Abstract
Prevention of orthopedic device related infection (ODRI) using antibiotics has met with limited amount of success and is still a big concern during post-surgery. As an alternative, use of silver as an antibiotic treatment to prevent surgical infections is being used due to the well-established antimicrobial properties of silver. However, in most cases silver is used in particulate form with wound dressings or with short-term devices such as catheters but not with load-bearing implants. We hypothesize that strongly adherent silver to load-bearing implants can offer longer term solution to infection in vivo. Keeping that in mind, the focus of this study was to understand the long term release study of silver ions for a period of minimum 6 months from silver coated surface modified porous titanium implants. Implants were fabricated using a LENS™ system, a powder based additive manufacturing technique, with at least 25% volume porosity, with and without TiO2 nanotubes in phosphate buffer saline (pH 7.4) to see if the total release of silver ions is within the toxic limit for human cells. Considering the fact that infection sites may reduce the local pH, silver release was also studied in acetate buffer (pH 5.0) for a period of 4 weeks. Along with that, the osseointegrative properties as well as cytotoxicity of porous titanium implants were assessed in vivo for a period of 12 weeks using a rat distal femur model. In vivo results indicate that porous titanium implants with silver coating show comparable, if not better, biocompatibility and bonding at the bone-implant interface negating any concerns related to toxicity related to silver to normal cells. The current research is based on our recently patented technology, however focused on understanding longer-term silver release to mitigate infection related problems in load-bearing implants that can even arise several months after the surgery.
Keywords: Load-bearing implants, osteomyelitis, Silver, infection control, additive manufacturing
Graphical abstract
Prevention of orthopedic device related infection using antibiotics has met with limited success and is still a big concern during post-surgery. Use of silver as an antibiotic treatment to prevent surgical infections is being explored due to the well-established antimicrobial properties of silver. However, in most cases silver is used in particulate form with wound dressings or with short-term devices such as catheters but not with load-bearing implants. We hypothesize that strongly adherent silver to load-bearing implants can offer longer-term solution towards infection in vivo. Keeping that in mind, the focus of this study was to understand the long-term release of silver ions, for a period of minimum 6 months, from silver coated surface modified porous titanium implants.
1.0 Introduction
Orthopedic implant failure as a result of infection is a growing concern commonly referred to orthopedic device related infection (ODRI). Although the estimated range is between 0.5 and 5% for incidents related to ODRI, the risk is increasingly becoming a serious concern with more patients undergoing implant surgeries like hip and knee joint replacements, fracture fixation devices, and trauma cases [1,2]. Often, bacterial infections can also lead to osteomyelitis, a condition affecting the bone or bone marrow area [3]. The occurrence of these infections is generally at the surgery site or at the site of implantation. If infected, the treatment for infection related to site of implantation is generally difficult and eventually results in undergoing revision surgeries if the antibiotics prove ineffective. A common way for treating infections is using antibiotics which has not been greatly effective over the years. Non-antibiotic remedies like chlorohexidine or nitrofurazone have also proved to be ineffective against some of these infections [4]. With increasing bacterial resistance against the antibiotics, use of silver coated medical devices, as an alternative measure, is proving to be an effective strategy, and is being considered seriously [5]. The use of silver as an antimicrobial agent dates to centuries where initially it was mainly used to prevent eye infections or as ointments to treat wounds [6–8]. Silver being biocidal in its ionic form, has a wide range of activity and a high chemotherapeutic ratio which is the ratio of toxic dose to its effective dose which makes it very effective [4,5]. Studies have shown use of silver in various forms such as ions, salts, metal itself and recent applications as nanoparticles loaded in titania nanotubes towards the prevention of bacterial infections [9,10].
Staphylococcus epidermidis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis and Enterobacter cloacae are some of the most common pathogens causing infection related issues resulting in failure of medical implants with Staphylococcus epidermidis and Staphylococcus aureus probably being the most common infection causing agents in orthopedic devices [11–14]. Significant research efforts have been devoted showing the antimicrobial effects of silver towards these infections causing pathogens [11–18]. Calcium phosphates like hydroxyapatite containing silver have shown to reduce the bacterial adhesion against Staphylococcus epidermidis, Pseudomonas aeruginosa and Staphylococcus aureus when compared to surfaces without silver [15–17]. Silver deposited on metallic surfaces such as titanium and stainless steel have also shown to have a toxic effect towards bacterial pathogens causing infections rather than the human cells within specified doses of silver [18–22]. Improved antibacterial efficacy has also been reported in silver-polymer composites, and even calcium phosphate and bioglass scaffolds containing silver [23–27]. Finally, antibacterial performance of titania (TiO2) nanotubes loaded silver nanoparticles have been reported showing prevention of bacterial adhesion without any cytotoxic effect to human cells [21,28–31]. Antimicrobial efficacy of silver coated megaprosthesis have also been reported showing no toxic effects against human [32,33]. With the antimicrobial nature of silver well established, FDA approved devices containing silver is already in use mostly in the form of catheters for short term applications and also as wound dressings [34–36]. The common pathways describing the antimicrobial nature are the affinity of silver ions towards Sulphur, oxygen and nitrogen forming silver salts results in disrupting the biochemical processes [37,38] and the interaction of silver ions with thiol and amino groups of proteins resulting in adverse effects [39–41], although the exact mechanism of silver resistance against bacteria is still debatable. The reported minimum inhibitory concentration (MIC) for various bacterial strains including Staphylococcus epidermidis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis and Enterobacter cloacae range from 0.2 to 20 µg/ml [20]. However, the minimum inhibitory concentration (MIC) limit for silver is 10 µg/ml (10ppm), the concentration below which it has been suggested to be effective and non-toxic for human cells [42,43].
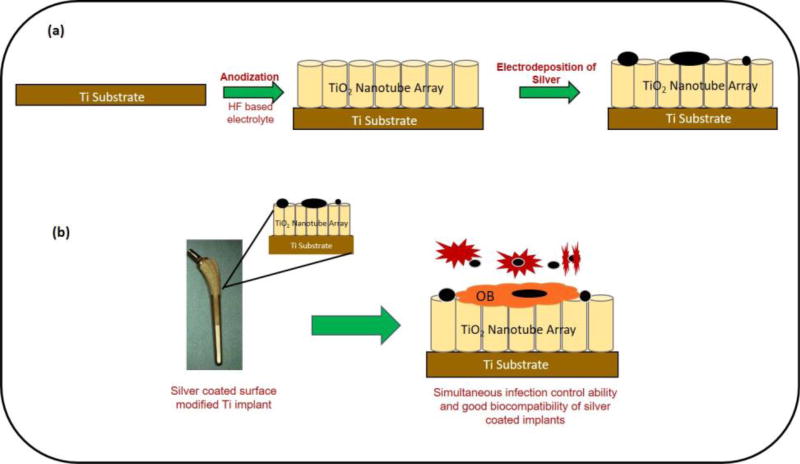
Porous titanium (Ti) are effectively used in orthopedic implants due to low effective modulus, good biocompatibility facilitating improved biological fixation and eventually improving the osseointegration between the implant and the bone [44,45]. Another technique to improve osseointegration of Ti implants is through nanoscale surface modifications in the form of TiO2 nanotubes [46]. Improved cell material interactions and bone bonding ability in vitro and in vivo have already been shown [47–49]. Also, good in vitro cell material interactions with no cytotoxic effects have also been observed for Ti surfaces with TiO2 nanotubes and silver [50,51]. With the antimicrobial nature of silver well established, there is still a knowledge gap that exists related to longer-term silver release from implant surface. And to our knowledge, most of the studies showing the release of silver ions concerning its toxicology and antimicrobial properties have been performed for a period of 2 to 4 weeks [52–54]. Keeping that in mind, the first aim of our research was to understand if silver ions can be released from the implants, where particulate silver is strongly adherent to the implant surface? The second aim was to understand the rate of silver release as a function of pH of the medium. The third aim was to measure if silver release rate can be controlled by tailoring the initial silver amount on the surface. And finally, our fourth aim was to study osseointegration properties of silver in vivo using porous titanium implants to determine if silver coating on TiO2 nanotubes can influence bone bonding ability due to inherent cytotoxicity of silver ions. We hypothesize that strongly adherent silver to load-bearing implants can offer longer term solution to infection in vivo along with good biocompatibility, and bonding at the bone-implant interface without any concerns related to the toxicity to bone cells. And to validate our hypothesis, long term silver release study was performed for a period of minimum 6 months using phosphate buffer saline (PBS) maintained at pH 7.4, and acetate buffer at pH 5 for 4 weeks to understand and measure if the cumulative release is within the toxic limit mentioned for human body. In vivo study was performed for 12 weeks in a rat distal femur model using male Sprague-Dawley rats. Histological analysis and SEM characterization was performed for in vivo samples whereas the silver ion concentrations were measured using Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS). The novelty of our work is not to show the antimicrobial properties of silver as it has already been well established, but to evaluate if silver coated load bearing implants can offer a long-term solution due to its antimicrobial properties in controlling infection in vivo at the surgery site along with maintaining good biocompatibility, improved osseointegrative properties at the implantation site without any toxic effects. Figure 1 represents a schematic layout demonstrating the research and novelty of this work. As shown in Fig. 1, strongly adherent silver should be effective in vivo towards infection control while TiO2 nanotubes will enhance biocompatibility. Together a simultaneous impact of enhanced early stage osseointegration and anti-microbial property via innovative surface modification approach. This work is based on our recent patent, US Patent 8,545,559 [46].
Figure 1.
A schematic showing fabrication steps of silver coated surface modified Ti implants and its simultaneous infection control ability as well as enhanced osseointegrative properties for load-bearing implants.
2.0 Materials and Methods
2.1 Processing of porous titanium samples
Porous titanium (Ti) rods of diameter 3mm were fabricated using a commercial laser engineered net shaping (LENS™, Optomec Inc., Albuquerque, NM USA) system, a powder based additive manufacturing method. LENS™ motion control software system was fed with the CAD design of the porous rod which converted it into a tool path file. The build jobs for LENS™ are controlled using parameters such as feeding rate of the powder, laser power and raster scan speed. Commercially pure titanium (cp-Ti) powder (ATI Powder Metals, Pittsburgh, PA, USA) of 99.99% purity and spherical particle size between 44–149 µm was used for processing the Ti rod on a cp-Ti substrate (President Titanium, Hanson, MA, USA). A detailed description of fabricating the porous Ti rods using LENS™ can be found elsewhere [50]. Porous cp-Ti rods were processed with 25 volume% porosity by varying different LENS™ parameters such as the powder feed rate, raster scan speed and laser power. The final processing parameters to obtain a 25 volume% porosity, which was measured using the Archimedes method were- laser power of 280W, raster scan speed between 60 and 80 cm/min and a powder feed rate of 20 g/min [50]. After LENS™ processing, rods were cut to necessary lengths for in vivo testing, and its outer layers were ground using silicon carbide papers ranging from 500 to 1000 grit. Samples were later cleaned ultrasonically in ethanol for 15 minutes then rinsed in deionized (DI) water and finally air dried to remove loose metal powders.
2.2 Fabrication of TiO2 nanotubes and electrodeposition of silver particle
Electrochemical anodization method was used to grow TiO2 nanotubes on porous Ti rods. The setup for anodization involved 1% hydrofluoric (HF) acid in deionized (DI) water as electrolyte, porous cp-Ti rods and platinum being anode and cathode respectively which were suspended using platinum wires. A DC power supply (Hewlett Packard 0–60 V/0–50 A, 1000 W) was used to apply a constant voltage of 20V throughout the process. Electrodeposition of silver was performed using the same setup with platinum foil as anode and porous Ti rods as cathode using a 0.1M silver nitrate (AgNO3) solution. For silver deposition, a DC current was applied at a constant voltage of 3V for 30 seconds and 3 minutes for the two-different amount of silver loading. Excess silver was washed using DI water after deposition followed by heat treatment of the coated samples at 500° C. Percent area coverage of the silver coating was measured using ImageJ software.
2.3 Long term silver release study
Long-term silver release study was performed to measure its release kinetics. A detailed outline of composition, number of samples and medium for the release study experiments is provided in Table 1. Silver release study was performed with two mediums, phosphate buffer saline (PBS-pH 7.4) and acetate buffer (pH 5.0). The total duration of the experiment was 27 weeks in PBS medium and 4 weeks in acetate buffer. For 27-weeks study, the measurement time-points were 1, 3, 5, 7, 14, 21, 28, 35, 42. 49, 56, 63, 70, 77, 84, 01, 98, 105, 175 and 189 days. Two different loading rates, based on the silver deposition time, were used - loading 1 and loading 2. Two different silver loading rates were used to study the effect, if any, of varying the silver deposition times over the release of silver ions from the surface. The deposition was performed with 0.1M AgNO3 solution at a constant voltage of 3V at room temperature. Samples were then placed in an orbital shaker maintained at a constant temperature of 37°C, and fresh medium was added after every time point. At the end of the study, all solutions were analyzed for silver ions using an Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS, Agilent model 7700 ICP-MS, Santa Clara, CA, USA). Calibration of the machine was performed using of known silver concentrations of 10 ppb, 50 ppb and 100 ppb. All samples after 27 weeks were also analyzed under FESEM (FEI Quanta 200, FEI Inc., OR, USA) at an operating voltage of 20kV to measure the presence of silver particles on the porous cp-Ti surface after the release study.
Table 1.
Experimental details for long-term silver release study using two loading rates and two different mediums. The end point for release study in PBS medium was 27 weeks whereas in acetate buffer was 4 weeks.
| Composition | Number of Samples |
Condition | Medium |
|---|---|---|---|
| Dense Ti (control) | 2 | Loading rate 1 | PBS (pH 7.4) |
| 2 | Loading rate 2 | Acetate Buffer (pH 5) | |
| PBS (pH 7.4) | |||
| Dense Ti-Ag | 3 | Loading rate 1 | Acetate Buffer (pH 5) |
| PBS (pH 7.4) | |||
| 3 | Loading rate 2 | Acetate Buffer (pH 5) | |
| PBS (pH 7.4) | |||
| LENS porous Ti-Ag | 3 | Loading rate 1 | Acetate Buffer (pH 5) |
| PBS (pH 7.4) | |||
| 3 | Loading rate 2 | Acetate Buffer (pH 5) | |
| PBS (pH 7.4) | |||
| LENS porous Ti-NT-Ag | 3 | Loading rate 1 | Acetate Buffer (pH 5) |
| PBS (pH 7.4) | |||
| 3 | Loading rate 2 | Acetate Buffer (pH 5) | |
| PBS (pH 7.4) | |||
| Acetate Buffer (pH 5) |
2.4 In vivo study
2.4.1 Surgery and implantation procedure
For the in vivo study, 6 male Sprague-Dawley rats weighing approximately 300g were used. Bilateral surgeries were performed on all 6 rats, and Table 2 lists the experimental details on number of implants with their respective compositions used for the in vivo study. Implant dimensions were 3mm in diameter and 5.5mm in length. For silver deposition on implants, loading rate 1 was used for deposition. Rats were housed individually in rooms with adequate humidity and temperature controlled environment prior to surgery for acclimatization. Rats were anesthetized with IsoFlo® (isoflurane, USP, Abbott Laboratories, North Chicago, IL, USA) along with the sufficient supply of oxygen controlled using a regulator. During surgery, a defect similar to the diameter of the implant was created using a drill bit in the distal femur of the rat and using the saline solution the defect cavity was washed to make sure no bone fragments remained which was followed by insertion of the implants into the defect site. Synthetic absorbable surgical suture was used to close the incision site following the surgery. Betadine solution as a disinfectant was applied at the surgery site to prevent any form of post-surgery infection. Meloxicam in the form of subcutaneous injection was given following surgery for pain reduction [50]. Rats were euthanized at the end of their time point by overdosing the bell jar with isoflurane. Experimental and surgical procedures were performed according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Washington State University (Pullman, WA).
Table 2.
Surgery details for the in vivo study using a rat distal femur model.
| Composition | Time Point | # of rats | Tests Performed |
|---|---|---|---|
| Dense Cp-Ti (Control-Both left and right femur) | 12 weeks | 1 | Histology and SEM characterization. |
| LENS Porous Cp-Ti (Left Femur) | 12 weeks | 2 | |
| LENS Porous Cp-Ti-NT (Right Femur) | |||
| LENS Porous Cp-Ti-NT (Left Femur) | 12 weeks | 3 | |
| LENS Porous Cp-Ti-NT-Ag (Right Femur) |
2.4.2 Histology and SEM characterization
Post necropsy, bone-implant samples were fixed using 10% formalin solution for histological evaluation. After fixing, the samples were then dehydrated using ethanol in increasing concentrations (70%, 95% and 100%), followed by a mixture of 1:1 ethanol-acetone and finally 100% acetone. After completing the dehydration process, samples were embedded in 1:1 acetone-spurrs mixture, followed by 100% spurrs resin embedding for a couple of days and then cured at 70°C. After curing, the spurrs embedded bone-implant samples were cut into thin sections which were mounted on glass slides and then stained using modified Masson Goldner’s trichrome staining method and were observed under light microscope (Olympus BH-2, Olympus America Inc., USA) for osteoid (new bone) formation [50]. To get a better idea about the interfacial bonding between the implant and the surrounding tissue area, the stained samples were characterized under FESEM (FEI Quanta 200, FEI Inc., OR, USA) maintained at low operating voltage of 10 kV and run under low vacuum.
2.5 Statistical Analysis
A repeated measure analysis was run in PROC GLIMMIX in SAS with 3 replicates observed over 20 timepoints for each composition. Our analysis yielded a significant (p-value<0.0001) time, treatment interaction. The results show a 95% confidence for all plots. The treatment effects were followed up using a Turkey adjustment which also showed to be significant (p-value<0.0001). Normality and equal variance were checked using diagnostic plots, which did not affect the significance for the distribution of the plots.
3.0 Results
3.1 Characterization of TiO2 nanotubes and silver coating on porous titanium surface
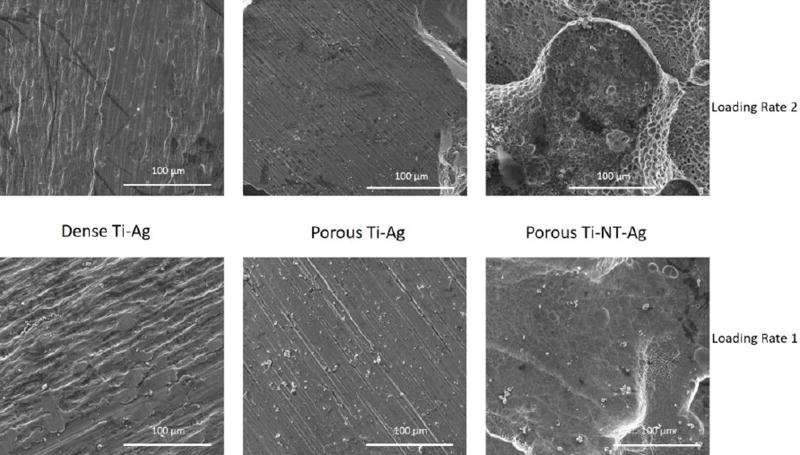
Figure 2 shows SEM images of LENSTM processed porous titanium surface of 25 volume% (Fig. 2a), with silver coating on the surface of nanotube arrays (Fig. 2b), and TiO2 nanotube arrays with diameter of 105nm ± 30nm, and length of 375 ± 35nm (Inset-Fig. 2c). Nanotube dimensions were measured using the ImageJ software. Care was taken to obtain the silver coating in the nanometer range by wiping of the excess loosely deposited large silver particles on the surface. Percent area coverage of silver deposition on surface was measured using ImageJ software based on Fig. 3 for silver coated porous Ti samples with nanotubes only for both loading rates. The results showed a coverage of 13.5% of silver particles on the surface of TiO2 nanotubes for loading rate 1, and 26.4% for loading rate 2.
Figure 2.
SEM images of (a) porous Ti surface, (b) nanotubes with silver deposition and (c) with nanotubes and silver deposition at a higher magnification.
Figure 3.
SEM images of samples with silver deposition with loading rate 1 (Figures 2(a–c)) and loading rate 2 (Figures 2(d–f)).
3.2 Silver release study
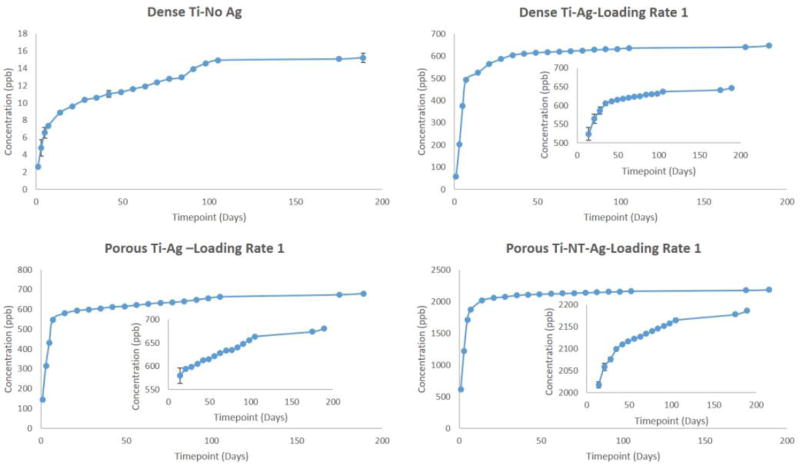
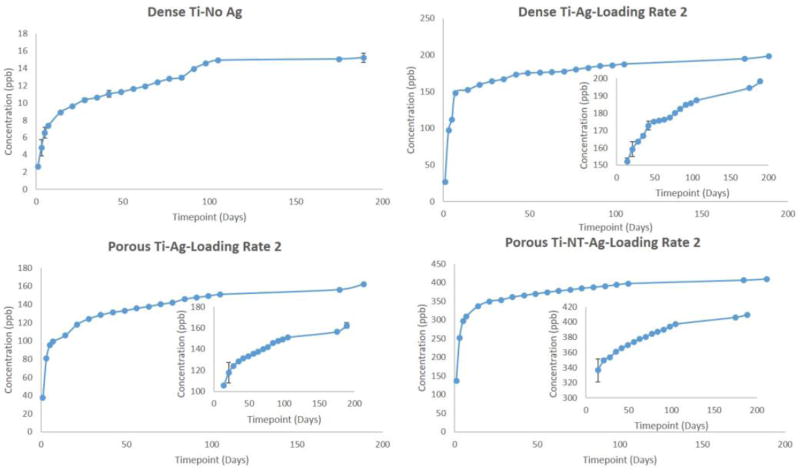
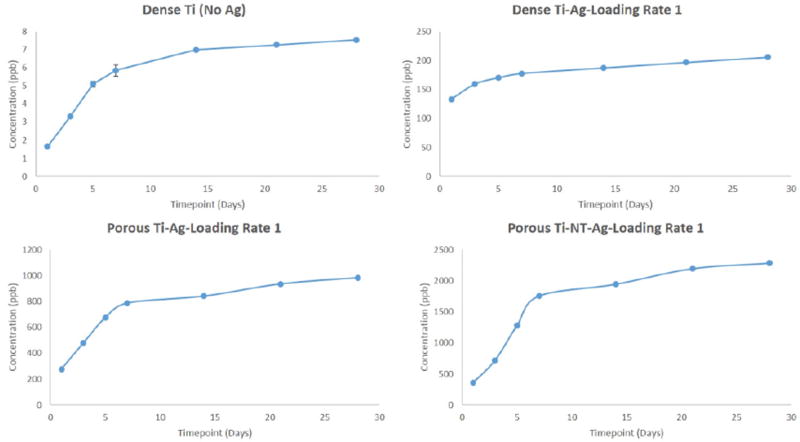
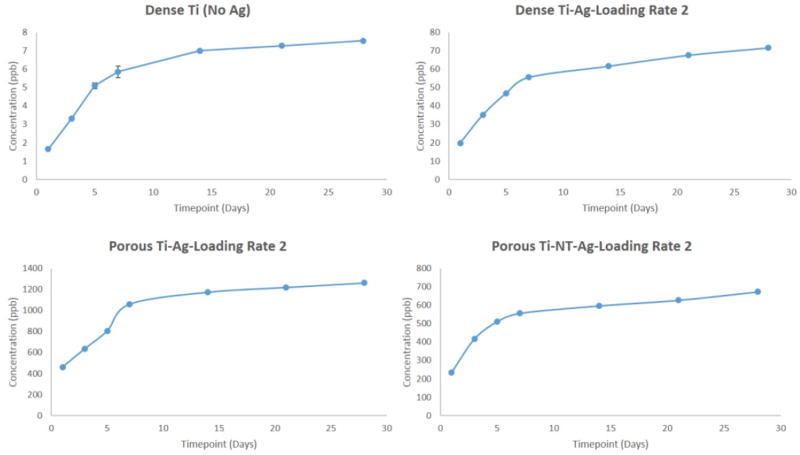
To understand the long-term release kinetics of silver, two different mediums namely phosphate buffer saline (PBS) and acetate buffer were used. PBS was used due to its similarity in ion concentrations with that of the human body and acetate buffer was used for its acidic nature which is the case during the healing of a wound or infection site in the body that can see a lower pH. Two different deposition rates were utilized to understand the effects of initial silver amount on rate of silver release from the surface. Figure 3 shows SEM images of silver deposited samples using two different loading rates for dense Ti, porous Ti and porous Ti with TiO2 nanotubes, respectively. Figures 4 and 5 show the cumulative release profiles for dense Ti with no silver which was used as control, dense Ti with silver, silver deposited porous Ti with no nanotubes, and porous Ti with nanotubes and silver in PBS (pH 7.4) after 27 weeks for both the loading rates, with an inset graph showing the gradual continuous release of silver ions at latter time points. A small amount, in the ppb range, of silver ions was detected for all solutions with dense Ti with no silver which indicates the limits of detection. The cumulative release profiles indicate the total silver ion release is well within the potential toxic limit mentioned for human cells which is 10 ppm (µg/ml). Also, the release of silver ions is more for porous samples as compared to the dense samples which is expected due to more silver deposition due to the presence of porous surfaces. Similar release profiles were observed for samples in acetate buffer with cumulative release of silver ions well below the potential toxic limit for human cells as shown in Figures 6 and 7 for both the loading rates. Figures 8 and 9 show SEM images of the surfaces of the silver released samples demonstrating the presence of silver particles even after 27 weeks in the media. Those images confirm that further silver release from those surfaces is still possible.
Figure 4.
Cumulative release profiles of silver ions from all samples in PBS medium at the end of 27 weeks using silver loading rate 1 with inset graphs showing continuous release of silver ions at latter time points. (Error bar values are very low and hence not properly visible for all time points although they are there for all time points; n=3)
Figure 5.
Cumulative release profiles of silver ions from all samples in PBS medium at the end of 27 weeks using silver loading rate 2 with inset graphs showing continuous release of silver ions at latter time points. (Error bar values are very low and hence not properly visible for all time points although they are there for all time points; n=3)
Figure 6.
Cumulative release profiles of silver ions from all samples in acetate buffer medium at the end of 4 weeks using silver loading rate 1. (Error bar values are very low and hence not properly visible for all time points; n=3).
Figure 7.
Cumulative release profiles of silver ions from all samples in acetate buffer medium at the end of 4 weeks using silver loading rate 2. (Error bar values are very low and hence not properly visible for all time points; n=3).
Figure 8.
SEM images of the surfaces of the samples showing the presence of silver particles after the end of 27 weeks in PBS medium.
Figure 9.
SEM images of the surfaces of the samples showing the presence of silver particles after the end of 4 weeks in acetate buffer medium.
3.3 Histological evaluation and SEM characterization
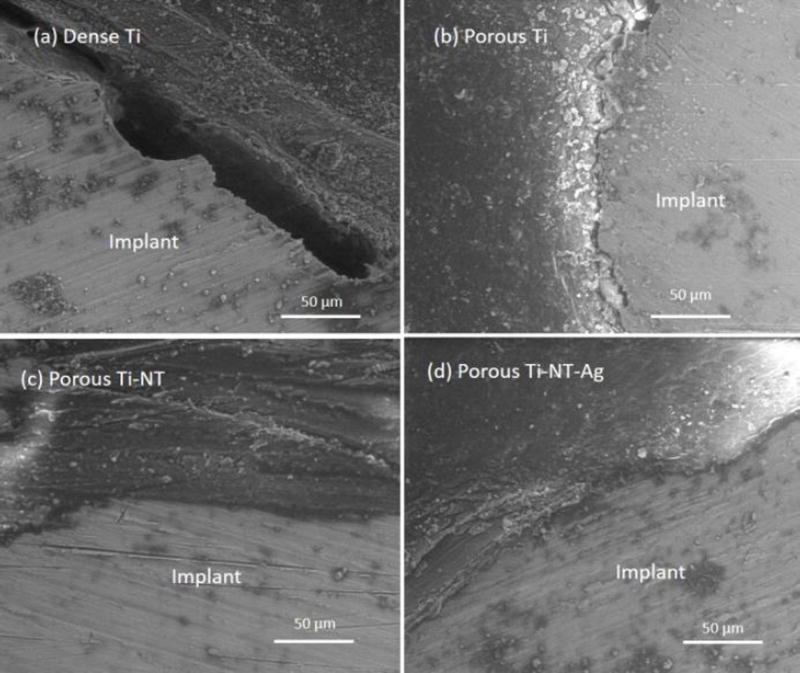
Histological evaluation was performed to understand osseointegrative properties of porous samples with silver coating, and also the cytotoxic effects, if any, due to silver coating as compared to uncoated samples. We hypothesized that addition of TiO2 nanotubes will further improve the bonding around the bone-implant area. Figure 10 shows histological evaluation of all samples after 12 weeks. First, silver deposition did not result in any cytotoxic effects as osteoid formation in orange reddish color could be seen around the implant. Also, higher amount of osteoid formation could be seen around the implant area as compared to other samples, which confirms osseointegrative properties with the implants. Moreover, less gaps around the implant area could be seen for samples with silver than without. Better understanding could be obtained from Figure 11, which shows SEM images for all samples indicating better bonding between implants and bone-tissue area. Porous Ti sample with nanotubes and silver coating shows good bonding with no gaps compared to porous sample with only nanotubes indicating excellent osseointegration and no cytotoxic effects due to the presence of silver particles on the surface.
Figure 10.
Photomicrographs showing the histological analysis after 12 weeks where signs of osteoid like new bone formation could be seen in orange/ red color. Modified Masson Goldner’s trichrome staining method was used.
Figure 11.
SEM images of stained samples after 12 weeks showing the interfacial bonding between the implant and the bone tissue.
4.0 Discussion
Porous titanium (Ti) implants have been preferred as a coating on orthopaedic implants due to good biocompatibility, excellent corrosion resistance and strong bonding with the dense implant. Porosity at the outer surface helps in improving the bonding between the implant and surrounding bone thereby increasing the in vivo life of the implants [44]. Porous Ti implants were fabricated using the LENS™, a powder based additive manufacturing system, with 25 volume% porosity. Porosity in the range of 20–30% is a minimum requirement for the implants to be effective by mimicking the properties of human bone along with a pore size greater than 200µm as it helps in reducing the stiffness between the bone and the implant thereby minimizing the mismatch between them [44, 45]. TiO2 nanotube arrays with diameter of 105nm ± 30nm and length of 375nm ± 35nm were fabricated on the LENS™ processed porous Ti surfaces using electrochemical anodization method with 1% HF in DI water as the electrolyte. LENS™ processing parameters were decided based on results from our previous work [53]. Interconnected porosity and nanoscale surface modification enhances bone-tissue integration and promotes early stage osseointegration thereby improving mechanical properties and interfacial bonding between bone and implant [49, 50]. Concerns related to post-surgical infections still remains in load-bearing implants mainly at the surgery site or site of implantation. Antimicrobial remedies have not been completely successful over the years, which has led to the possibility of using silver as an antimicrobial agent. Silver being biocidal in nature has proved to be effective when used under appropriate doses. The common pathways describing the antimicrobial nature are the affinity of silver ions towards Sulphur, oxygen and nitrogen forming silver salts results in disrupting the biochemical processes [37,38], and the interaction of silver ions with thiol and amino groups of proteins resulting in adverse effects [39–41].
Osteomyelitis, a condition caused due to bacterial infection affecting the bone tends to arise even after several weeks to months post-surgery [3]. Constant release of antimicrobial agent at the implantation site for several weeks to months is required at appropriate doses in order to prevent any sort of infection or further complication arising at the surgery/implantation site. Silver has been proved to be effective as an antimicrobial agent against the bacterial pathogens causing osteomyelitis [11–22]. Keeping the antimicrobial properties of silver in mind, the main objective of this study was to understand the long-term release study of silver ions from the surface modified implants with and without nanotubes in phosphate buffer saline (pH 7.4). Since there is a change in the pH at the site of infection which reduces the pH and generally makes it acidic in nature, silver release kinetics was also measured in acetate buffer (pH 5.0) for a period of 4 weeks. Another important aspect that was observed during this study was if silver release rate can be controlled by tailoring the initial silver amount on the surface. And finally, the osseointegrative properties of porous titanium implants with and without silver was assessed for its biocompatibility as well as cytotoxicity in vivo for 12 weeks using a rat distal femur model. We hypothesize that strongly adherent silver coatings on porous titanium implants have the ability for long-term sustained action along with good osseointegrative properties in vivo helping in prevention of implantation failure arising due to potential toxic effects. This research mainly aims at demonstrating our recently patented approach, US patent # 8545559, to show effects of antimicrobial silver coating on surface modified porous load-bearing implants towards the promotion of tissue integration post-surgery while preventing any cytotoxic effects [46].
Silver was electrodeposited on the surface of titanium implants with two different loading rates based on the deposition times. Aim was to obtain the coating in nanoparticulate range, but due to the uneven surface nature of the porous implants, the resultant coating resulted from nanoscale to micron scale. Two different mediums, phosphate buffer saline (PBS) maintained at pH 7.4 and acetate buffer maintained at pH 5 were used. PBS was used due to the similarity in ion concentrations with that of the human body, and acetate buffer was used due to its acidic pH which tends to be the nature of the environment at the infected site. It could be seen from the release profiles that the cumulative release of silver ions in PBS is well within the potential toxic limit mentioned for the human cells which is 10 µg/ml (10ppm) [42,43]. An initial burst release can be seen during the initial time points for all silver profiles with gradual stabilization with time, generally observed in all silver release profiles. This can be seen due to the rapid leaching of silver ions leading to its initial accumulation. The stabilization times vary depending on various factors such as experimental conditions, particle size, concentration, pH gradient and aggregation kinetics. The slow stabilization times is generally observed due to the absence of complexing agents such as Cl− or S− in pH solutions ≤ 8 [56,57]. Cumulative release of silver ions in acetate buffer is also within the potential toxic limit, however a faster release is observed due to the low pH of the solution, which results in higher dissolution of silver particles [58]. Steady and prolonged release of silver ions is observed, a minimum requirement for the coatings to be effective [59]. The presence of silver particles on porous cp-Ti implants even after 27 weeks (more than 6 months) shows the long-term effectiveness of the silver coatings. The release of silver ions observed from porous surfaces, especially surfaces with nanotubes, is higher as compared to the release of silver ions from the dense surfaces. A possible explanation for higher release from porous surfaces could be due to the porous nature which could result in accumulation of silver particles into the pores resulting into higher release. Surface properties also play an important role in the release kinetics. The porous morphology of nanotubes increases the surface area resulting in higher deposition of silver particles onto the surface of TiO2 nanotubes. Another important observation that needs to be noticed is the effects of initial silver concentration on the silver release kinetics.
In vivo study using rat distal femur model was performed to measure bone-tissue integration at the bone- implant interface after 12 weeks to understand the effects of silver coating towards early stage osseointegration. Histological evaluations revealed better osteoid like new bone formation in the form of orange reddish color around the implant area for porous samples with nanotubes and with silver coating than other samples. No cytotoxic effects due to the presence of silver coating was observed as the osteoid formation could be nicely seen around the bone-implant interface for silver coated implants. To better understand the interfacial bonding between the implant and the surrounding bone, SEM characterization of stained samples were performed that showed improved bonding between porous samples with nanotubes and silver. No gaps in any form could be observed in porous samples with nanotubes and silver coating, which further signifies the importance of nanotubes and silver coating. TiO2 nanotubes in general are known to have better surface wettability properties, which results in better bonding between the bone and the implant [49]. Silver coatings on the surface of TiO2 nanotubes have also shown good surface wettability with results shown to decrease the contact angles further resulting an increase in surface energy i.e., better biocompatibility. Thus, along with good antimicrobial properties, silver coatings on surface modified porous titanium implants have shown goods signs of early stage osseointegration with no cytotoxic effects.
5.0 Conclusions
Porous titanium implants, having 25 volume% porosity, were fabricated using LENS™ system. TiO2 nanotubes with diameter of 105nm ± 30nm and length of 375nm ± 35nm were fabricated on porous titanium implants using electrochemical anodization method. Silver particles were electrodeposited onto the porous titanium implants using two different loading rates by varying the deposition time. Long term efficacy of these coatings was studied for a period of minimum 6 months (27 weeks) using PBS medium and for 4 weeks using acetate buffer. Cumulative release of silver ions in both the mediums, and for both the loading rates, were well within the potential toxic limit of 10 µg/ml (10ppm) specified for human cells. Osseointegrative properties of silver coating on porous titanium implants was assessed using an in vivo rat distal femur model for 12 weeks. In vivo results indicate good interfacial bonding between the implant and the surrounding bone-tissue area for porous titanium implants with TiO2 nanotubes and silver coating similar to the porous implants with only nanotubes. Based on the results it can be concluded that porous titanium implants with strongly adherent silver coatings have good long term efficacy. Also, no cytotoxic effects were observed irrespective of the pH of the medium or due to the change in the initial deposition conditions of the silver coating. Finally, porous titanium implants with particulate silver coatings show good osseointegrative properties along with its well established antimicrobial ability.
Acknowledgments
Authors would like to acknowledge financial support from the Washington State University Gap Fund from the Office of Commercialization. Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR067306-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Authors would also like to acknowledge guidance towards statistical analysis from Prof. Nairanjana (Jan) Dasgupta of Department of Mathematics at Washington State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsaras G, Osmon DR, Mabry T, Lahr B, St Sauveur J, Yawn B, Kurland R, Berbari EF. Incidence, secular trends, and outcomes of prosthetic joint infection: a population-based study, olmsted county, Minnesota, 1969–2007. Infect Control Hosp Epidemiol. 2012;33:1207–12. doi: 10.1086/668421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poultsides LA, Liaropoulos LL, Malizos KN. The socioeconomic impact of musculoskeletal infections. J Bone Joint Surgery [Am] 2010;92:e13. doi: 10.2106/JBJS.I.01131. [DOI] [PubMed] [Google Scholar]
- 3.Haas DW, McAndrew MP. Bacterial osteomyelitis in adults: Evolving considerations in diagnosis and treatment. Am J Med. 1996;101(5):550–561. doi: 10.1016/s0002-9343(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 4.Thefreelibrary.com/Antimicrobial silver in orthopedic and wound care products (accessed July 2016)
- 5.Schierholz J, Lucas L, Rump A, Pulverer G. Efficacy of silver coated medical devices. J Hosp Inf. 1998;40:257–62. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 6.Alexander JW. History of the medical use of silver. Surgical infections. 2009;10(3):289–292. doi: 10.1089/sur.2008.9941. [DOI] [PubMed] [Google Scholar]
- 7.Maillard JY, Hartemann P P. Silver as an antimicrobial: facts and gaps in knowledge. Crit Rev Microbiol. 2013;39:373–83. doi: 10.3109/1040841X.2012.713323. [DOI] [PubMed] [Google Scholar]
- 8.Klasen HJ. Burns. 2000;26:117. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 9.Moriarty TF, Kuehl R, Coenye T, Metsemakers W-J, Morgenstern M, Schwarz EM, Riool M, Zaat SAJ, Khana N, Kates SL, Richards RG. Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Reviews. 2016;1(4):89–99. doi: 10.1302/2058-5241.1.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angewandte Chemie International Edition. 2013;52(6):1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2(4):176–194. doi: 10.4161/biom.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goel SC. Infection following implant surgery. Indian Journal of Orthopaedics. 2006;40(3):133. [Google Scholar]
- 13.Khosravi AD, Ahmadi F, Salmanzadeh S, Dashtbozorg A, Abasi Montazeri E. Study of bacteria isolated from orthopedic implant infections and their antimicrobial susceptibility pattern. Research Journal of Microbiology. 2009;4(4):158–163. [Google Scholar]
- 14.Mah TFC, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends in microbiology. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 15.Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S. Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS applied materials & interfaces. 2012;4(3):1341–1349. doi: 10.1021/am201610q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Liu Y, Courtney HS, Bettenga M, Agrawal CM, Bumgardner JD, Ong JL. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials. 2006;27(32):5512–5517. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Fielding GA, Roy M, Bandyopadhyay A, Bose S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta biomaterialia. 2012;8(8):3144–3152. doi: 10.1016/j.actbio.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosetti M, Masse A, Tobin E, Cannas M. Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials. 2002;23(3):887–892. doi: 10.1016/s0142-9612(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;91(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 20.DeVasConCellos P, Bose S, Beyenal H, Bandyopadhyay A, Zirkle LG. Antimicrobial particulate silver coatings on stainless steel implants for fracture management. Materials Science and Engineering: C. 2012;32(5):1112–1120. doi: 10.1016/j.msec.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roguska A, et al. Evaluation of the Antibacterial Activity of Ag-Loaded TiO2 Nanotubes. European Journal of Inorganic Chemistry. 2012;32:5199–5206. [Google Scholar]
- 22.Necula BS, Fratila-Apachitei LE, Zaat SA, Apachitei I, Duszczyk J. In vitro antibacterial activity of porous TiO 2–Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomaterialia. 2009;5(9):3573–3580. doi: 10.1016/j.actbio.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26(14):2081–2088. doi: 10.1016/j.biomaterials.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Hoover S, Tarafder S, Bandyopadhyay A, Bose S. Silver Doped Resorbable Tricalcium Phosphate Scaffolds for Bone Graft Applications. Materials Science and Engineering C. 2017;79:763–769. doi: 10.1016/j.msec.2017.04.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vimala K, Mohan YM, Sivudu KS, Varaprasad K, Ravindra S, Reddy NN, Padma Y, Sreedhar B, MohanaRaju K. Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application. Colloids and Surfaces B: Biointerfaces. 2010;76(1):248–258. doi: 10.1016/j.colsurfb.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Jones JR, Ehrenfried LM, Saravanapavan P, Hench LL. Controlling ion release from bioactive glass foam scaffolds with antibacterial properties. Journal of Materials Science: Materials in Medicine. 2006;17(11):989–996. doi: 10.1007/s10856-006-0434-x. [DOI] [PubMed] [Google Scholar]
- 27.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25(18):4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim Y-K, Lee Y-S, Jeong DH, Cho M-H. Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2007;3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32(24):5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Li Y, Huo K, Gao B, Xiong W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. Journal of Biomedical Materials Research Part A. 2014;102(10):3488–3499. doi: 10.1002/jbm.a.35019. [DOI] [PubMed] [Google Scholar]
- 31.Gao A, Hang R, Huang X, Zhao L, Zhang X, Wang L, Tang B, Ma S, Chu PK. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35(13):4223–4235. doi: 10.1016/j.biomaterials.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 32.Hardes J, Ahrens H, Gebert C, Streitbuerger A, Buerger H, Erren M, Gunsel A, Wedemeyer C, Saxler G, Winkelmann W, Gosheger G. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials. 2007;28(18):2869–2875. doi: 10.1016/j.biomaterials.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Goshegera G, Hardesa J, Ahrensa H, Streitburgera A, Buergerb H, Errenc M, Gunseld A, Kemperd FH, Winkelmanna W, von Eiffe C. Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomaterials. 2004;25(24):5547–5556. doi: 10.1016/j.biomaterials.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Eby DM, Luckarift HR, Johnson GR. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS applied materials & interfaces. 2009;1(7):1553–1560. doi: 10.1021/am9002155. [DOI] [PubMed] [Google Scholar]
- 35.Pollini M, Paladini F, Catalano M, Taurino A, Licciulli A, Maffezzoli A, Sannino A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. Journal of Materials Science: Materials in Medicine. 2011;22(9):2005–2012. doi: 10.1007/s10856-011-4380-x. [DOI] [PubMed] [Google Scholar]
- 36.Thomas S, McCubbin R. An in vitro analysis of the antimicrobial properties of 10 silver-containing dressings. Journal of wound care. 2003;12(8):305–308. doi: 10.12968/jowc.2003.12.8.26526. [DOI] [PubMed] [Google Scholar]
- 37.Olson ME, Harmon BG, Kollef MH. Silver-coated endotracheal tubes associated with reduced bacterial burden in the lungs of mechanically ventilated dogs, CHEST Journal. 2002;121(3):863–870. doi: 10.1378/chest.121.3.863. [DOI] [PubMed] [Google Scholar]
- 38.Brutel de la Riviere A, Dossche KM, Birnbaum DE, Hacker R. First clinical experience with a mechanical valve with silver coating. J Heart Valve Dis. 2000;9(1):123–9. discussion 129–30. [PubMed] [Google Scholar]
- 39.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of biomedical materials research. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Lansdown ABG. Silver in healthcare: its antimicrobial efficacy and safety in use. 6. Royal Society of Chemistry; 2010. [Google Scholar]
- 41.Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology. 2010;4(3):319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, de Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. International journal of antimicrobial agents. 2009;34(2):103–110. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Hidalgo E, Domınguez C. Study of cytotoxicity mechanisms of silver nitrate in human dermal fibroblasts. Toxicology letters. 1998;98(3):169–179. doi: 10.1016/s0378-4274(98)00114-3. [DOI] [PubMed] [Google Scholar]
- 44.Balla VK, Bose S, Bandyopadhyay A. Low stiffness porous Ti structures for load-bearing implants. Acta biomaterialia. 2007;3(6):997–1006. doi: 10.1016/j.actbio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Xue W, Balla VK, Bandyopadhyay A, Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomaterialia. 2007;3(6):1007–1018. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Bandyopadhyay A, Bose S. Modified metal materials, surface modifications to improve cell interactions and antimicrobial properties, and methods for modifying metal surface properties. 8,545,559 US Patent #.
- 47.Thieme M, Wieters K-P, Bergner F, Scharnweber D, Worch H, Ndop J, Kim TJ, Grill W. Titanium powder sintering for preparation of a porous FGM destined as a skeletal replacement implant. Mater. Sci. Forum. 1999:308–374. doi: 10.1023/a:1008958914818. [DOI] [PubMed] [Google Scholar]
- 48.Das K, Bose S, Bandyopadhyay A. Surface modifications and cell–materials interactions with anodized Ti. Acta Biomaterialia. 2007;3(4):573–585. doi: 10.1016/j.actbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Das K, Bose S, Bandyopadhyay A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell–materials interaction. Journal of Biomedical Materials Research Part A. 2009;90(1):225–237. doi: 10.1002/jbm.a.32088. [DOI] [PubMed] [Google Scholar]
- 50.Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S. In Vivo Response of Laser Processed Porous Titanium Implants for Load-Bearing Implants. Annals of Biomedical Engineering. 2017;45(1):249–260. doi: 10.1007/s10439-016-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das K, Bose S, Bandyopadhyay A, Karandikar B, Gibbins BL. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;87(2):455–460. doi: 10.1002/jbm.b.31125. [DOI] [PubMed] [Google Scholar]
- 52.Mei S, Wang H, Wang W, Tong L, Pan H, Ruan C, Ma Q, Liu M, Yang H, Zhang L, Cheng Y, Zhang Y, Zhao L, Chu PK. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35(14):4255–4265. doi: 10.1016/j.biomaterials.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Wang R, Neoh KG, Kang E-T, Tambyah PA, Chiong E. Antifouling coating with controllable and sustained silver release for long- term inhibition of infection and encrustation in urinary catheters. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015;103(3):519–528. doi: 10.1002/jbm.b.33230. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Murphy WL. Functionalizing calcium phosphate biomaterials with antibacterial silver particles. Advanced Materials. 2013;25(8):1173–1179. doi: 10.1002/adma.201203370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivaram A, Bose S, Bandyopadhyay A. Mechanical degradation of TiO2 nanotubes with and without nanoparticulate silver coating. Journal of the mechanical behavior of biomedical materials. 2016;59:508–518. doi: 10.1016/j.jmbbm.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environmental science & technology. 2010;44(6):2169–2175. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- 57.Molleman B, Hiemstra T. Surface structure of silver nanoparticles as a model for understanding the oxidative dissolution of silver ions. Langmuir. 2015;31(49):13361–13372. doi: 10.1021/acs.langmuir.5b03686. [DOI] [PubMed] [Google Scholar]
- 58.Peretyazhko TS, Zhang Q, Colvin VL. Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: kinetics and size changes. Environmental science & technology. 2014;48(20):11954–11961. doi: 10.1021/es5023202. [DOI] [PubMed] [Google Scholar]
- 59.Nandi SK, Shivaram A, Bose S, Bandyopadhyay A. Silver nanoparticle deposited implants to treat osteomyelitis. Journal of Biomedical Materials Research: Part B -Applied Biomaterials. 2017 doi: 10.1002/jbm.b.33910. [DOI] [PMC free article] [PubMed] [Google Scholar]