Abstract
We aimed to describe our experience with metaplastic breast carcinoma (MBC), evaluate its clinical outcome compared with triple negative breast cancer (TNBC), and provide a through and comprehensive review of the literature to date. We reviewed MBC cases (n=46) from our institution. The following variables were recorded, tumor histologic subtype, Nottingham grade, tumor size, lymph node status, TNM-stage, biomarkers profile, patient’s age and race, therapy modality (chemotherapy and radiation), and survival [disease free survival (DFS) and overall survival (OS)]. The clinical and pathological data for TNBC (n=508) cases was extracted from the BC database. In order to compare the survival between MBC and TNBC, a subgroup of MBC (n=40) were matched with TNBC (n=40) cases based on known prognostic confounders. There were 17 of 46 (37%) cases with mesenchymal differentiation, 12 (26.1%) squamous cell carcinoma, 14 (30.4%) spindle cell carcinoma, and 3 (6.5%) mixed type. MBC presented at a more advanced stage than TNBC (p=0.014) and was more likely to recur 34% vs. 15.5% (p= 0.004). More MBC patients died from disease than TNBC, 29% vs. 16% (p= 0.05). In the multivariate analysis, MBC had about twice the risk of local recurrence than TNBC (95% CI 1.01–3.83, p=0.05). MBC patients had worse DFS and OS than the matched TNBC patients (p <0.001 and p=0.033, respectively). A review of literature comparing MBC vs. TNBC is presented. Our results suggest that MBC is clinically more aggressive than TNBC. Further studies may help delineate the differences between these two entities.
Keywords: metaplastic breast carcinoma, triple negative breast carcinoma, survival
INTRODUCTION
Metaplastic breast cancer (MBC) is a rare and morphologically diverse group of tumors in which a variable proportion or the entire tumor is composed of non-glandular epithelium or mesenchymal cells. The 2011 World Health Organization (WHO) Working Group recognizes the following five subtypes: squamous cell carcinoma (SCC), MBC with mesenchymal differentiation, low grade adenosquamous carcinoma, spindle cell carcinoma and fibromatosis-like metaplastic carcinoma. MBC accounts for about 0.2–5% of all BC. However, the prevalence varies to such an extent, given the different definitions adopted by different authors. Pure metaplastic carcinoma has been reported to account for about 1% of all BC. In this study we adopted the criteria set by the WHO (1).
Metaplastic breast carcinomas present similar clinical features and age distribution to estrogen receptor (ER) negative invasive carcinoma of no special type (IC-NST) (2–3). A relatively consistent observation across many studies suggests that MBC tends to present with negative biomarkers [ER, progesterone receptor (PR) and HER2] (4–7). This has often led to its generalization with TNBC, which is a separate and distinct category of BC with different clinical behavior and treatment. Although most MBC have triple negative phenotype, anecdotally, the clinical outcomes seem different from TNBC.
Nevertheless, few studies have compared the two entities side by side and the existing literature may overlook the differences in these two groups, largely in part due to small numbers and the inclusion of the favorable subtypes (4,8–15). In our experience the clinical outcome seems to be worse than TNBC. While some studies agree with this observation (4,9,11,12), other studies showed that there was no significant difference between these two subgroups (7,8,10,15). One of the issues in these previously published studies is how close the cases (MBC vs. TNBC) were matched. Therefore, in this study we aimed to carefully match these two groups in terms of patient’s age, Nottingham grade, tumor stage, and therapy modality including chemotherapy (CT) and radiation therapy (RT) with relatively long follow-up time. Then, we compared the disease free survival (DFS) and overall survival (OS).
MATERIAL AND METHODS
Institutional review board approved this retrospective study (BDR #040413).
Metaplastic Breast Carcinoma Cases
A retrospective review of a prospectively collected database was performed for MBC at Roswell Park Cancer Institute from 1992 to 2013, including all cases of MBC diagnosed between 1992 and 2013. This resulted in 81 cases which were then re-reviewed jointly by two pathologists (TK, DE) according to the 2011 WHO classification (1). The Hematoxylin and Eosin slides were reviewed (1 to 25 slides per case with median of 9 slides per case) and included if both of the following criteria were met; 1) the tumor had to have a component of invasive carcinoma or ductal carcinoma in situ (DCIS) with at least 10% metaplastic component. 2) When the tumor is pure sarcomatoid, it had to have at least one epithelial marker expression by immunohistochemistry (pancytokeratin, cytokeratin 5/6, high molecular weight cytokeratin, and/or p63).
The percentage of each metaplastic component (mesenchymal differentiation, SCC, spindle cell) and the non-metaplastic component was estimated. The non-metaplastic components were described as IC-NST and/or lobular. The case was considered metaplastic when the metaplastic component comprised >10% of the tumor cells. The case was considered mixed metaplastic type when at least two metaplastic components were recognized, each comprising at least 10% of the tumor.
The following clinicopathologic data were recorded; patient’s age, race, therapy modality (adjuvant, neoadjuvant; CT and RT), tumor size, lymph node status, AJCC TNM stage, biomarkers profile (ER, PR, and HER2), and survival data including DFS and OS.
Triple Negative Breast Cancer cases
All TNBC cases were retrieved from the prospectively maintained BC database in the same time frame. The inclusion criteria were 1) ER-/PR-/HER2-, 2) tumor size larger than 1-mm, 3) not a metaplastic carcinoma, and 4) have clinical, pathologic and treatment data available. The search yielded 508 cases. The clinical data including patient age, AJCC TNM stage, therapy modality (adjuvant, neoadjuvant, CT, RT), and survival data including DFS and OS were extracted from the database. For the TNBC matched cases (n=40) to the MBC cases, the slides were reviewed confirming the pathology diagnosis.
Statistical analysis
The clinicopathologic factors were compared between selected cases of MBC and TNBC subtypes. Fisher’s exact test was used for categorical variables while Wilcoxon non-parametric test for continuous variables. All comparisons were two-sided and a p-value of 0.05 was considered significant. Univariate cox proportional hazard analysis is performed to assess the risk of breast tumor recurrence and mortality relative to the clinicopathologic factors in breast cancer cases. The significant clinicopathologic factors in the univariate analysis were then included in the multivariate analysis.
The cumulative survival differences for MBC and TNBC cases were assessed using the log-rank method. Case matching was used to exclude the cofounding factors in survival analysis. We included only the MBC cases with complete survival data (n=40) and matched these to the TNBC group. The matching included patient age using 50 years as the cutoff, as well as pathology stage (I, II and III), Nottingham grade (1, 2, 3), therapy type (adjuvant, neoadjuvant) and finally therapy modality (CT, RT). When multiple patients were matched to a single patient, the patient with closest age was chosen. Cumulative survival rates of the matched breast cancer cases were analyzed using the Kaplan-Meier (KM) estimate method. All statistical analysis was performed using R version 3.2.2 (http://www.r-project.org).
RESULTS
Clinicopathologic Characteristics of MBC and TNBC
There were a total of 81 cases reported as MBC. Upon histologic review 28 cases were re-classified to IC-NST. We further excluded four cases with incomplete data and three cases which were stage IV disease at presentation. Therefore, the final number included in this analysis was 46. There were 17 of 46 (37%) cases with mesenchymal differentiation, 12 (26.1%) SCC, 14 (30.4%) spindle cell carcinoma, and 3 (6.5%) mixed type.
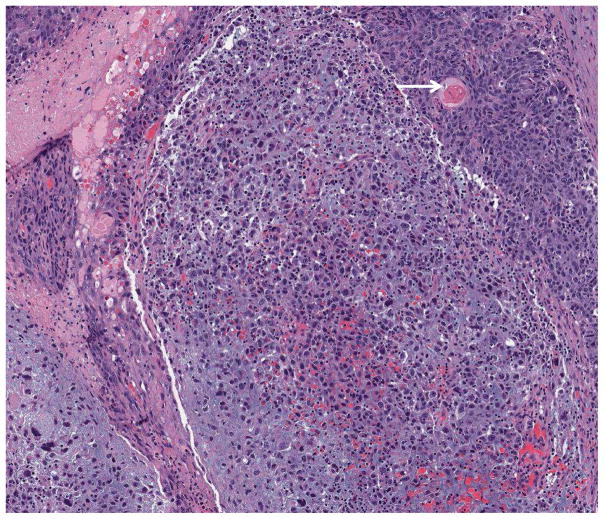
There were 37 (80.4%) cases mixed with non-metaplastic BC (35 IC-NST and2 lobular). Mixed MBC was seen in 3 cases; one mesenchymal (20%)/spindle cell (30%)/non-metaplastic (50%); one SCC (60%)/spindle cell (30%)/non-metaplastic (10%); and one with SCC (80%)/mesenchymal (20%) (figure 1). Squamous cell carcinoma was keratinizing in 4 cases and non-keratinizing in 8 cases. Chondroid differentiation was detected in 14 cases and osteoid in 3.
Figure 1.
Histologic example of mixed MBC, mesenchymal differentiation (chondroid) and SCC (arrow marks squamous pearl) (4x).
Table 1 illustrates the clinicopathologic characteristics of MBC and TNBC cases. There was no significant difference in the mean age between MBC and TNBC patients. MBC cases were more likely to present in an advanced stage (p=0.014). Lymph node involvement on the other hand was not statistically different between the two groups. The median and range of tumor size was 1.7-cm (0.2-cm to 13-cm) for TNBC vs. 3.1-cm (0.5-cm to 14-cm) for MBC (p<0.001). Comparing to TNBC, patients with MBC tend to more often have mastectomy procedure (63% vs. 31%, p<0.001) or CT (91% vs. 76%, p=0.024). On the other hand patients with TNBC tend to receive RT more often (80% vs. 59%, p=0.002). The histologic subtypes of the TNBC cases were 460 (90.6%) IC-NST, 28 (5.5%) mixed IC-NST/lobular, 16 (3.1) lobular and 4 (0.8%) apocrine.
Table 1.
Clinicopathologic characteristics of MBC and TNBC cases.
| Variables | TNBC (n=508) | MBC (n=46) | P-value | |
|---|---|---|---|---|
| Age (years) | Median (range) | 57(25,91) | 54.5(42,90) | 0.51 |
| Race | African American | 95(94.1)* | 6(5.9) | 0.637 |
| Others | 12(92.3) | 1(7.7) | ||
| Caucasian | 401(91.1) | 39(8.9) | ||
| Tumor Size(cm) | Median(range) | 1.7(0.2,13) | 3.1(0.5,14) | <0.001 |
| T-stage | 1 | 317(95.8) | 14(4.2) | <0.001 |
| 2 | 160(88.9) | 20(11.1) | ||
| 3 | 28(73.7) | 10(26.3) | ||
| 4 | 3(60.0) | 2(40.0) | ||
| No. positive nodes | Median (range) | 0(0,32) | 0(0,14) | 0.486 |
| N-stage | Negative | 340(91.4) | 32(8.6) | 0.622 |
| Positive | 168(92.8) | 13(7.2) | ||
| Stage | I | 242(95.3) | 12(4.7) | 0.014 |
| II | 201(88.2) | 27(11.8) | ||
| III | 65(90.3) | 7(9.7) | ||
| Nottingham Grade | 1 | 5(100.0) | 0(0.0) | 0.064 |
| 2 | 47(100.0) | 0(0.0) | ||
| 3 | 456(90.8) | 46(9.2) | ||
| Surgery | Lumpectomy | 351(95.4) | 17(4.6) | <0.001 |
| Mastectomy | 157(84.4) | 29(15.6) | ||
| Neoadjuvant | No | 439(92.4) | 36(7.6) | 0.128 |
| Yes | 69(87.3) | 10(12.7) | ||
| Radiation Therapy | No | 100(84.0) | 19(16.0) | 0.002 |
| Yes | 403(93.7) | 27(6.3) | ||
| Chemotherapy | No | 119(96.8) | 4(3.2) | 0.024 |
| Yes | 386(90.4) | 41(9.6) |
N(%);
MBC Metaplastic breast cancer, TNBC Triple negative breast cancer
Thirty-three of 46 (78.6%) MBC patients were treated with doxorubicin hydrochloride (adriamycin) and cyclophosphamide, with or without paclitaxel (taxol) (AC/T) or docetaxel (taxotere). Two patients were given cyclophosphamide, methotrexate, and 5-fluorouracil (CMF). A single patient was given a sarcoma regimen of mesnex, adriamycin, ifosfamide, and dacarbazine (MAID) who developed lung metastases and died from disease 12 month after initial diagnosis. Three patients received carboplatin based chemotherapy and two patients received gemcitabine based chemotherapy. Herceptin was given as a single agent to a single patient, while it was given in combination with AC/T to another patient.
Estrogen receptor was positive in 4 (9%) cases, PR in 6 (13%) and HER2 in 3 (6.5%). Two cases were positive for both ER and PR, two for ER but not PR and four for PR but not ER. Six patients were treated with HT, one of which was triple negative. One of three patients with HER2 positive tumor was treated with trastuzumab.
Univariate and multivariate analyses of DFS and OS of MBC vs. TNBC
The median follow up for MBC patients was 41 months (range 3 to168) and for TNBC patients, 67 months (range 5 to 219). Disease recurrence occurred in 14 (30%) patients with MBC and 78 (15%) with TNBC (p= 0.004). Death due to disease occurred in 12 of 42 (28.6%) patients with MBC and 80 of 508 (15.7%) with TNBC (p=0.05). In univariate analysis, patients with MBC had worse 5-year DFS than patients with TNBC (30% vs. 89.9%, <0.001). Patients with MBC had worse 5-year OS than patients with TNBC (65.3% vs. 86.6%, p=0.002) (Table 2).
Table 2.
Univariate analysis of the DFS and OS of MBC and TNBC
| Characteristic | Patients (n) | 5-year DFS (%) | P-value | 5-year OS (%) | P-value | |
|---|---|---|---|---|---|---|
| Age | <50 | 169(31.4)* | 86.1 | 0.272 | 82.7 | 0.261 |
| ≥50 | 370(68.6) | 89.9 | 86.4 | |||
| Race | African American | 99(18.4) | 88.7 | 0.628 | 84.9 | 0.855 |
| Others | 11(2.0) | 90.9 | 78.8 | |||
| Caucasian | 429(79.6) | 88.7 | 85.4 | |||
| Histologic Type | MBC | 40(7.4) | 73.7 | <0.001 | 65.3 | 0.002 |
| TNBC | 499(92.6) | 89.9 | 86.6 | |||
| T-Stage | 1 | 324(60.1) | 94.6 | <0.001 | 90.4 | <0.001 |
| 2 | 174(32.3) | 84 | 80.4 | |||
| 3 | 36(6.7) | 65.4 | 69 | |||
| 4 | 5(0.9) | 40 | 40 | |||
| N-Stage | Negative | 365(67.8) | 93.5 | <0.001 | 91.6 | <0.001 |
| Positive | 173(32.2) | 78.9 | 73.1 | |||
| Pathology-Stage | I | 249(46.2) | 97.5 | <0.001 | 94.2 | <0.001 |
| II | 221(41.0) | 86 | 82.4 | |||
| III | 69(12.8) | 66.4 | 62.8 | |||
| Nottingham Grade | 1 | 5(0.9) | 100 | 0.417 | 100 | 0.689 |
| 2 | 46(8.5) | 93.3 | 90 | |||
| 3 | 488(90.5) | 88.2 | 84.5 | |||
| Surgery | Lumpectomy | 360(66.8) | 93.7 | <0.001 | 89.6 | <0.001 |
| Mastectomy | 179(33.2) | 78.6 | 76.1 | |||
| Neoadjuvant | No | 462(85.7) | 92.1 | <0.001 | 88.8 | <0.001 |
| Yes | 77(14.3) | 68.5 | 64.5 | |||
| Radiation Therapy | No | 113(21.2) | 83.3 | 0.108 | 79.9 | 0.044 |
| Yes | 421(78.8) | 90.1 | 86.5 | |||
| Chemotherapy | No | 122(22.7) | 92.1 | 0.177 | 89.8 | 0.151 |
| Yes | 415(77.3) | 87.7 | 84 |
N(%);
MBC Metaplastic breast cancer, TNBC Triple negative breast cancer; DFS, disease free survival; OS, overall survival
In the multivariate analysis patients with MBC had almost twice (1.99, 95% CI 1.01–3.83) the risk of disease recurrence than patients with TNBC (p=0.05). However, there was no statistical difference between the two diseases in terms of OS. The other variables that had significant effect on both DFS and OS were T-stage, lymph node status, neoadjuvant therapy, and RT. Chemotherapy had only a borderline effect on the DFS but not the OS (Table 3).
Table 3.
Multivariate analysis of DFS and OS of MBC and TNBC
| Characteristic | DFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| MBC vs. TNBC | 1.99 | (1.01–3.83) | 0.05 | 1.50 | (0.75–2.97) | 0.250 |
| T2 vs. T1 | 2.34 | (1.44–3.94) | 0.001 | 2.34 | (1.41–3.88) | 0.001 |
| T3 vs. T1 | 2.07 | (1.01–4.28) | 0.048 | 2.52 | (1.22–5.19) | 0.012 |
| T4 vs. T1 | 7.35 | (1.96–27.56) | 0.003 | 10.39 | (2.88–37.51) | <0.001 |
| N (Positive vs. Negative) | 3.53 | (2.21–5.65) | <0.001 | 2.80 | (1.75–4.47) | <0.001 |
| Neoadjuvant, Yes vs. No | 3.39 | (2.01–5.74) | <0.001 | 3.45 | (2.07–5.75) | <0.001 |
| Radiation, No vs. Yes | 2.19 | (1.30–3.71) | 0.003 | 2.17 | (1.29–3.67) | 0.004 |
| Chemotherapy, No vs. Yes | 1.84 | (0.94–3.61) | 0.076 | 1.67 | (0.84–3.33) | 0.144 |
MBC Metaplastic breast cancer, TNBC Triple negative breast cancer; DFS, disease free survival; OS, overall survival
Survival results for MBC vs. overall TNBC
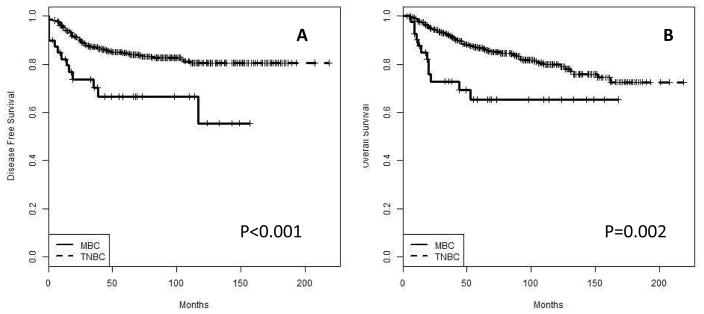
Metaplastic breast carcinoma had worse DFS and OS than TNBC (p<0.001 and 0.002, respectively) (figure 2a and b). Disease recurrence occurred in 30 % of MBC vs. 15.5 % for TNBC. Death due to disease occurred in 28.6% for patients with MBC vs. 15.7% for patients with TNBC.
Figure 2.
Disease free (A) and overall (B) survival of MBC vs. TNBC (overall).
Survival results for matching group (MBC vs. TNBC)
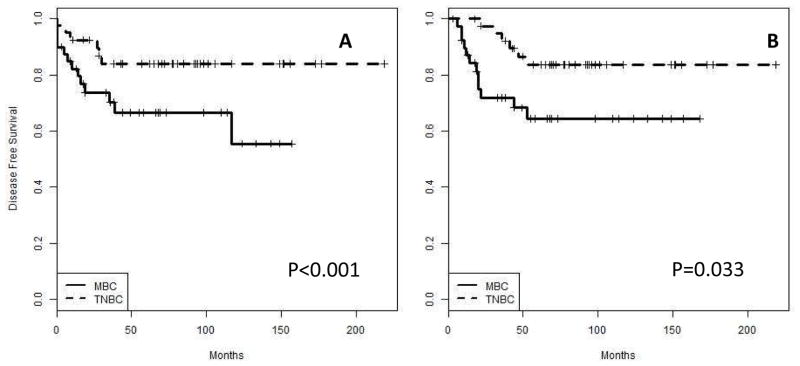
There were almost perfect matching between MBC (n=40) and the selected TNBC cases (n=40) (table 4). When we performed KM curves for DFS, MBC had worse DFS than TNBC (p<0.001) (figure 3a). Disease recurrence occurred in 32.5% of MBC vs. 15% for TNBC. Similarly, patients with MBC had worse OS than patients with TNBC (p=0.03, figure 3b). Death due to disease occurred in 30% for patients with MBC vs. 15% for patients with TNBC.
Table 4.
Comparison of clinicopathologic characteristics of MBC (n=40) and matched TNBC (n=40)
| Variables | Overall (n=80) | TNBC (n=40) | MBC (n=40) | P-value | |
|---|---|---|---|---|---|
| Age (years) | median(range) | 56.5(28,90) | 59(28,77) | 55(42,90) | 0.281 |
| Stage | I | 24(30) | 12(50.0) | 12(50.0) | 1 |
| II | 46(57.5) | 23(50.0) | 23(50.0) | ||
| III | 10(12.5) | 5(50.0) | 5(50.0) | ||
| Tumor Grade | 1 | 0(0) | 0(0) | 0(0) | 1 |
| 2 | 0(0) | 0(0) | 0(0) | ||
| 3 | 80(100) | 40(50.0) | 40(50.0) | ||
| Surgery | Lumpectomy | 39(48.8) | 22(56.4) | 17(43.6) | 0.371 |
| Mastectomy | 41(52.2) | 18(43.9) | 23(56.1) | ||
| Neoadjuvant | No | 62(77.5) | 31(50.0) | 31(50.0) | 1 |
| Yes | 18(22.5) | 9(50.0) | 9(50.0) | ||
| Radiation Therapy | No | 30(37.5) | 15(50.0) | 15(50.0) | 1 |
| Yes | 50(62.5) | 25(50.0) | 25(50.0) | ||
| Chemotherapy | No | 8(10) | 4(50.0) | 4(50.0) | 1 |
| Yes | 72(90) | 36(50.0) | 36(50.0) |
MBC Metaplastic breast cancer, TNBC Triple negative breast cancer
Figure 3.
Disease free (A) and overall (B) survival of MBC vs. TNBC (matched).
Survival results for MBC subgroups
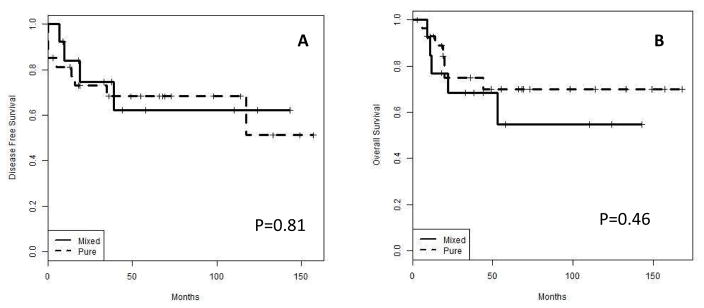
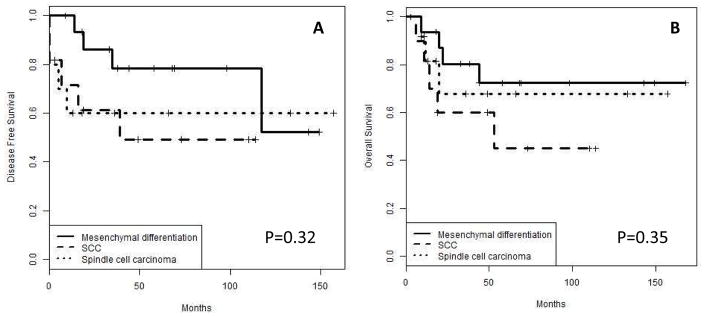
Pure MBC (n= 9) cases are compared to mixed with non-metaplastic (n=37). There was no statistical significant difference between these groups (figure 4). Also, the major three MBC groups [SCC (n=12), mesenchymal (n=17) and spindle cell (n=14)] were also compared (figure 5). There was a trend towards better local control for MBC with mesenchymal differentiation when compared with SCC or spindle cell carcinoma with no statistical significance (p=0.32).
Figure 4.
Disease free (A) and overall (B) survival of MBC, pure vs. mixed with non-metaplastic component.
Figure 5.
Disease free (A) and overall (B) survival of MBC, MBC with mesenchymal differentiation vs. SCC vs. spindle cell carcinoma. Note a trend towards less aggressive phenotype for MBC with mesenchymal differentiation.
DISCUSSION
Metaplastic breast carcinoma is increasingly perceived as an aggressive tumor with a poor prognosis. Several recent studies have focused on outcome in this rare and morphologically diverse malignancy. The studies, all retrospective cohorts, show conflicting results and are considerably different in design and analysis. One of the major issues in comparing survival is that MBC appears to have very different clinicopathologic parameters than other types of BC. These include larger tumor size, higher tumor grade at presentation, negative biomarkers and lower rates of lymph node metastases (4–7). In order to investigate the aggressive nature of this tumor we chose to compare its clinical outcome with TNBC. We chose TNBC for two reasons, first, it is the most aggressive known phenotype among all other phenotypes (16); 2, the majority of MBC is triple negative (4–7). Comparisons between the two groups may be subject to bias based on the differing presentations, and a matching or case control study is a reasonable way to decrease this bias. Moreover, having relatively higher number of cases would allow performing multivariate analysis.
We reviewed the literature to explore the clinical behavior of MBC compared to TNBC. The review is summarized in table 5. We recorded if matching was performed, the type of BC (all types combined or TNBC) that matched to MBC, the variables that the authors used to match MBC to TNBC, and finally if multivariate analysis was performed and what the outcome of the multivariate analysis. There was inconsistency in data handling and analysis. While some authors performed matching (4,8–13,15), some did not match instead they compared with either all TNBC (14) or with BC regardless of tumor molecular subtype (5). There was also inconsistency in the chosen variables for matching, ranging from only one variable such as Nottingham grade (4), or stage (12), to a more comprehensive matching including age, stage, and therapy modality (11 and current study). Multivariate analysis was not routinely performed by authors, where only three studies have (4,10,12). One study did not perform matching instead multivariate analysis was performed (10). All studies included all MBC types except Lester et al who included only sarcomatoid carcinoma (11) and Nayak et al who included only SCC (17). Some studies did not record the histologic subtypes (10,15). While some studies (4,9,11,12) showed a significant survival difference, others (8,10,13,15) concluded that while MBC tumors have poor prognostic indicators at presentation, they behave similarly to matched controls.
Table 5.
Review of studies that compared between MBC vs. TNBC or IDC-NST with similar biomarker profile
| Author [Ref] | Cases (No.) | Histologic subtypes (%) | - Matching criteria - No. case-to-case |
Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| DFS | OS | Performed (Yes/No) | DFS (HR) | OS (HR) | ||||
| Jung et al (4) | 35 | - SCC (60) - With mesenchymal differentiation (11.4) - Spindle cell carcinoma (11.4) - Mixed (14.3) - Low grade adenosquamous (2.9) |
- Grade - One-to-one |
MBC>TNBC | MBC>TNBC | Yes | 3.99 | 3.14 |
| Beaty et al (8) | 24 | - SCC (50) - With mesenchymal differentiation (12.5) - Spindle cell carcinoma (25) - Not stated (16.5)^ |
- Date of diagnosis, age, tumor size, node status, ER, PR, and HER2 (all cases TNBC) - Three-to-one |
NS | NS | No | NA | NA |
| Luini et al (9) | 37 | - SCC (18.9) - With mesenchymal differentiation (51.4) - Spindle cell carcinoma (8.1) - Carcinosarcoma (24.3) - With osteoclastic giant cell (2.7) |
- Grade, year of surgery, T-stage, N-stage - One-to-two |
NS | MBC>TNBC HR=5.0 |
No | NA | NA |
| Bae et al (10) | 47 | - Not recorded | No matching | Not calculated | Not calculated | Yes | NS | NS |
| Lester et al (11) | 47 | - Sarcomatoid carcinoma (100) | - Age, stage, therapy (CT, RT) - One-to-one |
MBC>TNBC | Not calculated | No | NA | NA |
| Lee et al (12) | 67 | - SCC (52.2) - With mesenchymal differentiation (23.9) - Spindle cell carcinoma (13.4) - Mixed (7.5)* |
- Stage - all cases |
MBC>TNBC | MBC>TNBC | Yes | 2.53 | 2.56 |
| Rakha et al (13) | 405 | - Not recorded | - Age, Nottingham grade, N-stage, ER and HER2 - 405 to 285 |
- Not recorded | No | NA | NA | |
| Zhang et al (14) | 90 | - SCC (31.1) - With mesenchymal differentiation (24.5) - Spindle cell carcinoma (34.4) - Mixed (5.6) - Fibromatosis-like (4.4) |
No matching | MBC>TNBC^^ | MBC>TNBC^^ | No | NA | NA |
| Barquet-Muñoz et al (15) | 24 | - Not recorded | - Age, clinical stage | NS | NS | No | NA | NA |
| Current study | 46 | - SCC (26.1) - With mesenchymal differentiation (37) - Spindle cell carcinoma (30.4) - Mixed (6.5) |
- Age, stage, Nottingham grade, therapy (CT, RT) - One-to-one |
MBC>TNBC | MBC>TNBC | Yes | 1.99 | NS |
cases don’t add up to 100% (reference 8);
comparison with overall cases (no matching);
MBC>TNBC, MBC had worse outcome than TNBC; SCC, squamous cell carcinoma; CT, chemotherapy; RT, radiation therapy; NS, not significant; NA, not available; HR, Hazard ratio
Here we present a summary of these studies. Lester et al (11) compared 47 cases of one subtype of MBC (spindle cell) to TNBC matched by age, tumor grade, clinical stage, and treatment modality. The study showed that spindle cell subtype had worse survival than matched TNBC when stages (I–III) were considered. The difference remained statistically significant when analysis was limited to earlier stage (I–II) cancers to further decrease any bias. This study focused on one subgroup and raised an important issue that MBC might be a heterogeneous group and not a single entity. For this reason, our present study performed subgroup analysis comparing the three major histologic subtypes (with mesenchymal differentiation vs., SCC vs. spindle call carcinoma). Although there was no statistical significant difference between the three subtypes, MBC with mesenchymal differentiation showed a trend towards better DFS than the other two subtypes. However, this finding should be interpreted with caution as the group is not uniform in terms of other clinical and pathological confounders. These findings are in part similar to the findings reported by Rakha et al (13) who found that MBC with mesenchymal differentiation and SCC had better outcome than spindle and mixed spindle and SCC.
Luini et al studied 37 cases and matched them with TNBC cases (one MBC to two TNBC) in terms of Nottingham grade, year of surgery, T-stage and N-stage. They found that patients with MBC had a hazard ratio of 5 times of dying from disease than patients with TNBC. There was no statistical significant difference between these two groups in terms of DFS. The limitation to this study is the lack of matching in terms of therapy modality and absence of multivariate analysis (9). Although Jung et al performed both matching and multivariate analyses; the study is limited by the small number of variables that were used for matching (only grade). They found MBC to have worse DFS and OS when the matching cohorts were compared and when multivariate analysis was performed (4). Similarly, Lee et al study suffers from the same issue of restricting the matching to a single variable (stage) (12). They had similar findings like the Luini et al study. Interestingly two studies by Beaty et al (8) and Barquet-Muñoz et al (15) had similar number of cases (n=24) and showed no statistical significant difference between MBC and matched TNBC. They both did not perform multivariate analysis most likely due to the small number of cases. The same reason (small number of cases) might be behind the lack of statistical significance.
Rakha et al (13) analyzed 405 cases of MBC and compared them to IC-NST (285 cases) matched in terms of age, grade, lymph node stage, ER and HER2 status. Treatment modality was not matched. MBC in European countries had worse DFS than patients in Asian countries or matched IC-NST. However when only patients with earlier stage (pT1–pT2) disease were considered in an attempt to further control the bias, no significant differences in the outcomes was seen between the three groups. The authors concluded that MBC as a group has worse prognostic variables at presentation but does not have an intrinsically worse biologic behavior than other similarly staged BC. Although this study has the largest number of cases it failed to have better matching methodology because the cases were not matched in terms of therapy and the number of cases were less than one-to-one (405-to-285). Moreover, the matching cases did not originate from the corresponding participating institution; instead, the authors chose to include cases from a single institution. Therefore, these results should be interpreted with caution.
In the present study, we found that the most common MBC subtype was mesenchymal differentiation (37%), followed by spindle cell carcinoma (30.4%) and then SCC (26.1%). In our cohort, 6.5% of the cases were mixed. These frequencies vary in different studies. The largest study by Rakha et al (13) found that the most common subtype in the western world was spindle cell carcinoma (34%) while SCC was the most common in the eastern world (34%). Luini et al (9) found that MBC with mesenchymal differentiation was the most common in European patients. Zhang et al (14) reported spindle cell carcinoma (34.4%) as the most common subtype in Chinese patients. This variation in frequencies might be explained by the relatively small number of cases in any series including ours, variation in tumor classification schemes, inter-observer variability and possibly different patient populations around the world. This variation may also be partly responsible for outcome differences reported in the literature. Therefore, we encourage conducting similarly designed studies from various centers around the world to enrich our literature with this relatively rare disease.
We reviewed 46 cases of MBC and compared the clinicopathologic characteristics and survival data of this group to all comers of TNBC cases (n=508). We then matched (one to one) 40 MBC cases to 40 TNBC cases in terms of age, clinical stage, Nottingham grade and therapy modality (CT, RT). Similar to previous findings (4,5,9,11,12,14), our study showed that MBC tends to present with larger tumor size and a more advanced stage. We found also that MBC had a worse DFS and OS when compared to TNBC (all comers) patients (p<0.001 and 0.002 respectively). This survival difference remained significant in our case matched cohort (p<0.001 for DFS and p= 0.03 for OS). In the multivariate analysis, MBC had worse DFS with hazard ratio of 1.99, while there was no statistical significance for the OS. Our carefully matched case control study reaffirms that despite being mostly hormone receptor negative, MBC is a distinct clinical entity from TNBC with worse long term clinical outcomes.
There was a significant difference in our study between the treatment received by MBC patients and that received by TNBC patients. MBC patients tended to receive mastectomy and CT more often than TNBC, while the latter had more RT (Table 1). This treatment difference might be a direct product of MBC presenting at a higher stage compared to TNBC. Since TNBC patients had less mastectomy procedure, they tend to have more RT as part of the breast conserving surgical approach. In univariate analysis, there was a significant outcome difference with treatment modalities. Patients who underwent mastectomy, neoadjuvant therapy, or were non-treated with RT all had significantly worse outcome (Table 2). The difference in treatment outcomes may be due to selection bias driven by the requirement of some therapies in advanced disease stage. It is worth noting that only three (7.5%) MBC patients received chemotherapy regimen that normally not given to TNBC. Two patients received Herceptin because they had positive HER2 and a single patient received a regimen normally given to sarcoma patients (MAID). It is unlikely that this difference had any effect on our matching process for two reasons, first, only 7.5% of the patients received non-TNBC regimen and second, MAID therapy likely did not alter tumor behavior as the patient died due to disease 12-month later. The other two patients who were treated with Herceptin had 38 months and 73 months survival with no recurrence or death due to disease.
Some of the limitations of our study are the small case number (n=46) and its retrospective nature which may introduce selection bias. In our case control matching we attempted to include factors that were significant in multivariate analysis of the two groups, including overall stage and treatment modality. DFS and OS were still significantly worse for MBC when compared to TNBC matched cases. Moreover, we were able to perform multivariate analysis, which also showed significant correlation in the DFS but not OS.
We conclude that MBC is an aggressive tumor that is more likely to present with worse prognostic indicators such as tumor size and stage. It is evident that more studies are needed to understand the true biologic potential of this tumor compared to other forms of BC, an endeavor that will always be challenged by the rarity of this entity. Our study adds to other studies delineating the clinicopathologic characteristics of this type of tumor, and highlighting its overall worse prognosis compared to TNBC. Additional studies similar to ours are encouraged to enrich the literature about this rare entity.
Footnotes
This data was presented in part in the United States and Canadian Academy of Pathology in Seattle, WA 2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reis-Filho JS, Lakhani SR, Gobbi H, Sneige N. Metaplastic Carcinoma. In: Lakhani S, Ellis I, Schnitt S, editors. World Health Organization Classification of Tumours of the Breast. 4. Lyon: IARC Press; 2012. pp. 48–52. [Google Scholar]
- 2.Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast: V. Metaplastic carcinoma with osteoclastic giant cells. Hum Pathol. 1990;21(11):1142–50. doi: 10.1016/0046-8177(90)90151-t. [DOI] [PubMed] [Google Scholar]
- 3.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6(12):718–30. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 4.Jung SY, Kim HY, Nam BH, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 5.Lai HW, Tseng LM, Chang TW, et al. The prognostic significance of metaplastic carcinoma of the breast (MCB) - a case controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22(5):968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Reis-Filho JS, Milanezi F, Carvalho S, et al. Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Res. 2005;7(6):1028–1035. doi: 10.1186/bcr1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibl S, Moinfar F. Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol. 2005;58(7):700–704. doi: 10.1136/jcp.2004.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: clinical significance. Am J Surg. 2006;191(5):657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101(3):349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 10.Bae SY, Lee SK, Koo MY, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126(2):471–478. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- 11.Lester TR, Hunt KK, Nayeemuddin KM, et al. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res Treat. 2012;131:41–48. doi: 10.1007/s10549-011-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Jung SY, Ro JY, et al. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65(5):441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 13.Rakha EA, Tan PH, Varga Z, et al. Prognostic factors in metaplastic carcinoma of the breast: a multi-institutional study. Brit J Cancer. 2014;112(2):283–289. doi: 10.1038/bjc.2014.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang LvF, Yang Y, et al. Clinicopathological Features and Prognosis of Metaplastic Breast Carcinoma: Experience of a Major Chinese Cancer Center. PLoS One. 2015;10(6):e0131409. doi: 10.1371/journal.pone.0131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barquet-Muñoz, Abraham Salim, et al. Metaplastic Breast Cancer: A Comparison between the Most Common Histologies with Poor Immunohistochemistry Factors. BMC Cancer. 2015;15:75. doi: 10.1186/s12885-015-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer - prognostic factors and survival. Radiol and Oncol. 2011;45(1):46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak A, Wu Y, Gilcrease MZ. Primary squamous cell carcinoma of the breast: predictors of locoregional recurrence and overall survival. Am J Surg Pathol. 2013;37:867–873. doi: 10.1097/PAS.0b013e3182877569. [DOI] [PubMed] [Google Scholar]