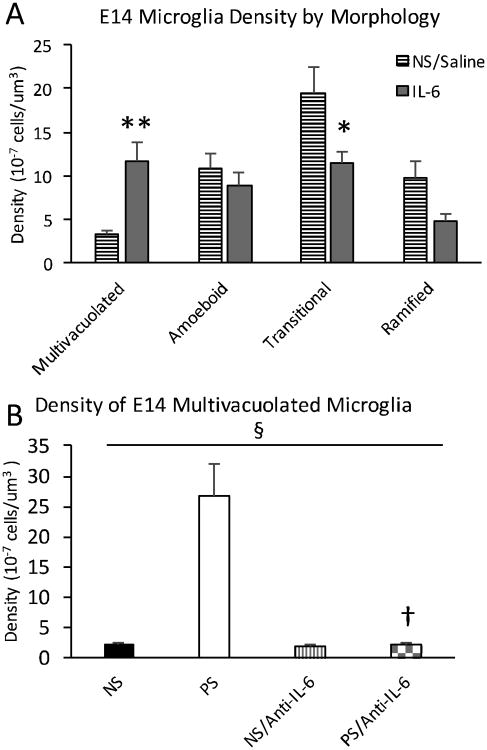
As with prenatal stress exposure, maternal IL-6 exposure resulted in increased multivacuolated microglia at E14 compared to saline-injected controls (NS/Saline) (Fig. 3), with no change in overall microglia density (NS/Saline=4.32 ±0.50 ×10-6 cells/μm3; NS/IL-6=3.53 ±0.27 ×10-6 cells/μm3, p=0.17). These data suggested that IL-6 was sufficient to recapitulate the effects of prenatal stress exposure on E14 microglia morphology, suggesting that IL-6 may be involved in the offspring neurobiology of prenatal stress.
Figure 3.
Prenatal stress (PS) and IL-6 both resulted in an increased density of multivacuolated microglia at E14 which was not present with anti-IL6. A, Prenatal IL-6 exposure resulted in an increased density of multivacuolated microglia at E14 over nonstress controls (NS/Saline) (p=0.00049). The density of transitional microglia was also increased (p=0.033). B, Anti-IL-6 concurrent with stress corrected the increase in multivacuolated microglia density resulting from prenatal stress exposure (ANOVA, interaction, F= 27.432, df=1, p<0.00005), with no difference between nonstress (NS) and nonstress/anti-IL-6 (NS/Anti-IL-6) or prenatal stress/anti-IL-6 (PS/Anti-IL-6) density (p=0.58, p=0.91). PS/Anti-IL-6 density was significantly lower than prenatal stress (p=0.0004). Means ± SEMs are shown. n= 6-7 animals per group. (* p<0.05, ** p<0.0005, † p<0.0005 by two-tailed student's t-test; § p<0.00005 interaction by one-way ANOVA)
Interestingly, while the multivacuolated morphology was most dramatically changed by both PS and IL-6, the transitional microglia morphology was significantly lower in the NS/IL-6 compared to the NS/Saline condition (Fig. 3A). The NS and PS groups initially assessed (Fig. 2B) also appeared lower in transitional microglia density than the NS/Saline group (Fig. 3A). Given the similarities across groups in total density of microglia, this difference in transitional morphology after the injection control manipulation, a minor stressor, may have reflected a shift from other morphology categories (i.e. ramified).
IL-6 was necessary for increased embryonic multivacuolated microglia
To test the role of IL-6 for neurodevelopmental prenatal stress effects, dams were exposed to neutralizing IL-6 antibody (anti-IL-6) just prior to restraint stress sessions. Offspring embryonic brain was then assayed for density of multivacuolated morphology. Anti-IL-6 with prenatal stress was found to return elevated levels of multivacuolated microglia to nonstress control levels (Fig. 3B).
IL-6 played a non-critical role in GABAergic progenitor delays in embryonic brain
As previously reported (Stevens et al., 2013), prenatal stress results in an additional neurodevelopmental endophenotype: delays in the migration of inhibitory, GABAergic progenitors from these cells' birthplace in the ventral telencephalon to their destination in the developing cortical plate. Here, IL-6 manipulation played a role in GABAergic progenitor migration. IL-6 exposure resulted in similar delays to these progenitors at E14 as occurs with prenatal stress (Fig. 4). Notably, unlike our findings on the mitigation of microglia changes, this migration delay was not fully corrected by anti-IL-6 exposure concomitant with prenatal stress (PS/Anti-IL-6 versus NS/Anti-IL-6; p<0.0001)—a trend difference between PS/Anti-IL-6 rescue and the NS/Saline control was also present (p=0.087). Additionally, anti-IL-6 advanced migration when administered in the absence of stress (NS/Saline vs NS/Anti-IL-6; p<0.0001).
Figure 4.
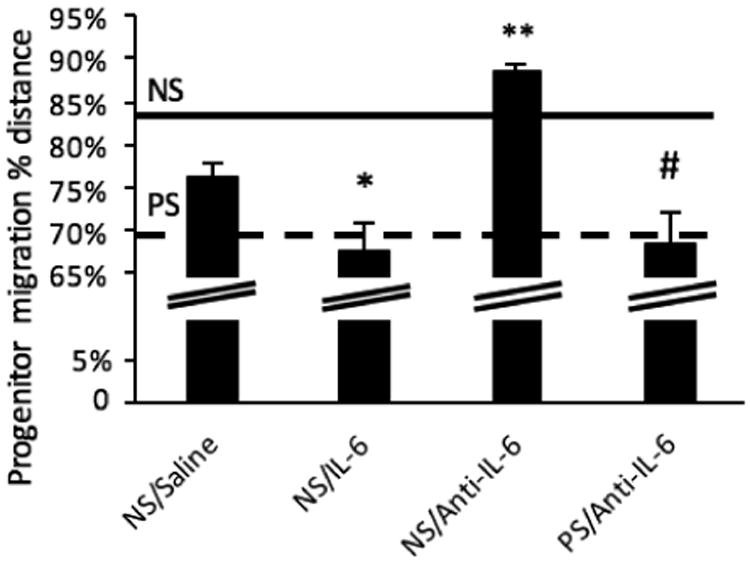
IL-6 and prenatal stress with anti-IL6 both resulted in delays to GABAergic progenitor migration as with prenatal stress alone. Dashed line indicates prenatal stress (PS) migration average (69%); solid line indicates nonstress (NS) migration average (83%), as previously reported (Stevens et al., 2013). IL-6 exposure resulted in similar delays to these progenitors at E14 as occurs with prenatal stress (one-way ANOVA, F=16.476, df=3, p<0.0001; t-test: NS/Saline vs NS/IL-6 p=0.033). PS delayed migration was not corrected by anti-IL-6 (NS/Anti-IL-6 vs PS/Anti-IL-6 p<0.0001). The NS/Saline control and PS/Anti-IL-6 rescue conditions showed a trend difference (p=0.087). Anti-IL-6 advanced migration above saline control levels (p<0.0001). Means ± SEMs are shown. n= 6-8 animals per group. (* p<0.05, ** p<0.0001, # p=0.087 by two-tailed student's t-test)
IL-6 was critical for persistent microglia changes
To assay long-term prenatal stress effects on microglia and the role of IL-6, adult brains were also analyzed for microglia morphologies. Unlike embryonic brains, in which immature microglia display a restricted morphological range (Dalmau et al., 1998), adult brains have a broad range of more ramified microglia morphologies, often displaying five or more processes (Fig. 5A). To determine differences between microglial function, as indicated by cell morphology in the adult cerebral cortex, microglia subtype percentages were assessed across four categories: amoeboid, lowly, moderately, and highly ramified (Diz-Chaves et al., 2012). Prenatal stress or IL-6 alone resulted in similar shifts in microglia morphology compared to controls, changes that were absent when anti-IL6 was administered (Fig 5B). Prenatal stress and prenatal IL-6 both produced significantly more highly ramified microglia (ANOVA: F=69.010, df=2, p=0.000001; post-hoc t-tests: p<0.00001 for PS and NS/IL-6 versus NS) and less amoeboid and lowly ramified microglia (ANOVA: amoeboid: F=20.928, df=2, p=0.00267, lowly: one way: F=9.872, df=2, p=0.004; post-hoc t-tests p<0.05 in both morphologies for PS and NS/IL-6 versus NS). In mice exposed to anti-IL6 during nonstress or prenatal stress experience, the shifts from amoeboid to highly ramified morphologies were absent and only the PS group significantly differed from both NS and PS/Anti-IL-6 for these morphologies (ANOVA interaction of stress and anti-IL6: amoeboid: F=17.089, df=1, p=0.001, highly ramified: F=29.284, df=1, p=0.000157; post-hoc t-tests p<0.05 in both morphologies for PS versus NS and PS/Anti-IL-6).
Figure 5.
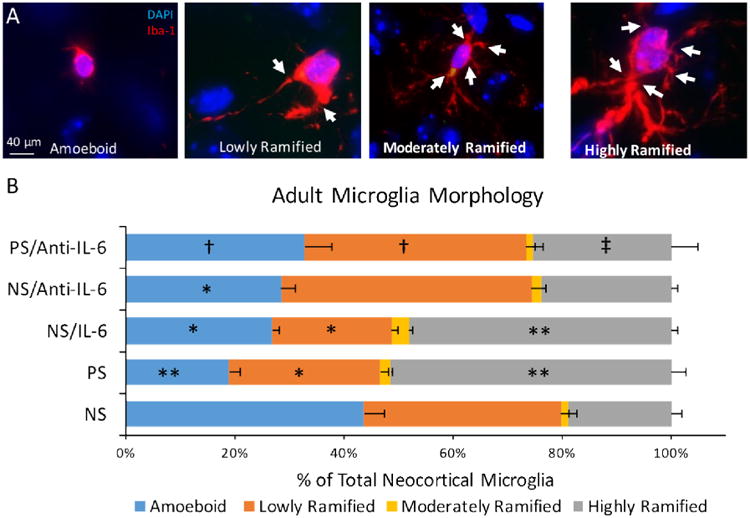
Changes to adult microglia morphology after prenatal stress, IL-6, and anti-IL6. A, Adult microglia were categorized among four subtypes. B, In adult brain, prenatal stress increased highly ramified microglia (vs NS, p<0.0001), as did NS/IL-6 (vs NS, p<0.0001). Prenatal anti-IL-6 concomitant with stress (PS/Anti-IL-6) corrected this increase (non-significant vs. NS p=0.20), with less highly ramified microglia in PS/Anti-IL-6 compared to PS alone (p=0.002). PS decreased levels of lowly ramified cells (vs NS p=0.03), as did NS/IL-6 (vs NS p=0.01), while PS/Anti-IL-6 increased levels of these cells compared to PS animals (p=0.005) and did not differ from controls (p=0.36). Finally, as with lowly ramified cells, NS/IL-6 alone (vs. NS p=0.02) and PS (vs. NS p=0.0004) decreased levels of amoeboid cells, while anti-IL-6 concomitant with PS restored levels of these cells to NS levels (p=0.13), significantly lower than PS levels (p=0.02). Means ± SEMs are shown. n= 3-5 animals per group. (for comparisons to NS: *p<0.05, **p<0.0005; for comparisons to PS: † p<.05, ‡ p<0.005 by two-tailed student's t-test; Arrows indicate cell processes.)
An effect of prenatal stress was still apparent in lowly-ramified microglia despite anti-IL-6 administration (ANOVA for Saline and anti-IL-6 groups: no interaction; stress main effect: F=7.156, df=1, p=0.020) but post-hoc tests demonstrated no difference between PS/Anti-IL-6 and NS controls (t-tests p<0.05 for PS versus NS only). Prenatal anti-IL-6 administration independently increased lowly-ramified microglia (ANOVA: anti-IL-6 main effect F=19.011, df=1, p=0.001: post-hoc t-tests p<0.05 for PS versus PS/Anti-IL-6; Fig. 5B). No significant differences were found for any comparisons for moderately-ramified microglia. The total densities of microglia in the cortex significantly differed across the NS, PS and NS/IL-6 groups (ANOVA: F=1.602, df=2, p=0.0249; no significant post-hoc tests; NS =4.03 ±0.66; PS=4.97 ±0.73; NS/IL-6=5.96 ±0.70 ×10-6 cells/μm3) but did not differ across other groups.
Behavioral phenotypes persist despite microglia morphology correction
While microglia morphology changes after prenatal stress in both the embryonic and adult brain were blocked when IL-6 antibody was administered, prenatal stress effects on animal behavior, as with GABAergic system development, were not. Prenatal stress resulted in an anxiety-like behavioral phenotype in adult male offspring, regardless of anti-IL-6 exposure, as evidenced by a decrease in time spent on the open arm of the EPM as compared to controls (Fig. 6A). Similarly, anti-IL-6 did not ameliorate the effect of prenatal stress on animal social behavior, as demonstrated by decreased sociability in both the PS/Anti-IL-6 and PS/Saline conditions (Fig. 6B). This finding was not explained by a generalized decrease in exploratory behavior, as evidenced by no significant deficit in PS animals' exploratory behavior ratio (data not shown: No main effect of stress by ANOVA, F=0.076, df=1, p=0.785). Finally, anti-IL-6 did not mitigate the effect of prenatal stress on offspring locomotor activity across the duration of the open field task, as demonstrated by a similar trend to decreased distance traveled in both PS/Anti-IL-6 and PS/Saline conditions (Fig. 6C). The independence of these behavioral changes from IL-6 mechanisms was also demonstrated in a cohort of prenatal IL-6 exposed mice (Fig. 7). This IL-6 cohort showed no significant differences from control mice in anxiety-like, sociability, or locomotor behaviors. Collectively, these behavioral data suggested that, while anti-IL-6 ameliorated prenatal stress-associated microglia morphological changes, such corrections were not seen in the domains of animal anxiety, sociability or locomotion, likely reflecting additional underlying mechanisms independent of IL-6. This resistance to the effects of IL-6 immunoblockade paralleled the persistence of delayed GABAergic progenitor migration in the embryonic cortical plate (Fig. 4).
Figure 6.
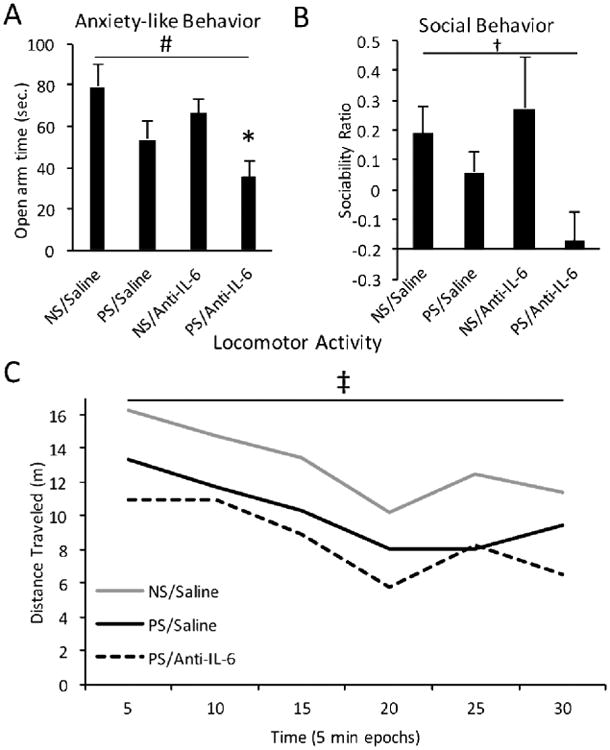
Anti-IL-6 did not alter prenatal stress-induced behavioral phenotypes. A, Prenatal stress and saline (PS/Saline) resulted in decreased time spent in the open arm of the elevated plus maze over nonstress, saline controls (NS/Saline) (ANOVA, main effect of prenatal stress, F=10.562, df=1, p=0.003), indicative of elevated anxiety-like behavior, which was not ameliorated with concurrent anti-IL-6 administration. B, Animal sociability was decreased by prenatal stress, regardless of anti-IL-6 exposure (ANOVA, main effect of prenatal stress, F= 5.591, df=1; p=0.0258). Sociability ratio indicated an increase (values >0) or decrease (values <0) in animal sociability over time. C, Inhibited locomotor activity resulting from prenatal stress, was also not affected by anti-IL-6 (repeated measures between-subjects ANOVA, trend effect of prenatal stress, F=3.882, df=1, p=0.063). Means ± SEMs are shown. n= 7-9 adult males. (*p<0.05 by t-test; #p<0.05 by one-way ANOVA; † trend effect by one-way ANOVA; ‡ trend effect by repeated measures ANOVA)
Figure 7.
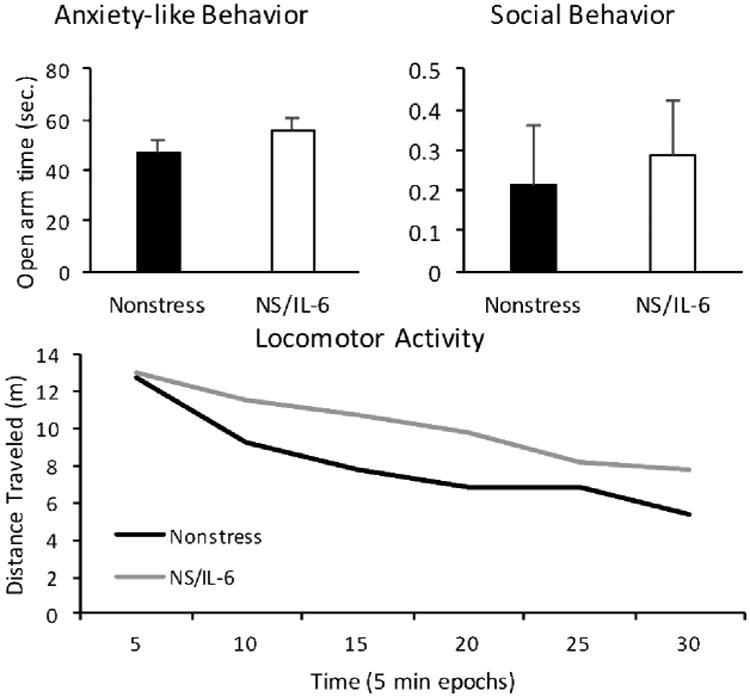
IL-6 did not alter behavioral phenotypes in an independent cohort. A, Prenatal interleukin-6 treatment in nonstressed (NS/IL-6) offspring did not change time spent in the open arm of the elevated plus maze over nonstress controls (p=0.24). B, Animal sociability was unchanged by IL-6 (p=0.72). C, Locomotor activity was also unchanged by IL-6 treatment (repeated measures between-subjects ANOVA, F=0.981, df=3.166, p=0.409). Means ± SEMs are shown. n=12-14 adult males.
Discussion
Prenatal stress is a physiological, maternal experience which has a broad range of possible mediators and likely influences offspring neurodevelopment in multiple ways. This study has begun to parse these effects. While previous studies have examined postnatal microglia morphology after prenatal stress (Diz-Chaves et al., 2012), here we examined early embryonic microglia morphology as well. Furthermore, this study addressed previously unanswered questions about the role of IL-6 in the conference of neurobiological and behavioral effects of prenatal stress on offspring.
Here, we showed that both prenatal stress and IL-6 exposure contributed to an increased density of multivacuolated microglia in the embryonic cortical plate at earlier stages of development—at E14 but not at E15—suggesting either a specific period of susceptibility or the influence of stress duration for these effects. From E14 to E15, multivacuolated and transitional microglia densities generally increased, a previously undescribed phenomena in line with their possible functions—phagocytosis of progenitors and gradual development into ramified forms (Dalmau et al., 1998; Parakalan et al., 2012; Cunningham et al., 2013). However, because densities also reflected changing cortical volume, there are limits to these comparisons across age. Interestingly, transitional microglia, a possible mid-point between immature amoeboid and more mature ramified microglia, were increased after prenatal stress at E15 and after IL-6 at E14 which may represent advancements of the normal developmental trajectory of these cells (Cunningham et al., 2013). The focus here remained on mulitvacuolated microglia, however, due to the less clear developmental role of the transitional morphology (Sanchez-Lopez et al., 2005).
Prenatal maternal anti-IL-6 exposure, concurrent with maternal restraint stress, blunted effects on embryonic microglia, restoring the density of multivacuolated microglia to control levels. Thus, this specific immunological blockade led to a phenotypic rescue of multivacuolated changes in embryonic microglia morphology. Furthermore, we showed that prenatal stress- and IL-6-linked microglia abnormalities, as well as rescue by anti-IL-6, persisted into adulthood. Morphological changes in microglia evolved over development, in part demonstrated by a lack of distinct morphological differences with prenatal stress at E15. Despite this evolution, prenatal stress effects in microglia did not resolve with time. There is currently little known about the developmental progression of microglia morphology and any associated functional changes. The persistence of abnormal microglia morphologies in this particular model may advance our understanding of the developmental trajectory of these crucial brain cells from embryogenesis to adulthood.
While these data suggest that some aspect of microglia respond in a lasting way to early-life insult, the literature is mixed with regard to the longevity and magnitude of these changes (Smolders et al., 2015; Antonson et al., 2016; Giovanoli et al., 2016). Additionally, there are only a few studies that address how embryonic microglia morphology reflects their function. Multivacuolated microglia cells, as described and assessed here embryonically, have been noted in the context of neurodegeneration and acute insult or injury in the adult brain, and may play a part in the neuroprotective and regulatory roles of adult microglia (Avellino et al., 1995; Mueller and Bale, 2007; Eyo and Dailey, 2013). More broadly, microglia activation states, including the appearance of phagocytic microglia, have been associated with developmental apoptosis, axonal growth, and synaptogenesis (Streit, 2001). Previous data on embryonic microglia form-to-function associations in vivo (Cunningham et al., 2013) demonstrate that their morphological change after prenatal stress may influence cortical development. It is important to note the possibility that some of these cells are themselves undergoing pyknosis, which would be a separate, significant effect of prenatal stress on microglia developmental trajectories. Future steps must involve direct exploration of the developmentally varied, functional roles of these cells and employ the use of molecular markers (e.g. IL-1β) that can be used to determine their activation status.
As previously reported by our group, prenatal stress causes delays to embryonic GABAergic progenitor migration, from their birthplace in the ventral telencephalon to their destination in the cortical plate (Stevens et al., 2013). Our results here demonstrate that prenatal IL-6 administration also caused smaller but still significant inhibitory progenitor migration delays. Others have also found that inhibitory progenitor development interacts with inflammatory molecules (Lysko et al., 2011). However, because delays were not fully rescued by prenatal anti-IL-6 administration concurrent with stress, prenatal stress effects on inhibitory progenitor migration likely involve multiple physiological players (Tamura et al., 2011), as we review below.
To further clarify whether IL-6 may be involved in the behavioral deficits of prenatal stress, we examined behaviors that our group has previously linked to prenatal stress exposure and GABAergic delays (Lussier and Stevens, 2016). We found that behavioral anxiety-like changes after prenatal stress did not involve IL-6. Animal performance on the elevated plus maze (EPM) indicated increased anxiety-like behavior among prenatal stress offspring, regardless of prenatal antibody exposure. Furthermore, decreased sociability and decreased locomotion, both associated with prenatal stress exposure here, persisted despite IL-6 blockade. These behavior changes were also not caused by repetitive prenatal IL-6 administration. These changes in behavior in male offspring after prenatal stress have relevance to the symptoms of male-predominant clinical outcomes with links to prenatal stress, including autism which features social deficits, anxiety-like behaviors, and altered activity (Davis and Pfaff, 2014). Our results did not address the high rates of anxiety disorders in women and anxiety-related behavioral changes in female rodents related to prenatal stress. It is also important to note that we utilized behavioral time-in-zone analyses to avoid the confound of altered overall locomotion influencing assayed performance more broadly. The combination of prenatal stress-linked behavioral deficits described here did not appear to be directly linked to IL-6 related processes.
These findings indicated that behavioral outcomes of prenatal stress aligned with delays to GABAergic development in terms of their resistance to anti-IL-6 rescue. These behavioral outcomes did not align with pre- or postnatal microglia morphology, however, which were rescued by IL-6 blockade. Some groups have demonstrated other behavioral alterations after prenatal stress or MIA that have unclear relationships to GABAergic development, microglia, or IL-6, including suppressed neonatal ultrasonic vocalizations, impaired learning, and increased stress reactivity (Morgan et al., 1999; Mueller and Bale, 2007; Green et al., 2011; Shanks and Lightman, 2001; Ito et al., 2010; Malkova et al., 2012). Existing findings on the role of IL-6 and microglia in the mediation of behavioral phenotypes after prenatal inflammatory insult are mixed. Some have found, as shown here, an uncoupling of behavioral alterations and microglial activation: our IL-6-linked changes in microglia but not behavior are contrasted by other findings of behavioral phenotypes without microglia changes (Antonson et al., 2016). Others have shown anti-IL-6 mediation of the effects of prenatal poly(I:C) exposure, a more severe, one-time inflammatory event compared to repetitive prenatal stress, on exploratory behaviors and social deficits, among other outcomes (Smith et al., 2007, Wu et al., 2017). Our results are also in contrast with other maternal immune activation studies which have shown the importance of IL-6 for locomotion and social behavior (Choi et al., 2016), but used distinctly different time courses and magnitude of maternal perturbations. Because of these differences, further parsing of maternal physiological changes, discussed in more detail below, and their role in offspring development are necessary.
Microglial development and aberrant embryonic progenitor migration after prenatal stress were differentially affected by concurrent anti-IL-6 blockade in our data. Other maternal physiological stress factors, ranging from hormones to neurosteroids to cytokines, may impact GABAergic progenitor migration (Hirst et al., 2014; Squarzoni et al., 2014). We did not examine the multiple other individual factors, or their combinations, that might mediate stress effects on the developing brain. For example, glucocorticoids interact with developing GABAergic systems: inhibitory neuron death occurs after prenatal dexamethasone and deficits in GAD67 during development lead to higher corticosterone peaks after restraint stress (Uchida et al., 2011; Zuloaga et al., 2011). The neurosteroid and stress factor, allopregnanolone, is a GABAergic agonist and may play a role during maternal stress in cell survival in the embryonic brain (Yawno et al., 2009; Brunton and Russell, 2010). Other maternal cytokines, such as TNF-alpha, may also subserve the role IL-6 plays during neurogenesis, although the effects of these cytokines are likely dose-dependent (Borsini et al., 2015). Due to the complexity of pregnancy physiology, further investigations should parse multiple maternal physiological factors (e.g. weight gain, food intake, circulating hormones, and IL-6 levels across multiple tissues).
Additionally, neurodevelopmentally-relevant prenatal maternal factors may come to light from investigating the range of maternal stresses or challenges that can occur during pregnancy. Understanding other maternal physiological challenges (e.g. prenatal ethanol exposure, metabolic or psychosocial stress) will also elucidate additional offspring neurodevelopmental changes of interest (Brunton and Russell, 2010; Krakowiak et al., 2012; Skorput and Yeh, 2016). Many maternal manipulations, even with diffusible factors, alter cardiovascular function and other nonspecific physiology, which may play a role in offspring changes. Much remains to be understood about how prenatal maternal psychological stress and other stressors influence offspring neurodevelopmental outcomes, including GABAergic progenitor migration and changes to microglia morphology.
These data also suggest that some adult animal behavior, changed as a function of prenatal stress, may not be related to microglia morphology at E14 or in adulthood. Additionally, because the present findings show that prenatal stress significantly affected both anxiety-related behavior and GABAergic progenitor migration, altered migration and overall development of GABAergic neurons may underlie this behavioral phenotype. Indeed, our group has previously associated behavioral inhibition after prenatal stress with alterations in GABAergic maturation (Lussier and Stevens, 2016). Other reports suggest that inter-related aspects of GABAergic development—epigenetic changes in GABAergic genes—and their correction, are also correlated with behavioral changes after prenatal stress (Matrisciano et al., 2013; Negron-Oyarzo et al., 2016).
Here, we show that prenatal stress affected microglia morphology, both in the embryonic and adult offspring brain, and that these changes involved IL-6. However, prenatal stress changes to animal behavior and more acute delays to GABAergic system development were not mediated by IL-6. IL-6 pathways, and their impact on microglia, are thus critically involved in some components of prenatal maternal psychological stress, but not all. These findings, taken collectively with others, indicate that the multiple maternal physiological components during the prenatal period may be critical for the pathogenesis of offspring neuropsychiatric disorders.
Highlights.
Microglia and maternal IL-6 may be involved in the effects of prenatal stress.
Prenatal stress effects on embryonic and adult microglia involved prenatal IL-6.
GABAergic progenitor migration was delayed by prenatal stress and IL-6.
Prenatal stress behaviors did not show a role for prenatal IL-6.
Prenatal stress behaviors correlated more with GABAergic changes than with microglia.
Acknowledgments
The authors are grateful to the Patterson Trust Award Program in Clinical Research and NIMH K08 MH086812 for making this work possible. The authors would also like to thank the Vaccarino and Stevens Labs for their support and Dr. Mike Dailey for his microglia expertise. Note: Ms Rebecca Fine's current affiliation is Harvard University.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akatsu S, Ishikawa C, Takemura K, Ohtani A, Shiga T. Effects of prenatal stress and neonatal handling on anxiety, spatial learning and serotonergic system of male offspring mice. Neurosci Res. 2015;101:15–23. doi: 10.1016/j.neures.2015.07.002. [DOI] [PubMed] [Google Scholar]
- AL-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. Journal of Neuroinflammation. 2012;9:1. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson AM, Radlowski EC, Lawson MA, Rytych JL, Johnson RW. Maternal viral infection during pregnancy elicits anti-social behavior in neonatal piglet offspring independent of postnatal microglial cell activation. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.09.019. [DOI] [PubMed] [Google Scholar]
- Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89:286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp Neurol. 1995;136:183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Jakob H. Prenatal disturbances of nerve cell migration in the entorhinal region: a common vulnerability factor in functional psychoses? J Neural Transm Gen Sect. 1991;84:155–164. doi: 10.1007/BF01249120. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques. 2005;38:702–704. 706. doi: 10.2144/05385BM05. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr NE, Earle K, Tam C, Romero LM. The effect of chronic psychological stress on corticosterone, plasma metabolites, and immune responsiveness in European starlings. Gen Comp Endocrinol. 2007;154:59–66. doi: 10.1016/j.ygcen.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation. 2012;9:71. doi: 10.1186/1742-2094-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Dailey ME. Microglia: key elements in neural development, plasticity, and pathology. J Neuroimmune Pharmacol. 2013;8:494–509. doi: 10.1007/s11481-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. 2010 doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Weber-Stadlbauer U, Schedlowski M, Meyer U, Engler H. Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Behav Immun. 2016;55:25–38. doi: 10.1016/j.bbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Green MK, Rani CS, Joshi A, Soto-Pina AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Hirst JJ, Kelleher MA, Walker DW, Palliser HK. Neuroactive steroids in pregnancy: key regulatory and protective roles in the foetal brain. J Steroid Biochem Mol Biol. 2014;139:144–153. doi: 10.1016/j.jsbmb.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, Gonzalez-Maeso J. Prenatal stress induces schizophrenia-like alterations of serotonin 2A a nd metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. J Neurosci. 2013;33:1088–1098. doi: 10.1523/JNEUROSCI.2331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houri-Haddad Y, Itzchaki O, Ben-Nathan D, Shapira L. The effect of chronic emotional stress on the humoral immune response to Porphyromonas gingivalis in mice. J Periodontal Res. 2003;38:204–209. doi: 10.1034/j.1600-0765.2003.20390.x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, Vaccarino FM. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009;123:1102–1107. doi: 10.1542/peds.2008-1734. [DOI] [PubMed] [Google Scholar]
- Lussier SJ, Stevens HE. Delays in GABAergic Interneuron Development and Behavioral Inhibition after Prenatal Stress. Dev Neurobiol. 2016 doi: 10.1002/dneu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko DE, Putt M, Golden JA. SDF1 regulates leading process branching and speed of migrating interneurons. J Neurosci. 2011;31:1739–1745. doi: 10.1523/JNEUROSCI.3118-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Camacho J, Fox E, Miller E, Ariza J, Kienzle D, Plank K, Noctor SC, Water JVd. Prenatal Exposure to Autism-Specific Maternal Autoantibodies Alters Proliferation of Cortical Neural Precursor Cells, Enlarges Brain, and Increases Neuronal Size in Adult Animals. 2014 doi: 10.1093/cercor/bhu291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, Nicoletti F, Guidotti A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–194. doi: 10.1016/j.neuropharm.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Patterson PH. Neuroimmunology in Brain Development and Disease. Dev Neurobiol. 2012;72:1269–1271. doi: 10.1002/dneu.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Moazzam S, Hussain MM, Ahmad TA. Effect of chronic restraint stress on immune status of male Sprague Dawley rats. J Coll Physicians Surg Pak. 2013;23:487–490. [PubMed] [Google Scholar]
- Morgan KN, Thayer JE, Frye CA. Prenatal stress suppresses rat pup ultrasonic vocalization and myoclonic twitching in response to separation. Dev Psycho biol. 1999;34:205–215. doi: 10.1002/(sici)1098-2302(199904)34:3<205::aid-dev5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Mehdawi H. Toll-like receptor 4-mediated immune stress in pregnant rats activates STAT3 in the fetal brain: role of interleukin-6. Pediatr Res. 2016;79:781–787. doi: 10.1038/pr.2015.86. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Muraki K, Tanigaki K. Neuronal migration abnormalities and its possible implications for schizophrenia. Front Neurosci. 2015;9:74. doi: 10.3389/fnins.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Negron-Oyarzo I, Lara-Vasquez A, Palacios-Garcia I, Fuentealba P, Aboitiz F. Schizophrenia and reelin: a model based on prenatal stress to study epigenetics, brain development and behavior. Biol Res. 2016;49:16. doi: 10.1186/s40659-016-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina H, Sudo N, Aiba Y, Oyama N, Koga Y, Kubo C. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J Neuroimmunol. 2001;115:46–52. doi: 10.1016/s0165-5728(01)00260-0. [DOI] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakalan R, Jiang B, Nimmi B, Janani M, Jayapal M, Lu J, Tay SS, Ling EA, Dheen ST. Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neurosci. 2012;13:64. doi: 10.1186/1471-2202-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA. Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods. 1999;18:493–507. doi: 10.1006/meth.1999.0818. [DOI] [PubMed] [Google Scholar]
- Petitto JM, McCarthy DB, Rinker CM, Huang Z, Getty T. Modulation of behavioral and neurochemical measures of forebrain dopamine function in mice by species-specific interleukin-2. J Neuroimmunol. 1997;73:183–190. doi: 10.1016/s0165-5728(96)00196-8. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R, Bagri A, Marin O, Lowenstein DH, Rubenstein JL. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Ponzio NM, Servatius R, Beck K, Marzouk A, Kreider T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci. 2007;1107:118–128. doi: 10.1196/annals.1381.013. [DOI] [PubMed] [Google Scholar]
- Pratt L, Ni L, Ponzio NM, Jonakait GM. Maternal inflammation promotes fetal microglial activation and increased cholinergic expression in the fetal basal forebrain: role of interleukin-6. Pediatr Res. 2013;74:393–401. doi: 10.1038/pr.2013.126. [DOI] [PubMed] [Google Scholar]
- Ratajczak P, Kus K, Murawiecka P, Slodzinska I, Giermaziak W, Nowakowska E. Biochemical and cognitive impairments observed in animal models of schizophrenia induced by prenatal stress paradigm or methylazoxymethanol acetate administration. Acta Neurobiol Exp (Wars) 2015;75:314–325. [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 2010;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N, Lakehayli S, Battas O, Hakkou F, Tazi A. Effects of prenatal stress on anxiety-like behavior and nociceptive response in rats. J Integr Neurosci. 2015;14:223–234. doi: 10.1142/S0219635215500107. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez AM, Cuadros MA, Calvente R, Tassi M, Marin-Teva JL, Navascues J. Activation of immature microglia in response to stab wound in embryonic quail retina. J Comp Neurol. 2005;492:20–33. doi: 10.1002/cne.20676. [DOI] [PubMed] [Google Scholar]
- Sebire G, Emilie D, Wallon C, Hery C, Devergne O, Delfraissy JF, Galanaud P, Tardieu M. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J Immunol. 1993;150:1517–1523. [PubMed] [Google Scholar]
- Shanks N, Lightman SL. The maternal-neonatal neuro-immune interface: are there long-term implications for inflammatory or stress-related disease? J Clin Invest. 2001;108:1567–1573. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorput AG, Yeh HH. Chronic Gestational Exposure to Ethanol Leads to Enduring Aberrances in Cortical Form and Function in the Medial Prefrontal Cortex. Alcohol Clin Exp Res. 2016;40:1479–1488. doi: 10.1111/acer.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders S, Smolders SM, Swinnen N, Gartner A, Rigo JM, Legendre P, Brone B. Maternal immune activation evoked by polyinosinic:polycytidylic acid does not evoke microglial cell activation in the embryo. Front Cell Neurosci. 2015;9:301. doi: 10.3389/fncel.2015.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Smith KM, Rash BG, Vaccarino FM. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Su T, Yanagawa Y, Vaccarino FM. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology. 2013;38:509–521. doi: 10.1016/j.psyneuen.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Takaki A, Huang QH, Somogyvari-Vigh A, Arimura A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation. 1994;1:335–342. doi: 10.1159/000097185. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tamura M, Sajo M, Kakita A, Matsuki N, Koyama R. Prenatal stress inhibits neuronal maturation through downregulation of mineralocorticoid receptors. J Neurosci. 2011;31:11505–11514. doi: 10.1523/JNEUROSCI.3447-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tearne JE, Allen KL, Herbison CE, Lawrence D, Whitehouse AJO, Sawyer MG, Robinson M. The association between prenatal environment and children's mental health trajectories from 2 to 14 years. European Child & Adolescent Psychiatry. 2015;24:1015–1024. doi: 10.1007/s00787-014-0651-7. [DOI] [PubMed] [Google Scholar]
- Uchida T, Oki Y, Yanagawa Y, Fukuda A. A heterozygous deletion in the glutamate decarboxylase 67 gene enhances maternal and fetal stress vulnerability. Neurosci Res. 2011;69:276–282. doi: 10.1016/j.neures.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013;8:e58488. doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun. 2017;62:11–23. doi: 10.1016/j.bbi.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawno T, Hirst JJ, Castillo-Melendez M, Walker DW. Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience. 2009;163:838–847. doi: 10.1016/j.neuroscience.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]