Abstract
The endolysosomal system is extremely dynamic, yet highly organized. The spatiotemporal distribution of endolysosomal organelles depends on transport driven by microtubule motors such as kinesins and dynein, and by actin-based myosin motors. It has recently become appreciated that interactions with motors are controlled by contacts with other organelles, particularly the endoplasmic reticulum (ER). The ER also controls the concentration of endolysosomal organelles in the perinuclear area, as well as their fission and fusion, through a complex system of tethering proteins. Dynamic interactions go both ways, as contacts with endosomes can influence the movement of the ER and peroxisomes. The dynamics of endolysosomal organelles should thus no longer be studied in isolation, but in the context of the whole endomembrane system.
Introduction
The steady-state distribution of organelles within the cell is the result of highly regulated, dynamic processes. Indeed, organelles move throughout the cytoplasm along cytoskeletal structures such as microtubules and actin filaments. In general, microtubule motors such as kinesins and dynein drive long-range transport, whereas actin-based myosin motors drive short-range transport. An assortment of adaptor proteins mediate coupling of organelles to the motor proteins. Various regulatory mechanisms ensure that organelle-motor interactions occur at the right place and time. One of these mechanisms involves contacts with other organelles. The endoplasmic reticulum (ER), in particular, is omnipresent throughout the cytoplasm and plays a major role in controlling the movement and overall positioning of other organelles. A prime example of an organelle ensemble that is subject to this dynamic control is the endolysosomal system. This system comprises various membrane-bound organelles, including early endosomes (EEs), recycling endosomes (REs), late endosomes (LEs), lysosomes, lysosome-related organelles (LROs), and other specialized organelles (Table 1). In this minireview, we discuss recent findings on the distribution and dynamics of endolysosomal organelles mediated by interactions with motor proteins and with the ER. These findings reveal that movement of endolysosomal organelles is highly interdependent with that of other organelles, and subject to cross-compartmental control mechanisms.
Table 1.
Examples of microtubule motors and adaptors/regulators involved in the movement of endolysosomal organelles
| Organelles | Motors | Cargo adaptors and regulators | References |
|---|---|---|---|
| EEs | KIF5B | KLC, Gadkin, AP-1 | 52 |
| KIF16B | Rab5, PtdIns3P, VPS34 | 2,53 | |
| Dynein-Dynactin | Rab5, FHF (FTS, Hook, FHIP) | 17,19,20 | |
| REs | KIF13A | BLOC-1, Rab11 | 31,54 |
| KIF13B | 55 | ||
| Dynein | Rab11, FIP3 | 56,57 | |
| LEs/lysosomes | KIF1A | BORC, Arl8 | 14,55 |
| KIF1Bβ | BORC, Arl8 | 9,14,55 | |
| KIF2A | 10 | ||
| KIF3A | KAP3 | 8 | |
| KIF5B | KLC, BORC, Arl8b, SKIP Rab7, FYCO1 | 11,12,38,58 | |
| Dynein-Dynactin | Rab7, RILP, ORP1L, JIP3, HPS6 | 23,24,59,60 | |
| Proto-lysosomes (ALR) | KIF5B | PtdIns(4,5)P2, clathrin | 7 |
| Apical REs | KIF3A KIF3B |
Rab11, FIP5 | 61 |
| Transcytotic endosomes | KIF16B | Rab11 | 4 |
| Sara signaling endosomes | Dmel Klp98 (KIF16B) | 3 | |
| Cytokinetic recycling endosomes | KIF5B | Arf6, JIP4 | 62 |
| Dynein-Dynactin | Arf6, JIP4 | 62 | |
| Melanosomes | KIF5B | Rab1A, SKIP | 6 |
| Dynein-Dynactin | Rab36, RILP Melanoregulin | 63, 64 | |
| Lytic granules | KIF5B | Rab27a, Slp3, KLC1 Arl8, SKIP | 65,66 |
| Dynein-Dynactin | HkRP3 Rab7, RILP | 67,68 |
Microtubule-dependent transport
Endolysosomal organelles move bidirectionally between the center and the periphery of the cell along microtubule tracks (Fig. 1). In non-polarized cells, microtubules are radially distributed, with their minus-ends at a juxtanuclear microtubule-organizing center (MTOC), and their plus-ends pointing towards the periphery. Most kinesin motors drive organelle transport from the minus-end to the plus-end (anterograde or centrifugal transport), while the cytoplasmic dynein motor drives organelle transport in the opposite direction (retrograde or centripetal transport). Polarized cells such as epithelial cells and neurons have more complex microtubule organizations, with some microtubules pointing their plus-ends towards the nucleus. Thus, whether transport is centrifugal or centripetal in these cells depends on the specific microtubules to which the organelles are attached.
Figure 1.
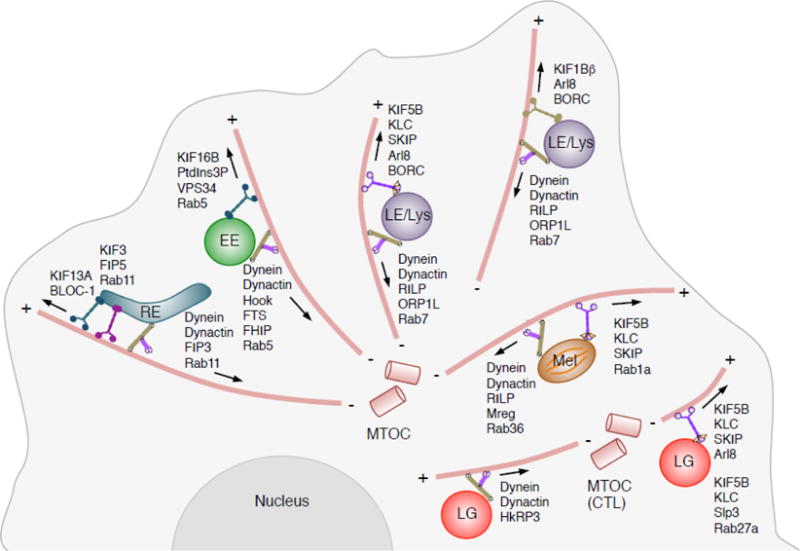
Microtubule-dependent transport of endolysosomal organelles. This cartoon depicts different endolysosomal organelles and the microtubule motors and adaptors that drive their movement. EE, early endosome; RE, recycling endosome; LE, late endosome; Lys, lysosome; Mel, melanosome; LG, lytic granule; MTOC, microtubule-organizing center. The minus (−) and plus (+) ends of microtubules are indicated. Arrows indicate the direction of movement driven by the corresponding motor-adaptor combinations. Notice that whereas in most cells the MTOC is located near the cell center, in activated cytotoxic T lymphocytes (CTL) it is relocated to an area under the immunological synapse. See Table 1 for references.
Anterograde transport
Mammalian genomes encode approximately 45 different kinesin heavy chains (KIFs), several of which drive movement of endolysosomal organelles [1] (Table 1) (Fig. 1). In general, there is not a simple correspondence of one organelle to one kinesin. In some cases, distinct organelles depend on the same kinesin for movement. For example, anterograde transport of EEs [2], SARA signaling endosomes [3] and transcytotic endosomes [4] depends on the kinesin-3 KIF16B. Similarly, anterograde transport of LEs and lysosomes [5], melanosomes [6] and proto-lysosomes in the process of autophagic lysosome reformation [7], involves the kinesin-1 KIF5B. In other cases, the same organelle can use multiple kinesins for motion, as exemplified by LEs and lysosomes, which use not only KIF5B [5], but also the kinesin-2 KIF3A [8], kinesin-3 KIF1A and KIF1Bβ [9], and kinesin-13 KIF2A [10] for anterograde transport.
The coupling of endolysosomal organelles to kinesins is often mediated by small GTPases and their effectors, as well as membrane phospholipids, all of which function as organelle (“cargo”) adaptors (Table 1) (Fig. 1). KIF16B, for example, is recruited to EEs through interaction of its PX domain with membrane phosphatidylinositol 3-phosphate (PtdIns3P), which is generated by the class III phosphatidylinositol 3-kinase VPS34, an effector of the small GTPase Rab5 [2]. The recruitment of KIF5B and KIF1A/KIF1Bβ to LEs/lysosomes, on the other hand, depends on the multisubunit complex BORC and the small GTPase Arl8 [11,12]. Arl8 engages these two kinesin types by different mechanisms. Whereas interactions with the Arl8-effector SKIP and the kinesin light chain (KLC) mediate coupling to KIF5B [12], Arl8 interacts directly with the CC3 domain of KIF1A/KIF1Bβ [13]. A recent study showed that KIF5B and KIF1A/KIF1Bβ preferentially couple LEs/lysosomes to distinct microtubule tracks localized to the perinuclear and peripheral regions of the cytoplasm, respectively [14]. In this case, the association of the same organelle with distinct kinesins enables movement in different regions of the cell.
Retrograde transport
In contrast to the many different kinesins, there is only one cytoplasmic dynein heavy chain [15]. Structural and functional diversification of dynein is achieved by the association of the heavy chain with various intermediate, light intermediate and light chains, as well as many cargo adaptors. Another multisubunit complex named dynactin associates with dynein to activate its transport towards microtubule minus-ends. Virtually all endolysosomal organelles can recruit dynein-dynactin following a template similar to that of the kinesins: a Rab GTPase that binds to the target organelle, and a Rab effector that directly or indirectly interacts with the motor (Table 1) (Fig. 1). Centripetal transport of EEs, for example, is mediated by Rab5 and the Rab5-effector FHF complex, composed of FHIP, Hook and FTS subunits [16,17,18,19,20]. FHIP is the subunit that directly interacts with Rab5 [20], whereas Hook simultaneously binds to dynein and dynactin, stabilizing this complex and enhancing the processivity of transport [21,22].
Centripetal transport of LEs/lysosomes depends on Rab7 and its effector RILP [23], which interacts with the dynactin subunit p150Glued [24] and the dynein light intermediate chain [25] (Figs. 1 and 2). The Rab7-RILP-dynein-dynactin complex associates with a cholesterol sensor named ORP1L, which adopts different conformations depending on cellular cholesterol levels. At low cholesterol levels, an FFAT motif in ORP1L interacts with the ER protein VAP-A at membrane contact sites (MCS) between LEs/lysosomes and the ER [68]. Interaction with VAP-A dissociates dynein-dynactin from the Rab7-RILP complex, resulting in peripheral localization of LEs. At high cholesterol levels, the FFAT motif is occluded, allowing long-term engagement of RILP with dynein-dynactin and thus promoting transport of LEs/lysosomes towards the MTOC [68]. This mechanism explains not only how LE/lysosome transport is controlled by cholesterol and the ER, but also the LE/lysosome clustering phenotype caused by endolysosomal cholesterol accumulation in cells from Niemann-Pick type C patients [68].
Figure 2.
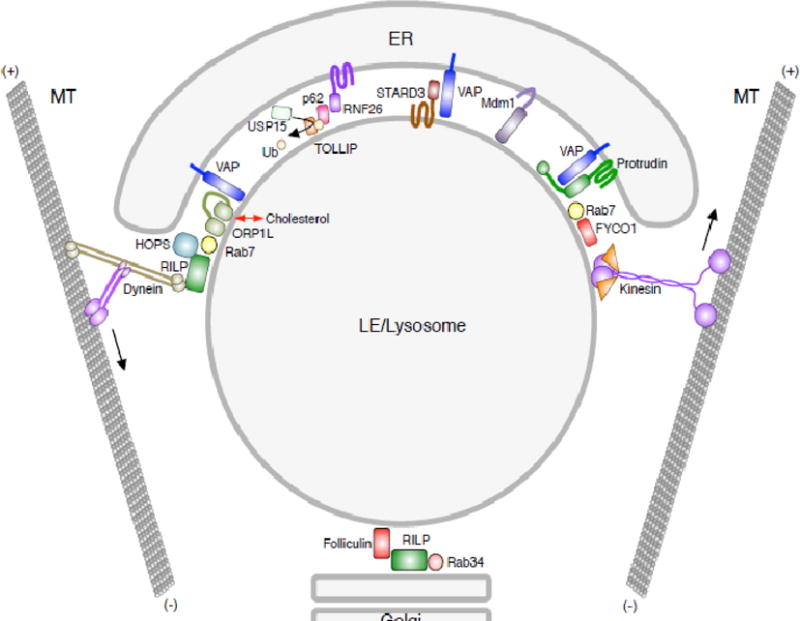
Contacts of LEs/lysosomes with the ER and Golgi complex. Schematic representation of interactions between LEs/lysosomes and other organelles. The multiple interactions with the ER often involve the ER protein VAP (A and B isoforms). These interactions control the overall positioning of the LEs/lysosomes, their association with kinesin and dynein motors, and their fission and fusion with other organelles. Also depicted are interactions of LEs/lysosomes with the Golgi complex. See Table 2 for references.
Role of the actin cytoskeleton
The actin cytoskeleton and associated myosin motors also modulate the transport of endolysosomal organelles by a variety of mechanisms. One of these mechanisms involves the dynamic capture of organelles within actin-rich regions of the cell such as the acto-myosin cortex that underlies the plasma membrane. This mechanism also fits a small GTPase-adaptor-motor model similar to that observed for organelle interactions with microtubule motors. This process has been extensively investigated for LROs such as melanosomes and cytotoxic T-cell lytic granules, which rely on a complex of Rab27a, a corresponding adaptor protein (melanophilin, munc13-4) and myosin Va for peripheral retention and/or exocytosis of the organelle content [26,27,28]. Late endosomal MHC class II compartments (MIIC) are also LROs that fuse with the plasma membrane upon maturation of dendritic cells (DC). In immature DC, MIIC are maintained intracellularly by the action of the small GTPase Arl14, the effector ARF7EP, and Myo1e, a single-leg myosin that is unable to move but can attach cargos to actin [29]. Inactivating this system yields immature DC with an MIIC distribution similar to that of mature DC. Another mechanism of actin-based motility involves the formation of actin comet tails on endosomes and lysosomes that propel them through the cytoplasm [30], and aid in the budding and fission of endosomal tubules [31,32]. The WASH complex plays a critical role in actin nucleation for this type of organelle motility [30,32]. The versatility of the actin cytoskeleton suggests that this system might contribute to endolysosomal motility and positioning in additional ways, functioning either in cooperation [33,31] or opposition to microtubules [34].
Regulation by organelle contacts
The overall distribution of endolysosomal organelles within the cytoplasm is not only determined by interaction with cytoskeletal motors but also by contacts with other organelles, particularly the ER (Table 2) (Fig. 2). In 1964, Alex Novikoff and colleagues coined the term “GERL” to describe the close apposition of ER, Golgi and lysosomes observed in electron micrographs of spinal ganglia neurons [35]. Beyond the fact that these organelles are connected by the biosynthetic/secretory pathway, the significance of their close apposition was not grasped until recently, with the recognition that organelles cross-control each other through membrane contact sites (MCS) [36,68]. The ER, in particular, plays a crucial role in this control because of its presence throughout the cytoplasmic space. Three basic endolysosomal processes are under control of the ER: motility, overall positioning, and fusion/fission.
Table 2.
Examples of organelle contacts involving endolysosomal organelles
| Organelle contacts | Contact molecules and regulators | References |
|---|---|---|
| ER-LEs/lysosomes | VAP, ORP1L | 69 |
| VAP, STARD3/MLN64, STARD3NL/MENTHO | 43 | |
| Protrudin, Rab7, PtdIns3P | 37,38 | |
| ER-retromer tubules | VAP, SNX2, PI4P, OSBP | 32 |
| ER-endolysosomal organelles | RNF26, p62/SQSTM1, EPS15, T6BP/TAX1BP1, TOLLIP, USP15 | 41 |
| ER-yeast vacuole | Mdm1, PtdIns3P | 70 |
| Golgi-LEs/lysosomes | Rab34, RILP, folliculin | 39,40 |
| Peroxisomes-lysosomes | Synaptotagmin VII, PtdIns(4,5)P2 | 71 |
| Peroxisomes-endosomes | PxdA | 49 |
| RNP-endosomes | Rrm4, Upa1 | 48 |
Regulation of LE/lysosome transport by the ER
Besides its role in regulating the cholesterol-dependent coupling of LEs/lysosomes to dynein-dynactin, as described above, the ER is also involved in controlling the kinesin-dependent transport of LEs. This latter mechanism involves an ER-anchored protein named protrudin that also has a VAP-A-interacting FFAT motif, and a FYVE domain that binds PtdIns3P on LEs (Fig. 2). These interactions allow protrudin to bridge the ER and endosomal membranes. In addition, protrudin delivers kinesin-1 (KIF5B2-KLC2) to a complex of Rab7 and its effector FYCO1 on LEs, thus promoting their plus-end-directed transport [37,38] (Fig. 2). The VAP-A-dependent control of dynein-dependent transport (see above) and kinesin-dependent transport of LEs could be functionally coupled, but the molecular details of such coupling are not known. The biology of intercompartmental control of endolysosomal transport is likely more complex, as recently illustrated by findings that the LE protein folliculin supports the binding of RILP to the Golgi-associated Rab34, also contributing to the perinuclear accumulation of LEs [39,40] (Fig. 2).
The ER organizes the positioning of the entire endolysosomal system
Although endolysosomal organelles can move bidirectionally between the center and the periphery of the cell, at steady state the majority is concentrated in a region around the MTOC (i.e., the “perinuclear cloud”). Recent studies have shown that this pericentrosomal concentration of endolysosomal organelles is controlled by the ubiquitin ligase RNF26, which localizes to the perinuclear ER [41]. RNF26 associates with and then ubiquitinates the adaptor protein p62/SQSTM1. Ubiquitinated p62/SQSTM1 in turn interacts with various endolysosomal adaptor proteins, including TOLLIP (LEs and phagosomes), EPS15 (EEs) and TAX1BP1 (TGN) (Fig. 2). These adaptor proteins are characterized by specific ubiquitin- and membrane-binding domains that allow tethering of their respective compartments to the perinuclear ER where RNF26 resides. These interactions are countered by a de-ubiquitinating enzyme named USP15, which releases the organelles from the ER, enabling their movement by kinesin and dynein motors [41] (Fig. 2). How endosomal vesicles are deemed ready for release to the cell periphery by USP15 is unclear, but may be related to their correct maturation state.
Regulation of endosomal fission and fusion by the ER
Another important aspect of endolysosomal dynamics is the ability of the organelles to undergo fission and fusion. Remarkably, a recent study showed that fission of EEs and LEs occurs at sites of contact with the ER [42]. Free diffusion of cargo is restricted at these sites, suggesting that this mechanism also plays a role in cargo sorting. Although the molecular mechanism of this particular process remains to be elucidated, another study showed how the ER contributes to the budding of retromer-containing tubules from endosomes [32]. This mechanism involves the same ER proteins that participate in the control of dynein and kinesin binding to LEs, VAP-A and its paralog VAP-B. These proteins establish a network of interactions with the PtdIns4P transporter OSBP (an ORP1L homolog) and the retromer-associated SNX2 protein. These interactions lead to PtdIns4P- and WASH-dependent actin nucleation and consequent budding of retromer-containing tubules. Like ORP1L and OSBP, two other lipid-transfer proteins on LEs, STARD3 and STARD3NL interact with VAP proteins via FFAT motifs, as well as with endosomes, providing yet another means for the ER to contact endosomes [43].
Fusion of LE/lysosomes with other organelles such as autophagosomes is also subject to regulation by interaction of ORP1L with VAP-A at the ER [44,45]. Release of ORP1L from VAP-A is required for a Rab7-RILP-PLEKHM1 complex to recruit the tethering complex HOPS to LEs/lysosomes so that they can fuse with other LEs/lysosomes and with autophagosomes [45]. These examples illustrate how endolysosomal transport is integrated with fission and fusion, and highlight the role of the ER in regulating these processes.
Hitchhiking on early endosomes
The dynamic interaction of the ER with endosomes goes both ways. In filamentous fungi, various organelles and particles such as the ER, lipid droplets, peroxisomes, polysomes and ribonucleoprotein particles exhibit some form of microtubule-dependent motility that results from tethering to moving EEs rather than direct coupling to motor proteins [46,47,48,49,50]. This process, termed “hitchhiking”, is also mediated by proteins that bridge an organelle or particle with the EE membrane. For example, the large coiled-coil protein PxdA links peroxisomes to EEs, partly through a predicted F-BAR domain that binds to the EE membrane [49]. Similarly, the RNA-binding protein Rrm4 links ribonucleoprotein particles to another protein named Upa1/rififylin, which in turn has a FYVE domain that binds to PtdIns3P on EEs [48]. It remains to be determined if hitchhiking also occurs in higher eukaryotes and what the corresponding tethers might be. In the case of ER hitchhiking, it would be interesting to test whether ER movements depend on the same proteins previously identified as components of ER-endosome MCS.
Concluding remarks
Our current view is that the movement and positioning of endolysosomal organelles involves not only interactions with cytoskeletal motors but also contacts with other cytoplasmic organelles. The endosomal system can thus no longer be considered in isolation, but as part of an integrated system with other organelles, particularly the ER. A picture is emerging of the cell as a social network, with a mother compartment (the ER) instructing the behavior of its offspring (the endolysosomal organelles). Interactions go both ways, though, and we can expect other organelles to influence ER behavior in return. Despite progress in the elucidation of these processes, the organelle interaction field is still in its infancy. Particularly lacking is information concerning the regulation of these processes by nutrients, signaling pathways, cellular stresses, developmental programs, cell proliferation, and other physiological and pathological conditions. These processes will be even more complex in polarized cell types such as epithelial cells and neurons, with cell-type specific components and pathways that do not exist in the non-polarized cells that have been most intensively studied to date. Finally, we anticipate greater interest in the importance of endolysosomal organelle movement and positioning for various cellular functions. There are already numerous examples of how the motor-driven translocation of endosomes and lysosomes contributes to processes such as autophagy, metabolic signaling, and cell adhesion and migration, to name a few [11,51]. Likewise, contacts of endolysosomal organelles with the ER in the perinuclear cloud are critical for the transfer of fluid phase materials from endosomes to lysosomes, and for attenuation of EGF receptor signaling [41]. Future work will likely expand the range of cellular functions that are dependent on the spatial and temporal dynamics of endolysosomal organelles.
Highlights.
Endolysosomal organelles are dynamically distributed throughout the cytoplasm
Movement of endolysosomal organelles depends on coupling to microtubule motors and actin
Contacts with the endoplasmic reticulum regulate organelle movement and positioning
Other organelles move by hitchhiking on endolysosomal organelles
Cellular functions depend on movement and positioning of endolysosomal organelles
Acknowledgments
We thank members of our laboratories for their contributions to original research covered in this article. We apologize to authors whose work we could not cite because of space limitations. Work in our laboratories was funded by the Intramural Program of NICHD, NIH (ZIA HD001607) (JSB) and an ERC Advanced Grant (JN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 2.Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Derivery E, Seum C, Daeden A, Loubéry S, Holtzer L, Jülicher F, Gonzalez-Gaitan M. Polarized endosome dynamics by spindle asymmetry during asymmetric cell division. Nature. 2015;528:280–285. doi: 10.1038/nature16443. [DOI] [PubMed] [Google Scholar]
- 4.Pérez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodríguez-Boulan EJ. The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J. 2013;32:2125–2139. doi: 10.1038/emboj.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakata T, Hirokawa N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J Cell Biol. 1995;131:1039–1053. doi: 10.1083/jcb.131.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida M, Ohbayashi N, Fukuda M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci Rep. 2015;5:8238. doi: 10.1038/srep08238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du W, Su QP, Chen Y, Zhu Y, Jiang D, Rong Y, Zhang S, Zhang Y, Ren H, Zhang C, et al. Kinesin 1 Drives Autolysosome Tubulation. Dev Cell. 2016;37:326–336. doi: 10.1016/j.devcel.2016.04.014. The kinesin-1 heavy chain KIF5B is shown to pull tubules from the autolysosomal membrane in the process of autophagic lysosome reformation. KIF5B is recruited to autolysosomes by membrane PtdIns(4,5)P2. [DOI] [PubMed] [Google Scholar]
- 8.Brown CL, Maier KC, Stauber T, Ginkel LM, Wordeman L, Vernos I, Schroer TA. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic. 2005;6:1114–1124. doi: 10.1111/j.1600-0854.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita M, Tanaka S, Nakamura N, Inoue H, Kanazawa H. A novel kinesin-like protein, KIF1Bbeta3 is involved in the movement of lysosomes to the cell periphery in non-neuronal cells. Traffic. 2004;5:140–151. doi: 10.1111/j.1600-0854.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 10.Santama N, Krijnse-Locker J, Griffiths G, Noda Y, Hirokawa N, Dotti CG. KIF2beta, a new kinesin superfamily protein in non-neuronal cells, is associated with lysosomes and may be implicated in their centrifugal translocation. EMBO J. 1998;17:5855–5867. doi: 10.1093/emboj/17.20.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multiprotein complex that regulates lysosome positioning. Dev Cell. 2015;33:176–188. doi: 10.1016/j.devcel.2015.02.011. This paper reports the identification of a multisubunit complex named BORC that recruits Arl8 to lysosomes, initiating a chain of interactions that leads to kinesin-dependent transport of lysosomes towards the cell periphery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosa-Ferreira C, Munro S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev Cell. 2011;21:1171–1178. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YE, Huo L, Maeder CI, Feng W, Shen K. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron. 2013;78:994–1011. doi: 10.1016/j.neuron.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guardia CM, Farías GG, Jia R, Pu J, Bonifacino J. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Reports. 2016;17:1950–1961. doi: 10.1016/j.celrep.2016.10.062. This study shows that BORC and Arl8 regulate the functions of two structurally different kinesins, the kinesin-1 KIF5B and the kinesin-3 KIF1B, in moving lysosomes towards the cell periphery. Each kinesin links lysosomes to microtubule populations localized to perinuclear and peripheral areas of the cell, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Sowa ME, Chen J, Li X, Gygi SP, Harper JW. An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol Biol Cell. 2008;19:5059–5071. doi: 10.1091/mbc.E08-05-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielska E, Schuster M, Roger Y, Berepiki A, Soanes DM, Talbot NJ, Steinberg G. Hook is an adapter that coordinates kinesin-3 and dynein cargo attachment on early endosomes. J Cell Biol. 2014;204:989–1007. doi: 10.1083/jcb.201309022. This study along with references 18–20 define the FHF complex (FTS-Hook-FHIP) as an adaptor that links Rab5 to dynein-dynactin, promoting movement of early endosomes and a population of axonal retrograde carriers towards microtubule minus-ends. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao X, Wang X, Xiang X. FHIP and FTS proteins are critical for dynein-mediated transport of early endosomes in Aspergillus. Mol Biol Cell. 2014;25:2181–2189. doi: 10.1091/mbc.E14-04-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Qiu R, Arst HNJ, Peñalva MA, Xiang X. HookA is a novel dynein-early endosome linker critical for cargo movement in vivo. J Cell Biol. 2014;204:1009–1026. doi: 10.1083/jcb.201308009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Farías GG, Mattera R, Bonifacino JS. Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci U S A. 2016;113:E5318–27. doi: 10.1073/pnas.1601844113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder CM, Vale RD. Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3. J Cell Biol. 2016;214:309–318. doi: 10.1083/jcb.201604002. This study and Ref 22 demonstrate that the Hook subunit of the FHF complex promotes assembly of a motile dynein-dynactin complex, explaining how cargo binding controls the processivity of early endosome movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olenick MA, Tokito M, Boczkowska M, Dominguez R, Holzbaur EL. Hook Adaptors Induce Unidirectional Processive Motility by Enhancing the Dynein-Dynactin Interaction. J Biol Chem. 2016;291:18239–18251. doi: 10.1074/jbc.M116.738211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 24.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder CM, Ostrem JM, Hertz NT, Vale RD. A Ras-like domain in the light intermediate chain bridges the dynein motor to a cargo-binding region. Elife. 2014;3:e03351. doi: 10.7554/eLife.03351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Rao K, Bowers MB, Copeland NG, Jenkins NA, Hammer JA. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- 27.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul P, van den Hoorn T, Jongsma ML, Bakker MJ, Hengeveld R, Janssen L, Cresswell P, Egan DA, van Ham M, Ten Brinke A, et al. A Genome-wide multidimensional RNAi screen reveals pathways controlling MHC class II antigen presentation. Cell. 2011;145:268–283. doi: 10.1016/j.cell.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L, Gokhale A, Morel E, Faundez V, et al. BLOC-1 Brings Together the Actin and Microtubule Cytoskeletons to Generate Recycling Endosomes. Curr Biol. 2016;26:1–13. doi: 10.1016/j.cub.2015.11.020. This study identifies another process under the control of the ER protein VAP: WASH-dependent actin nucleation and retromer tubule budding to control endosome-to-Golgi trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, De Camilli P. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell. 2016;166:408–423. doi: 10.1016/j.cell.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordonnier MN, Dauzonne D, Louvard D, Coudrier E. Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol Biol Cell. 2001;12:4013–4029. doi: 10.1091/mbc.12.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C, Schuster M, Guimaraes SC, Ashwin P, Schrader M, Metz J, Hacker C, Gurr SJ, Steinberg G. Active diffusion and microtubule-based transport oppose myosin forces to position organelles in cells. Nat Commun. 2016;7:11814. doi: 10.1038/ncomms11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novikoff AB. GERL, its form and function in neurons of rat spinal ganglia. Biol Bull. 1964;127:358. [Google Scholar]
- 36.Gatta AT, Levine TP. Piecing Together the Patchwork of Contact Sites. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Matsuzaki F, Shirane M, Matsumoto M, Nakayama KI. Protrudin serves as an adaptor molecule that connects KIF5 and its cargoes in vesicular transport during process formation. Mol Biol Cell. 2011;22:4602–4620. doi: 10.1091/mbc.E11-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Hong W. Interorganellar regulation of lysosome positioning by the Golgi apparatus through Rab34 interaction with Rab-interacting lysosomal protein. Mol Biol Cell. 2002;13:4317–4332. doi: 10.1091/mbc.E02-05-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starling GP, Yip YY, Sanger A, Morton PE, Eden ER, Dodding MP. Folliculin directs the formation of a Rab34-RILP complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. 2016;17:823–841. doi: 10.15252/embr.201541382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jongsma ML, Berlin I, Wijdeven RH, Janssen L, Janssen GM, Garstka MA, Janssen H, Mensink M, van Veelen PA, Spaapen RM, Neefjes J. An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport. Cell. 2016;166:152–166. doi: 10.1016/j.cell.2016.05.078. Description of an ER-located E3 ligase that links ubiquitin receptors to control the perinuclear positioning of the entire endolysosomal system. This organization is required for efficient transport through the endosomal system and for termination of receptor-based signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER Contact Sites Define the Position and Timing of Endosome Fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. Live-cell imaging study demonstrating that the position and timing of endosome fission events are determined by contacts with the ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, et al. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci. 2013;126:5500–5512. doi: 10.1242/jcs.139295. [DOI] [PubMed] [Google Scholar]
- 44.van der Kant R, Fish A, Janssen L, Janssen H, Krom S, Ho N, Brummelkamp T, Carette J, Rocha N, Neefjes J. Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J Cell Sci. 2013;126:3462–3474. doi: 10.1242/jcs.129270. [DOI] [PubMed] [Google Scholar]
- 45.Wijdeven RH, Janssen H, Nahidiazar L, Janssen L, Jalink K, Berlin I, Neefjes J. Cholesterol and ORP1L-mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat Commun. 2016;7:11808. doi: 10.1038/ncomms11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumann S, Pohlmann T, Jungbluth M, Brachmann A, Feldbrügge M. Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. J Cell Sci. 2012;125:2740–2752. doi: 10.1242/jcs.101212. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi Y, Ashwin P, Roger Y, Steinberg G. Early endosome motility spatially organizes polysome distribution. J Cell Biol. 2014;204:343–357. doi: 10.1083/jcb.201307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohlmann T, Baumann S, Haag C, Albrecht M, Feldbrügge M. A FYVE zinc finger domain protein specifically links mRNA transport to endosome trafficking. Elife. 2015;4 doi: 10.7554/eLife.06041. This study reports the identification of an endosome-associated protein named Upa1 that interacts with the mRNA-associated protein Rrm4 to enable hitchhiking of ribonucleoprotein particles on endosomes in the filamentous fungus Ustilago maydis. Upa1 contains a FYVE domain that interacts with endosomal PtdIns3P and a PAM2-like domain that interacts with Rrm4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salogiannis J, Egan MJ, Reck-Peterson SL. Peroxisomes move by hitchhiking on early endosomes using the novel linker protein PxdA. J Cell Biol. 2016;212:289–296. doi: 10.1083/jcb.201512020. The endosome-associated PxdA protein is shown to enable peroxisome hitchhiking on endosomes by linking the membranes of both organelles in another filamentous fungus, Aspergillus nidulans. A predicted F-BAR domain in PxdA mediates association with early endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guimaraes SC, Schuster M, Bielska E, Dagdas G, Kilaru S, Meadows BR, Schrader M, Steinberg G. Peroxisomes, lipid droplets, and endoplasmic reticulum “hitchhike” on motile early endosomes. J Cell Biol. 2015;211:945–954. doi: 10.1083/jcb.201505086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt MR, Maritzen T, Kukhtina V, Higman VA, Doglio L, Barak NN, Strauss H, Oschkinat H, Dotti CG, Haucke V. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc Natl Acad Sci U S A. 2009;106:15344–15349. doi: 10.1073/pnas.0904268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farkhondeh A, Niwa S, Takei Y, Hirokawa N. Characterizing KIF16B in neurons reveals a novel intramolecular “stalk inhibition” mechanism that regulates its capacity to potentiate the selective somatodendritic localization of early endosomes. J Neurosci. 2015;35:5067–5086. doi: 10.1523/JNEUROSCI.4240-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep. 2014;6:445–454. doi: 10.1016/j.celrep.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentley M, Decker H, Luisi J, Banker G. A novel assay reveals preferential binding between Rabs, kinesins, and specific endosomal subpopulations. J Cell Biol. 2015;208:273–281. doi: 10.1083/jcb.201408056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 57.Le Droguen PM, Claret S, Guichet A, Brodu V. Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Development. 2015;142:363–374. doi: 10.1242/dev.113472. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 59.Drerup CM, Nechiporuk AV. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 2013;9:e1003303. doi: 10.1371/journal.pgen.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li K, Yang L, Zhang C, Niu Y, Li W, Liu JJ. HPS6 interacts with dynactin p150Glued to mediate retrograde trafficking and maturation of lysosomes. J Cell Sci. 2014;127:4574–4588. doi: 10.1242/jcs.141978. [DOI] [PubMed] [Google Scholar]
- 61.Li D, Kuehn EW, Prekeris R. Kinesin-2 mediates apical endosome transport during epithelial lumen formation. Cell Logist. 2014;4:e28928. doi: 10.4161/cl.28928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montagnac G, Sibarita JB, Loubéry S, Daviet L, Romao M, Raposo G, Chavrier P. ARF6 Interacts with JIP4 to control a motor switch mechanism regulating endosome traffic in cytokinesis. Curr Biol. 2009;19:184–195. doi: 10.1016/j.cub.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 63.Matsui T, Ohbayashi N, Fukuda M. The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J Biol Chem. 2012;287:28619–28631. doi: 10.1074/jbc.M112.370544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohbayashi N, Maruta Y, Ishida M, Fukuda M. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J Cell Sci. 2012;125:1508–1518. doi: 10.1242/jcs.094185. [DOI] [PubMed] [Google Scholar]
- 65.Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, de Saint Basile G, Ménasché G. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood. 2012;119:3879–3889. doi: 10.1182/blood-2011-09-382556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuli A, Thiery J, James AM, Michelet X, Sharma M, Garg S, Sanborn KB, Orange JS, Lieberman J, Brenner MB. Arf-like GTPase Arl8b regulates lytic granule polarization and natural killer cell-mediated cytotoxicity. Mol Biol Cell. 2013;24:3721–3735. doi: 10.1091/mbc.E13-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ham H, Huynh W, Schoon RA, Vale RD, Billadeau DD. HkRP3 is a microtubule-binding protein regulating lytic granule clustering and NK cell killing. J Immunol. 2015;194:3984–3996. doi: 10.4049/jimmunol.1402897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniele T, Hackmann Y, Ritter AT, Wenham M, Booth S, Bossi G, Schintler M, Auer-Grumbach M, Griffiths GM. A role for Rab7 in the movement of secretory granules in cytotoxic T lymphocytes. Traffic. 2011;12:902–911. doi: 10.1111/j.1600-0854.2011.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henne WM, Zhu L, Balogi Z, Stefan C, Pleiss JA, Emr SD. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J Cell Biol. 2015;210:541–551. doi: 10.1083/jcb.201503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, Song BL. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 2015;161:291–306. doi: 10.1016/j.cell.2015.02.019. [DOI] [PubMed] [Google Scholar]


