Abstract
A unique cohort of military personnel exposed to isolated blast was studied to explore acute peripheral cytokine levels, with the aim of identifying blast-specific biomarkers. Several cytokines, including interleukin (IL) 6, IL-10 and tumor necrosis factor alpha (TNFα) have been linked to pre-clinical blast exposure, but remained unstudied in clinical blast exposure. To address this gap, blood samples from 62 military personnel were obtained at baseline, and daily, during a 10-day blast-related training program; changes in the peripheral concentrations of IL-6, IL-10 and TNFα were evaluated using an ultrasensitive assay. Two groups of trainees were matched on age, duration of military service, and previous history of blast exposure(s), resulting in moderate blast cases and no/low blast controls. Blast exposures were measured using helmet sensors that determined the average peak pressure in pounds per square inch (psi). Moderate blast cases had significantly elevated concentrations of IL-6 (F1,60 =18.81, p < 0.01) and TNFα (F1,60 =12.03, p < 0.01) compared to no/low blast controls; levels rebounded to baseline levels the day after blast. On the day of the moderate blast exposure, the extent of the overpressure (psi) in those exposed correlated with IL-6 (r = 0.46, p < 0.05) concentrations. These findings indicate that moderate primary blast exposure results in changes, specifically acute and transient increases in peripheral inflammatory markers which may have implications for neuronal health.
Keywords: blast, traumatic brain injury (TBI), military, inflammation, cytokines
1. Introduction
Blast exposures during deployment are a signature injury of the Operation Iraqi Freedom/Operation Enduring Freedom campaigns (Tanielian, 2008), which are linked to chronic neurological symptoms and impairments (Mac Donald et al., 2016a; Yurgil et al., 2014); yet, the mechanisms underlying these impairments remain poorly understood, thereby limiting clinical management. During deployments, there is a high overlap in blast and blunt-force traumatic brain injuries (TBIs) (Chandra and Sundaramurthy, 2015; Manners et al., 2016), obscuring the physiological impact of isolated blast exposure in humans (Mac Donald et al., 2016b; MacDonald et al., 2014). This is concerning, because in animal model blast-induced TBI (biTBI) has different signature features than blunt-force TBI (Courtney and Courtney, 2015). Previously, we have linked chronic neurological symptoms related to a combination of blast and TBIs in military personnel to greater inflammation, suggesting that inflammation may contribute to blast related pathology, yet no acute clinical studies to understand this relationship have been undertaken (Devoto et al., 2016). Therefore, clinical studies such as the one presented here are necessary to better understand how inflammatory changes shape both recovery, as well as pathology, following blast exposure without the impact of physical injuries, including blunt force TBIs.
In pre-clinical models of biTBI, insufficient regulation of inflammation negatively affects neuronal integrity and function (Elder et al., 2015). Cytokine elevations in the brain tissue of rodents exposed to blast are well-established (Cho et al., 2013; Simard et al., 2014), as are central and peripheral cytokine elevations after clinical blunt-force trauma (Ferreira et al., 2014; Kumar et al., 2016; Plesnila, 2016; Santarsieri et al., 2015). However, there remains a gap in the knowledge surrounding inflammatory consequences of primary blast exposure in humans. To address this unknown, a unique military cohort exposed to primary blast during a 10-day training course was examined for correlations within peripheral cytokine levels to blast exposure data using helmet-mounted sensors. Inflammatory proteins (TNFα, IL-6 and IL-10) were compared over time across two matched groups: moderate blast exposed cases and no/low blast exposed controls. We assayed the temporal profile of the aforementioned cytokines due to the close interaction network that exists between the proinflammatory cytokines, IL-6 and TNFα and the anti-inflammatory cytokine IL-10 (Black, 2002; Hansel et al., 2010). Furthermore, these cytokines are well-characterized and well-studied biomarkers within pre-clinical and clinical studies of TBI classification (Woodcock and Morganti-Kossmann, 2013).
2. Methods
2.1 Participants
The protocol was approved by the Naval Medical Research Center and Walter Reed Army Institute of Research Institutional Review Boards (NMCR#2011.0002; WRAIR#1796) (Carr et al., 2016). All participants provided informed consent prior to data collection. Methods are described in detail elsewhere (Carr et al., 2015). Briefly, military personnel involved in a 10-day blast-related training program had daily blood draws. Two groups were compared: 1) moderate blast exposed cases (n = 30) characterized by a peak pressure greater than 5 psi on day 7 of training, and 2) no/low blast controls (n = 32), who had no blast exposure exceeding 2 psi throughout the course. Cases and controls were matched based on self-reported age, duration of military service, and prior blast exposure(s).
2.2 Blast Characteristics
Participants wore helmets with bilateral pressure sensors (micro Data Acquisition System, μDAS; Applied Research Associates, Inc.) mounted above the ear cups. The average of peak overpressure from both left and right sensors were used as raw data for analyses.
2.3 Mood Changes
A questionnaire described in depth previously (Carr et al., 2016), was used to determine daily changes in symptoms including the following: irritability, feelings of depression or sadness, frustration, and feeling anxious or tense. Changes in reporting were compared between the groups.
2.4 Blood Sampling
Blood was drawn and processed for serum. Serum was aliquoted and stored at −80°C until batch processing. Plasma samples were obtained on all training days between 1600–1800 hours to control for known diurnal variation in circulating cytokine levels (Altara et al., 2015; Petrovsky et al., 1998; Scheff et al., 2010; Vgontzas et al., 2005).
2.5 Laboratory Methods
Concentrations of TNFα, IL-6 and IL-10 in plasma samples were analyzed using a high-definition-1 (HD-1) immunoassay analyzer, Simoa™, which runs ultra-sensitive paramagnetic bead-based enzyme-linked immunosorbent assays (ELISAs) (Mondello et al., 2014). The TNFα, IL-6 and IL-10 assays have low limits of detection (0.126 pg/ml, 0.330 pg/ml and 0.034 pg/ml, respectively). The intra- and inter-plate concentration values (CV) were < 7% for all analytes.
2.6 Statistical Methods
Statistical analyses were conducted with SPSS version 22 (IBM Corporation, Chicago, IL), and figures were developed using GraphPad Prism version 6.02 (Graph Pad Software, San Diego, CA). Baseline characteristics were compared between moderate blast cases and no/low blast controls using ANOVA (age; duration of service) and chi-square tests (number previous blast exposures).
TNFα, IL-6 and IL-10 were treated as continuous data, and the Shapiro-Wilk test was used to test the assumption of normality. Repeated measures ANOVA was used to determine if concentrations differed across the two groups and explore the effects of time. Mean change from baseline (i.e. day 1 of the study, during which neither group was exposed to blast) to training day 7 (the day when some participants experienced a moderate blast exposure) was also compared across the cases and controls using ANOVA. The relationship between cytokine levels and overpressure exposures were examined using Pearson’s correlation. Change in mood symptoms was examined using chi-square to compare the groups on the number of participants with an increase in any of the 4 symptoms from baseline to day 7.
3. Results
3.1 Participants
This sample included male, active duty Army members from a blast-related training course. The mean age was 30.55 years (SD = 4.88) and the majority of the participants reported a previous blast exposure, with 43.75% reporting more than 100 exposures. The two groups did not significantly differ at baseline with respect to age, service duration, and number of previous blast exposures (Table 1).
Table 1.
Comparison of baseline characteristics in the moderate blast cases and no/low blast controls.
| No/Low Blast (n= 32) | Moderate Blast (n= 30) | Significance | |
|---|---|---|---|
| Mean Age in Years (SD) | 29.91 (4.00) | 31.33 (4.88) | F1,61= 1.80; p= 0.20 |
| Mean Years of Service (SD) | 10.40 (4.59) | 8.56 (4.77) | F1,61= 3.01; p= 0.08 |
| Number of Prior Breaches and Artillery Fires, % (#) | 6.25% (2) | 13.30% (4) | χ2= 3.38; p= 0.28 |
| 0 | |||
| 1–9 | 12.50% (4) | 16.70% (5) | |
| 10–39 | 37.50% (12) | 26.70% (8) | |
| 40–99 | 12.5% (4) | 30.00% (9) | |
| 100–199 | 31.25% (10) | 20.00% (6) | |
| 200–399 | 6.25% (2) | 26.70% (8) | |
| 400+ | 6.25% (2) | 10.00% (3) |
Cases and controls had different average peak overpressures recorded on training day 7 (F61,1 = 565.03, p < 0.01), with the moderate blast cases having a mean peak overpressure of 8.52 psi (SD = 1.65) compared to 0.52 psi (SD = 0.49) in the no/low blast controls. In the moderate blast cases the range of average peak overpressure exposure was 5.06–11.73 psi.
3.2 Inflammatory Protein Changes Following Blast Exposure
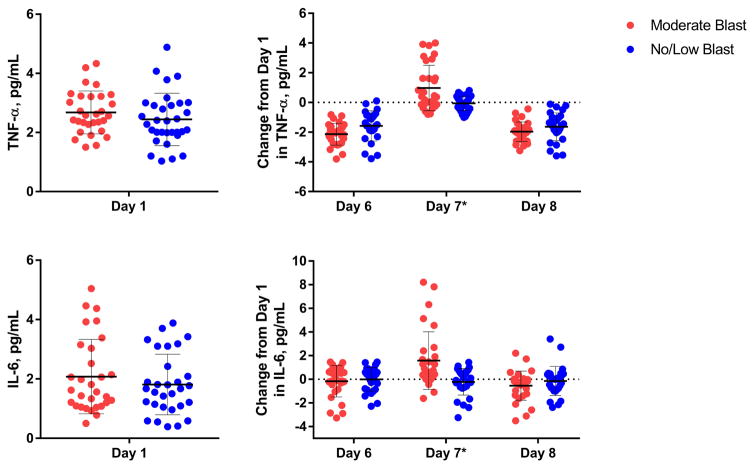
There were no participants with cytokine levels below the detectable limit of the assay. Significant differences in the longitudinal pattern of TNFα (F1,60 = 12.03, p < 0.01) and IL-6 on day 7 (F1,60 = 18.81, p < 0.01) were observed using repeated measures ANOVA, with levels in moderate blast cases increasing significantly from baseline (Figure 1).
Figure 1.
Comparison of moderate blast cases and no/low blast controls on inflammatory cytokine levels. Baseline (day 1) levels of IL-6 and TNFα are shown, as well as changes from these baseline levels to days 6, 7 and 8. There was no significant change in the total number of mood symptoms (p=0.22), or a single symptom (p’s= 0.12–0.37) between the groups.
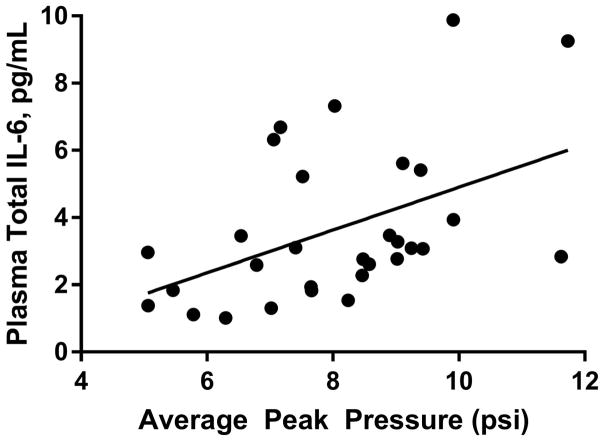
There were no significant differences in pattern of IL-10 across the groups. On day 7 of training, TNFα increased by 1.22 pg/mL (SD = 0.82) in moderate blast cases, whereas levels significantly decreased (F1,59 = 8.54, p < 0.01) by 0.18 pg/mL (SD = 0.41) in the no/low blast controls. Among moderate blast cases, IL-6 levels significantly increasedF1,59 = 4.668, p < 0.05) from their own baseline measurements (day 1) to levels on the day of moderate blast exposure (day 7); day 7 IL-6 levels were 1.48 pg/mL (SD = 1.01) higher than baseline levels. Conversely, in the no/low blast control group IL-6 levels reduced by 0.024 pg/mL (SD = 0.89). In the moderate blast exposed cases, the average peak overpressure on day 7 was significantly correlated with total IL-6 (r = 0.46, p < 0.05) (Figure 2), with TNFα trending toward significance (r = 0.32, p = 0.08).
Figure 2.
Correlation between IL-6 and average peak overpressure on the day 7 (when cases experienced moderate blast exposure (training day 7).
4. Discussion
This study is the first to report that moderate primary blast exposure results in elevated peripheral cytokines (TNFα and IL-6), which correlated to the peak overpressure. Examining biomarker levels in isolated moderate blast cases for the first time makes an important contribution to the evidence; this line of inquiry may eventually lead to better detection of military personnel who were exposed to blast while not wearing a helmet with pressure sensors. This observation of increased IL-6 after moderate blast exposure in humans is unique, and consistent with the pre-clinical evidence which suggest central and peripheral IL-6 activity occurs after primary blast exposure (Kamnaksh et al., 2011). Increased IL-6 after blast exposure may have clinical ramifications, since pre-clinical blast studies and clinical studies of blunt-force TBIs report associations between central and peripheral IL-6 levels and poor outcomes (Kumar et al., 2015a; Kumar et al., 2015b; Minambres et al., 2003; Yang et al., 2013). Established physiological consequences of elevated IL-6 include ultrastructural changes in endothelial cells (Vajtr et al., 2009) and neuroinflammation (Erta et al., 2012; Kumar et al., 2015b; Yang et al., 2013). Neuroinflammation is one of the major secondary injury processes following TBI that has been identified as a potentially promising therapeutic target (Lozano et al., 2015). Notably, inflammation can lead to further damage to the brain by contributing to processes such as edema (Stamatovic et al., 2006) and cellular death (Carson et al., 2006; Wallach et al., 2014).
A limitation of this study is that it is impossible to currently define the direct source of inflammatory activity, whether it stems from central or peripheral processes. While the helmet sensors confirm an overpressure to the head, the periphery was also exposed, though this was not measured with body sensors. This study is further limited by the relatively small sample size, limiting our ability to determine the influence of previous blast exposures on inflammatory activity. Therefore, additional studies are needed to confirm these findings and examine whether past exposures confound results. Another issue that should be considered is the impact of blast on behavioral, neurological and psychological symptoms, and other training related factors other than blast that can contribute to cytokine changes. Substantial evidence exists to support the release of proinflammatory cytokines, such as IL-6 and TNFα in response to physiological stressors through the neuroendocrine pathway (Black, 2002; Hansel et al., 2010). More long-term studies are needed to further determine whether physiological stressors, resulting from blast exposure, alters circulating cytokine levels, or if blast exposure, independent of stress, influences the inflammatory response observed in our findings. This information could also be used to identify individuals in need of assessment and, when warranted, treatment. Still, given this is the first published study, it presents an important contribution to the literature, especially considering the novel sample, objective measure of blast exposure, and use of ultra-sensitive laboratory methods. This work can be built on with additional clinical studies aimed at clarifying and expanding upon the present findings by inclusion of additional biomarkers, clinical outcomes, and other biomarker changes.
5. Conclusion
The findings of this study suggest that primary moderate blast exposure in military personnel is associated with acute inflammatory responses, indicated by increases in both TNFα and IL-6 levels following blast. The cytokine levels in moderate blast cases were significantly higher than individual baseline levels, and also higher than matched no/low blast controls engaged in similar training. Future studies should replicate and expand on this work to assess neuronal pathology, symptoms, and long-term outcomes following both isolated and repeated blast exposures, with and without concomitant blunt-force trauma.
Highlights.
Pro-inflammatory cytokines are, IL-6 and TNFα, elevated acutely following blast exposure.
Increased cytokines correlate to the degree the peak overpressure of blast exposure.
Increased cytokine levels may have implications for neuronal health.
Acknowledgments
This study was funded by the National Institute of Nursing Research, Intramural Program, and by the Walter Reed Army Institute of Research. We would like to thank the service members who participated in this study.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altara R, Manca M, Hermans KC, Daskalopoulos EP, Brunner-La Rocca HP, Hermans RJ, Struijker-Boudier HA, Blankesteijn MW. Diurnal rhythms of serum and plasma cytokine profiles in healthy elderly individuals assessed using membrane based multiplexed immunoassay. J Transl Med. 2015;13:129. doi: 10.1186/s12967-015-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Carr W, Stone JR, Walilko T, Young LA, Snook TL, Paggi ME, Tsao JW, Jankosky CJ, Parish RV, Ahlers ST. Repeated Low-Level Blast Exposure: A Descriptive Human Subjects Study. Military medicine. 2016;181:28–39. doi: 10.7205/MILMED-D-15-00137. [DOI] [PubMed] [Google Scholar]
- Carr W, Yarnell AM, Ong R, Walilko T, Kamimori GH, da Silva U, McCarron RM, LoPresti ML. Ubiquitin carboxy-terminal hydrolase-l1 as a serum neurotrauma biomarker for exposure to occupational low-level blast. Front Neurol. 2015;6:49. doi: 10.3389/fneur.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res. 2006;6:237–245. doi: 10.1016/j.cnr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra N, Sundaramurthy A. Acute Pathophysiology of Blast Injury-From Biomechanics to Experiments and Computations: Implications on Head and Polytrauma. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): 2015. [Google Scholar]
- Cho HJ, Sajja VS, Vandevord PJ, Lee YW. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience. 2013;253:9–20. doi: 10.1016/j.neuroscience.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Courtney A, Courtney M. The Complexity of Biomechanics Causing Primary Blast-Induced Traumatic Brain Injury: A Review of Potential Mechanisms. Front Neurol. 2015;6:221. doi: 10.3389/fneur.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto C, Arcurio L, Fetta J, Ley M, Rodney T, Kanefsky RZ, Gill J. Inflammation Relates to Chronic Behavioral and Neurological Symptoms in Military with Traumatic Brain Injuries. Cell Transplant. 2016 doi: 10.1177/0963689717714098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Gama Sosa MA, De Gasperi R, Stone JR, Dickstein DL, Haghighi F, Hof PR, Ahlers ST. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front Neurol. 2015;6:48. doi: 10.3389/fneur.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LC, Regner A, Miotto KD, Moura S, Ikuta N, Vargas AE, Chies JA, Simon D. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain injury. 2014;28:1311–1316. doi: 10.3109/02699052.2014.916818. [DOI] [PubMed] [Google Scholar]
- Hansel A, Hong S, Camara RJ, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kamnaksh A, Kovesdi E, Kwon SK, Wingo D, Ahmed F, Grunberg NE, Long J, Agoston DV. Factors affecting blast traumatic brain injury. J Neurotrauma. 2011;28:2145–2153. doi: 10.1089/neu.2011.1983. [DOI] [PubMed] [Google Scholar]
- Kumar RG, Boles JA, Wagner AK. Chronic Inflammation After Severe Traumatic Brain Injury: Characterization and Associations With Outcome at 6 and 12 Months Postinjury. J Head Trauma Rehabil. 2015a;30:369–381. doi: 10.1097/HTR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- Kumar RG, Diamond ML, Boles JA, Berger RP, Tisherman SA, Kochanek PM, Wagner AK. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav Immun. 2015b;45:253–262. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Kumar RG, Rubin JE, Berger RP, Kochanek PM, Wagner AK. Principal components derived from CSF inflammatory profiles predict outcome in survivors after severe traumatic brain injury. Brain, behavior, and immunity. 2016;53:183–193. doi: 10.1016/j.bbi.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Adam OR, Rivet DJ, Flaherty CS, Oh LC, Zonies LC, Fang CR, Brody DL. Outcome Trends after US Military Concussive Traumatic Brain Injury. J Neurotrauma. 2016a doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, Werner NJ, Adam OR, Rivet DJ, Flaherty SF, Oh JS, Zonies D, Fang R, Brody DL. Outcome Trends after US Military Concussive Traumatic Brain Injury. Journal of neurotrauma. 2016b doi: 10.1089/neu.2016.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CL, Johnson AM, Nelson EC, Werner NJ, Fang R, Flaherty SF, Brody DL. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma. 2014;31:889–898. doi: 10.1089/neu.2013.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners JL, Forsten RD, Kotwal RS, Elbin RJ, Collins MW, Kontos AP. Role of Pre-Morbid Factors and Exposure to Blast Mild Traumatic Brain Injury on Post-Traumatic Stress in United States Military Personnel. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minambres E, Cemborain A, Sanchez-Velasco P, Gandarillas M, Diaz-Reganon G, Sanchez-Gonzalez U, Leyva-Cobian F. Correlation between transcranial interleukin-6 gradient and outcome in patients with acute brain injury. Crit Care Med. 2003;31:933–938. doi: 10.1097/01.CCM.0000055370.66389.59. [DOI] [PubMed] [Google Scholar]
- Mondello S, Buki A, Barzo P, Randall J, Provuncher G, Hanlon D, Wilson D, Kobeissy F, Jeromin A. CSF and plasma amyloid-beta temporal profiles and relationships with neurological status and mortality after severe traumatic brain injury. Scientific reports. 2014;4:6446. doi: 10.1038/srep06446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Plesnila N. The immune system in traumatic brain injury. Curr Opin Pharmacol. 2016;26:110–117. doi: 10.1016/j.coph.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Santarsieri M, Kumar RG, Kochanek PM, Berga S, Wagner AK. Variable neuroendocrine-immune dysfunction in individuals with unfavorable outcome after severe traumatic brain injury. Brain, behavior, and immunity. 2015;45:15–27. doi: 10.1016/j.bbi.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff JD, Calvano SE, Lowry SF, Androulakis IP. Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol. 2010;264:1068–1076. doi: 10.1016/j.jtbi.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Simard JM, Pampori A, Keledjian K, Tosun C, Schwartzbauer G, Ivanova S, Gerzanich V. Exposure of the thorax to a sublethal blast wave causes a hydrodynamic pulse that leads to perivenular inflammation in the brain. Journal of neurotrauma. 2014;31:1292–1304. doi: 10.1089/neu.2013.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl. 2006;96:444–450. doi: 10.1007/3-211-30714-1_91. [DOI] [PubMed] [Google Scholar]
- Tanielian T, Jaycox L. Invisible wounds of war. RAND Corperation; 2008. [Google Scholar]
- Vajtr D, Benada O, Kukacka J, Prusa R, Houstava L, Toupalik P, Kizek R. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol Res. 2009;58:263–268. doi: 10.33549/physiolres.931253. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol. 2014;14:51–59. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Gangidine M, Pritts TA, Goodman MD, Lentsch AB. Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock. 2013;40:471–475. doi: 10.1097/SHK.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, Litz BT, Nash WP, Baker DG Marine Resiliency Study T. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA psychiatry. 2014;71:149–157. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]