Abstract
Novel synthetic opioids (NSOs) include various analogs of fentanyl and newly emerging non-fentanyl compounds. Together with illicitly manufactured fentanyl (IMF), these drugs have caused a recent spike in overdose deaths, whereas deaths from prescription opioids have stabilized. NSOs are used as stand-alone products, as adulterants in heroin, or as constituents of counterfeit prescription medications. During 2015 alone, there were 9580 deaths from synthetic opioids other than methadone. Most of these fatalities were associated with IMF rather than diverted pharmaceutical fentanyl. In opioid overdose cases, where the presence of fentanyl analogs was examined, analogs were implicated in 17% of fatalities. Recent data from law enforcement sources show increasing confiscation of acetylfentanyl, butyrylfentanyl, and furanylfentanyl, in addition to non-fentanyl compounds such as U-47700. Since 2013, deaths from NSOs in the United States were 52 for acetylfentanyl, 40 for butyrylfentanyl, 128 for furanylfentanyl, and 46 for U-47700. All of these substances induce a classic opioid toxidrome, which can be reversed with the competitive antagonist naloxone. However, due to the putative high potency of NSOs and their growing prevalence, it is recommended to forgo the 0.4 mg initial dose of naloxone and start with 2 mg. Because NSOs offer enormous profit potential, and there is strong demand for their use, these drugs are being trafficked by organized crime. NSOs present major challenges for medical professionals, law enforcement agencies, and policymakers. Resources must be distributed equitably to enhance harm reduction though public education, medication-assisted therapies, and improved access to naloxone.
Keywords: acetylfentanyl, AH-7921, butyrylfentanyl, carfentanil, furanylfentanyl, illicitly manufactured fentanyl, MT-45, new psychoactive substances, novel synthetic opioids, U-47700, valerylfentanyl, W-18
New psychoactive substances (NPS) are defined as “substances of abuse, either in pure form or a preparation, that are not controlled by the 1961 Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances, but which may pose a public health threat (UNODC, 2013; Madras, 2017).” In the context of this definition, the term “new” does not necessarily refer to new chemical entities, but rather those compounds that have recently become available in the recreational (ie, nonmedical) drug market. Many NPS are created by modifying the chemical structures of illegal drugs or prescribed medications to generate substances which circumvent existing drug control laws. As governments pass legislation to render specific NPS illegal, new replacement analogs are synthesized and marketed to stay 1 step ahead of regulators and law enforcement. In recent years, there has been explosive growth in the market for NPS, fueled by entrepreneurs and organized crime groups who have exploited both the manufacturing capacity in Asian countries and the emergence of globalized trade (Brandt et al., 2014).
Phenethylamines and piperazine derivatives were present in the recreational drug market during the 1990s and early 2000, but it was not until synthetic cannabinoids (eg, “incense” or “spice”) appeared around 2004 that misuse of NPS became widespread (Vardakou et al., 2010; UNODC, 2013). The emergence of synthetic cannabinoids was followed by the appearance of synthetic cathinones (eg, “bath salts”) in 2010 (Baumann et al., 2013). When the United Nations (UN) Office on Drugs and Crime released its report on NPS in 2013, synthetic cannabinoids and cathinones accounted for the vast majority of new street drugs, and the only opioids mentioned were O-desmethyltramadol and kratom (UNODC, 2013). Since then, illicitly manufactured fentanyl (IMF) and a number of novel synthetic opioids (NSO) have appeared in the recreational drug market in the United States and elsewhere. Fentanyl is a prescribed opioid medication which is 50 to 100 times more potent than morphine, but IMF is synthesized in Asian laboratories and marketed via the Internet much like other NPS. Figure 1 depicts the chemical structures of fentanyl, morphine, and some commonly encountered NSOs. For the purposes of this review, the term NSO includes emerging analogs of fentanyl and various non-fentanyl compounds. At present, IMF and NSO are causing an alarming spike in overdose deaths. This increase in fatalities seems partially due to the fact that many users are unknowingly consuming these compounds as adulterants in products sold as heroin, or as counterfeit pain killers (Amlani et al., 2015; DEA, 2016b). It is estimated that a single kilogram of NSO can be used to manufacture hundreds of thousands of counterfeit prescription tablets, which can produce millions of dollars in revenue for traffickers (DEA, 2016b). The substances are usually imported through the mail, but the small quantities of compounds being shipped are difficult to detect and intercept when compared with typical illicit drug freight. There is incredible financial incentive for traffickers and counterfeiters to import and sell these substances, so the trend is expected to continue or even intensify.
FIGURE 1.
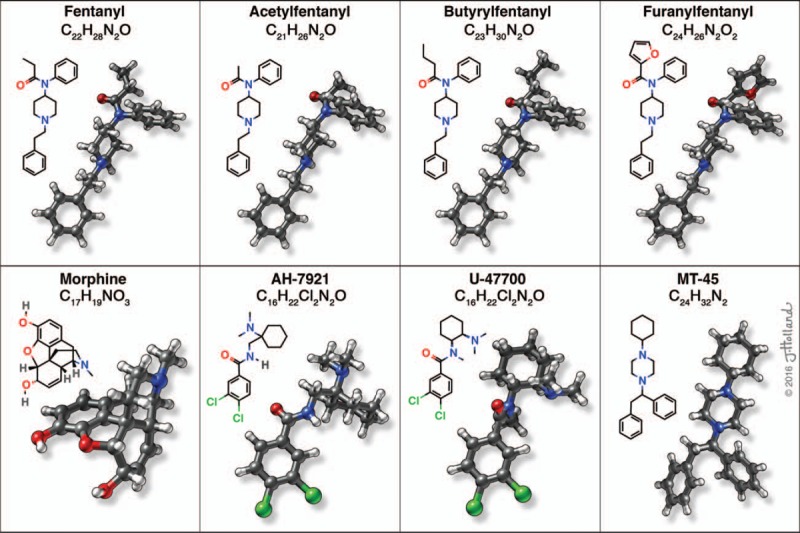
Chemical structures of novel synthetic opioids. Each compound is represented as molecular formula (top), skeletal formula (left), and ball-and-stick model (right) (used with permission of Jessica Holland, 2016).
This article will present a brief review of the epidemiology of use, pharmacology and toxicology, clinical management, forensic detection, and regulatory issues associated with NSOs. Recent data from the National Forensic Laboratory Information System (NFLIS) will also be presented. NFLIS collects data pertaining to the psychoactive constituents of drug products confiscated by local, state, and Federal law enforcement agencies in the United States. This review is intended to inform healthcare providers of this dangerous new class of drugs, and serve as a starting point for basic scientists and policymakers looking to explore this problem further.
EPIDEMIOLOGY OF USE
The widespread availability of NPS is a global phenomenon, but the prevalence of use remains enigmatic (UNODC, 2013). With regard to NSOs, information about the prevalence of misuse is scarce, because the drug landscape is constantly changing, the substances are not detected by standard toxicology screens, and users are often unknowingly exposed to the drugs. In the United States, trends in availability and use of NPS can be inferred from information about confiscated drug products such as the NFLIS database, and from overdose death data provided by the Center for Disease Control (CDC). Table 1 depicts the number of drug encounters for fentanyl and selected NSOs as reported from 2011 to 2016 by NFLIS. Before 2015, fentanyl was the main synthetic opioid encountered by law enforcement, but more recently, fentanyl analogs and non-fentanyl compounds have appeared.
TABLE 1.
Law Enforcement Seizures of Selected Synthetic Opioids From 2011 to 2016
| Drug | 2011 | 2012 | 2013 | 2014 | 2015 | 2016* |
| Fentanyl | 671 | 694 | 1041 | 5494 | 15,154 | 28,781 |
| Acetylfentanyl | — | — | 8 | 63 | 2001 | 1584 |
| Butyrylfentanyl | — | — | — | 7 | 204 | 91 |
| Furanylfentanyl | — | — | — | — | 4 | 1505 |
| U-47700 | — | — | — | — | 1 | 320 |
| AH-7921 | — | — | 2 | — | — | — |
| MT-45 | — | 1 | 2 | — | — | — |
Source: NFLIS database (personal communication).
*Dataset for 2016 is incomplete.
In 2015, the Drug Enforcement Administration (DEA) and CDC both issued nationwide alerts identifying fentanyl, particularly IMF, as a threat to public safety (Peterson et al., 2016). In a study which examined synthetic opioid overdose deaths in 27 states from 2013 to 2014, the number of confiscated drug products containing fentanyl (ie, fentanyl submissions) increased by 426%, whereas the number of deaths due to synthetic opioids increased by 79% (Gladden et al., 2016). Importantly, the increase in synthetic opioid deaths was strongly correlated with the rise in fentanyl submissions (r = 0.95), but not correlated with fentanyl prescriptions which remained stable (Gladden et al., 2016). Eight states carry particularly high synthetic opioid death burdens including 3 in the Northeast (Massachusetts, Maine, and New Hampshire), 4 in the South (Florida, Kentucky, Maryland, and North Carolina), and 1 in the Midwest (Ohio). The increase in synthetic opioid deaths is disproportionately affecting the same demographic associated with heroin use, namely non-Hispanic white men aged 25 to 44 years. Drug products containing fentanyl increased a further 160% between 2014 and 2015 to 13,822, and deaths from synthetic opioids other than methadone increased 72% to 9580 over that same time interval (Rudd et al., 2016).
Whereas pharmaceutical fentanyl is diverted for abuse in the United States at low levels, the recent rise in synthetic opioid overdose deaths is largely due to IMF (DEA, 2016e). It is estimated the true numbers of synthetic opioid-related deaths could be much higher than noted above, because many medical examiners and state crime laboratories do not test for fentanyl or NSOs unless given a specific reason to do so. IMF is often mixed with heroin and then sold as a heroin product in the illicit market. Thus, the role of fentanyl adulterants in purported heroin-overdose deaths could be underestimated. More recently, a trend of counterfeit prescription pills containing IMF or NSO has been observed (DEA, 2016b; Green and Gilbert, 2016; Armenian et al., 2017). A single kilogram of IMF or NSO can be used to produce hundreds of thousands of counterfeit prescription pills, yielding tens of millions of dollars for traffickers. In 1 recent study where the presence of fentanyl analogs was examined in forensic cases, fentanyl analogs were implicated in 17% of fentanyl-related deaths between January and June 2015 (Peterson et al., 2016).
The DEA reports that the current fentanyl crisis is being fueled by fentanyl and fentanyl precursor chemicals coming from Asian laboratories, principally in China (DEA, 2016b). Seizures of fentanyl, pills containing NSOs, and clandestine pill press operations from across North America indicate that the availability of NSOs is becoming a trend, rather than just isolated incidents. The 2015 National Survey on Drug Use and Health estimates 12.5 million Americans misused pain relievers in the past year (SAMHSA, 2016). This high demand, combined with the tremendous profit potential, provides strong incentives for traffickers to produce counterfeit pills to meet market needs. The DEA reports the pattern of abuse of fentanyl analogs mirrors that of heroin and prescription opioid analgesics misuse (DEA, 2016b).
PHARMACOLOGY AND TOXICOLOGY
Morphine is the prototypical opioid receptor agonist, and the standard to which all other opioid analgesics are compared (Pasternak and Pan, 2013; Schumacher et al., 2015). Opioid agents include not only the natural and semisynthetic alkaloid derivatives from opium, but also synthetic surrogates and other opioid-like drugs whose actions are blocked by the opioid receptor antagonist naloxone. It is well-established that opioid drugs can interact with 3 major opioid receptor subtypes in the brain and spinal cord (ie, μ, δ, and κ subtypes), and receptor selectivity influences in vivo drug actions (Law et al., 2000). The discovery of μ-δ opioid heteroreceptors (Fujita et al., 2015) and μ-opioid receptor gene splice variants (Pasternak, 2014) adds tremendous complexity to the endogenous opioid system. Nevertheless, several lines of evidence, including studies with opioid receptor knockout mice, confirm the major pharmacologic actions of morphine such as euphoria, analgesia, respiratory depression, and dependence are all due to agonist actions at the μ-opioid receptor (Williams et al., 2013; Charbogne et al., 2014).
Among the major classes of μ-opioid receptor agonists, 4-anilidopiperidines (ie, fentanyl analogs) have a prominent place in clinical usage because of their high potency, low cardiovascular toxicity, rapid onset, and short duration of action (Vucković et al., 2009). These properties arise from their high lipophilicity, which allows them to distribute rapidly across the blood-brain barrier (Williams et al., 2013). Fentanyl is the prototypic 4-anilidopiperidine, and a large number of fentanyl analogs have been synthesized since the 1960s (Vucković et al., 2009). The same properties which give fentanyl its therapeutic attributes can lead to life-threatening adverse effects when the drug is consumed illicitly, especially at high doses.
In this section, we review the pharmacology and toxicology of specific NSOs including fentanyl analogs and non-fentanyl μ-opioid agonists that have entered the recreational drug marketplace in recent years. A detailed discussion of structure–activity relationships (SAR) is beyond the scope of this review, but it should be noted that subtle alterations in chemical structure can markedly affect drug potency, duration of action, and receptor selectivity (Vardanyan and Hruby, 2014). It is also well-known that many opioid drugs exhibit stereo-selectivity in their interactions at opioid-binding sites (Law, 2011). Because none of the newly emerging NSOs has been studied in controlled clinical settings, their pharmacological properties in humans can only be inferred from animal experiments, or from studies of related compounds in man and toxicological case reports. Most opioid analgesics are well-absorbed when given by subcutaneous, intramuscular, and oral routes (Schumacher et al., 2015). However, due to first-pass metabolism, the effective oral dose may need to be much higher than the parenteral dose. There is significant interindividual variability in both first-pass and subsequent metabolism related to variety of factors including genetics and history of drug or medication exposure.
For opioid drugs, the demonstration of pain relief (ie, antinociception) in laboratory animals is usually indicative of analgesic actions in man (Cox, 2011). With respect to the NSOs discussed here, estimating effective drug doses in humans based on animal studies presents significant challenges because: various opioid drugs have been tested using diverse antinociception assay methods that are not always comparable; drugs have been administered via different routes of administration or in different species across studies; there is significant variability in antinociceptive dose–effect curves even among different strains of the same species (Elmer et al., 1998). Thus, extrapolation of data from rodents to man is not straightforward. In Table 2, ED50 values for various NSOs in mouse antinociception assays are compared with corresponding values for morphine and fentanyl, where appropriate. Keeping in mind the caveats noted above, these ratios could be cautiously applied to estimate dosing in humans.
TABLE 2.
Analgesic Potencies of Selected Synthetic Opioids in Mice
| Drug | Assay Method | ED50 Dose, Route [Citation] | Potency Relative to Morphine | Potency Relative to Fentanyl |
| Morphine | Acetic acid writhing | 0.33 mg/kg, p.o.* | 1 | 1/54* |
| Tail flick | 0.83 mg/kg, s.c.† | 1 | 1/46† | |
| Phenylquinone writhing | 1.10 mg/kg, p.o.‡ | |||
| Tail flick | 1.50 mg/kg, s.c.§ | |||
| Tail pinch | 5.90 mg/kg, s.c.¶ | |||
| Fentanyl | Acetic acid writhing | 0.0061 mg/kg, p.o.* | 54* | 1 |
| Tail flick | 0.018 mg/kg, s.c.† | 46† | 1 | |
| Acetylfentanyl | Acetic acid writhing | 0.021 mg/kg p.o.* | 16* | 1/3* |
| Butyrylfentanyl | Acetic acid writhing | 0.047 mg/kg, p.o.* | 7* | 1/8* |
| Furanylfentanyl | Hot plate | 0.02 mg/kg, i.v.|| | — | — |
| AH-7921 | Phenylquinone writhing | 0.85 mg/kg, p.o.‡ | 1.3‡ | — |
| U-47700 | Tail flick | 0.20 mg/kg, s.c.§ | 7.5§ | 1/11†,§ |
| MT-45 | Tail pinch | 1.70 mg/kg, s.c.¶ | 3.5¶ | — |
*Higashikawa and Suzuki, 2008.
†Narita et al., 2002.
‡Brittain et al., 1973.
§Cheney et al., 1985.
¶Fujimura et al., 1978.
||Huang et al., 1986.
Acetylfentanyl
Acetylfentanyl (IUPAC name: N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]acetamide) is an analog also known as des-methylfentanyl, since its chemical structure is characterized by the removal of a single methyl group from the N-propionyl of fentanyl. The synthesis of acetylfentanyl was first described in 1964 by Janssen and Gardocki (Janssen and Gardocki, 1964). In the mouse acetic acid writhing assay, acetylfentanyl is approximately 1/3 the potency of fentanyl and 16 times more potent than morphine. Importantly, the drug has a narrow therapeutic index with LD50/ED50 ratios 23 times and 3 times less than fentanyl and morphine, respectively (Higashikawa and Suzuki, 2008). The first submission of acetylfentanyl to NFLIS was recorded in Maine in April 2013 (DEA, 2015). Concurrently, in March to May 2013, a series of fatal intoxications in the region was reported (Lozier et al., 2015). Since its initial appearance, the DEA reports at least 52 confirmed fatalities, and numerous acetylfentanyl overdose cases are described in the forensic literature (DEA, 2015; McIntyre et al., 2015; Poklis et al., 2015; Cunningham et al., 2016). Clinical presentation of acetylfentanyl intoxication and overdose is similar to that of other opioid analgesics, and the drug was placed into temporary schedule I control in the United States in May 2015.
Butyrylfentanyl
Butyrylfentanyl (IUPAC name: N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]butanamide), or butyrfentanyl, is an analog with a methyl group added to the N-propionyl of fentanyl. It was first mentioned in the scientific literature in the late 1980s, where it was tested for opioid activity in variety of assays (Woods et al., 1988). In the acetic acid writhing assay, butyrylfentanyl displays 1/8 the potency of fentanyl and is about 7 times more potent than morphine (Higashikawa and Suzuki, 2008). The drug first appeared in the NFLIS database in March 2014 when it was seized in Kansas (DEA, 2016f). To date, the DEA confirmed at least 40 deaths related to butyrylfentanyl, and several fatal cases with post mortem drug concentrations have been described in the literature (McIntyre et al., 2016; Poklis et al., 2016). Its clinical presentation is similar to other fentanyls, and the drug was placed into temporary schedule I control in the United States in May 2016.
Furanylfentanyl
Furanylfentanyl (IUPAC name: N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]-2-furamide) is an analog characterized by the presence of a furan ring on the carboxamide moiety. The drug was initially described in 1986 patent literature by scientists at the BOC group (Huang et al., 1986). Based on the limited data available from mice, furanylfentanyl displays an antinociceptive potency of 0.02 mg/kg after i.v. administration; however, this potency value is difficult to compare with other studies summarized in Table 2, which employed different assay methods and routes of drug administration. The first report of furanylfentanyl in NFLIS was in January 2016 in Ohio; however, it was reported in toxicological data as early as 2015 (DEA, 2016g). There have been at least 128 confirmed fatalities associated with furanylfentanyl according to the DEA. Mohr et al. (2016) recently described a series of fatal furanylfentanyl cases, with post mortem tissue concentrations. The drug was placed into temporary schedule I status in November 2016.
AH-7921
AH-7921 (IUPAC name: 3,4-dichloro-N-{[1(dimethylamino)cyclohexyl]methyl} benzamide) was initially described in 1974 by a drug discovery team from Allen & Hanburys Ltd, and belongs to a series of compounds known as cyclohexylamines (Harper et al., 1974; Harper and Veitch, 1976). The drug exhibits similar potency to morphine in the mouse hot plate and phenylquinone writhing assays (Brittain et al., 1973). AH-7921 was first noted in NFLIS during 2013, but has been encountered by American law enforcement only a few times (Table 1). There has been 1 confirmed fatality from AH-7921 in the United States, but a number of deaths have been associated with the drug in Europe (Karinen et al., 2014; Kronstrand et al., 2014). It should be noted that AH-7921 is 1.7-fold more potent than morphine at inducing respiratory depression in mice, suggesting greater risk for adverse effects in man (Hayes and Tyers, 1983). The substance was placed into schedule I control in May 2016.
U-47700
U-47700 (IUPAC name: 3,4-dichloro-N-[(1R,2R)-2-(dimethylamino)cyclohexyl]-N-methylbenzamide) was developed by research scientists at the Upjohn Company in the late 1970s (Szmuszkovicz, 1978). In the recreational drug market, U-47700 is sometimes referred to as “pink,” because impurities in its synthesis cause the drug powder to be slightly pink in color. The drug is also known as “U4.” It is a structural isomer of AH-7921 that contains 2 chiral centers, 1 at each of its nitrogen atoms (Szmuszkovicz, 1999), and the trans-racemic mixture is the form being sold online (Elliott et al., 2016). U-47700 has much higher binding affinity for the μ-opioid receptor when compared with its affinity for the δ and κ-opioid receptors (Loew et al., 1988). In the mouse tail flick assay, U-47700 is about 1/10 as potent as fentanyl, but 7.5 times more potent than morphine (Cheney et al., 1985; Narita et al., 2002). U-47700 was first reported to NFLIS in October 2015. Since then, there has been an uptick in confiscated products and at least 46 overdose deaths in the United States (DEA, 2016h). The majority of deaths took place in New York or North Carolina, and a number of overdose cases are reported in the literature (Mohr et al., 2016; Ruan et al., 2016; Jones et al., 2017). Online reports from users indicate U-47700 induces significant euphoria, which is short-lived and causes an urge to keep “re-dosing” (Elliott et al., 2016). As of November 14, 2016, U-47700 is placed under temporary schedule I status (DEA, 2016h).
MT-45
MT-45 (IUPAC name: 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine) was developed in the 1970s as an analgesic agent at the Dainippon Pharmaceutical Co. in Japan (Nishimura et al., 1976). It is an N,N-di-substituted piperazine that was part of a series of chemicals investigated as alternatives to morphine for analgesia (Natsuka et al., 1987). MT-45 is a chiral molecule with 1 asymmetric center. The S(+) enantiomer is responsible for most of the analgesic activity of the racemic mixture (Nakamura and Shimizu, 1976), which is the form most often seized by law enforcement (EMCDDA, 2014). In the mouse tail pinch assay, MT-45 is 3.5 times more potent than morphine (Fujimura et al., 1978). It was first reported in NFLIS during 2013, but there have been few seizures of the drug and only isolated fatalities in the United States (Papsun et al., 2016). By contrast, MT-45 has been associated with many reports of fatal intoxications in Europe, with Sweden reporting 28 analytically confirmed deaths between November 2013 and July 2014 alone (EMCDDA, 2014; Siddiqi et al., 2015).
When compared with other NSOs, MT-45 displays unique pharmacological properties including long-term ototoxicity and a deep level of unconsciousness (Helander et al., 2014). It produces a low mitotic effect, which could lead to misdiagnosis and compromised treatment (Coppola and Mondola, 2014). MT-45 exhibits significant agonism at both δ and κ-opioid receptors, which might explain its unique effects (EMCDDA, 2014). Reports from online forums indicate a slow onset of action, greater than 1 to 2 hours when taken orally, which may increase the risk of toxic overdose from re-dosing before peak effect is reached (Helander et al., 2014). Taken intravenously, it is 11 times more lethal than morphine on the basis of LD50 data in mice (EMCDDA, 2014). It is currently unregulated in the United States.
INTOXICATION AND MANAGEMENT
Intoxication with all of the aforementioned NSOs is characterized by a reduced level of consciousness, ranging from drowsiness to stupor, which resembles that produced by more classic opioid agents (Fareed et al., 2011). Under conditions of overdose, NSOs induce an opioid toxidrome associated with loss of consciousness, bradycardia, respiratory depression, cyanosis, and miosis (Holstege and Borek, 2012; Zimmerman, 2014). Additional clinical features may include hypotension, pulmonary edema, ileus, nausea, vomiting, and pruritus. Death is usually from respiratory depression. Because many NSOs display chemical structures that are closer to fentanyl rather than morphine, it is expected that properties of these substances would be more akin to fentanyl as well. Thus, one would predict low oral bioavailability, high potency, and short duration of action, especially with the fentanyl analogs (MacKenzie et al., 2016). All routes of administration including oral, sublingual, nasal insufflation, nasal spray, inhalation via burning powder on aluminum foil, inhalation via a “vaporizer,” and rectal and intravenous injection have been reported (Helander et al., 2014; WHO, 2015; Papsun et al., 2016; WHO, 2016). Diagnosis of opioid overdose is made by characteristic clinical findings, exposure history, qualitative urine toxicology assay, and response to naloxone (Zimmerman, 2014). Immediate priorities in opioid toxicity are support of ventilation, correction of hypotension, and reversal of toxic effects with an opioid antagonist. If reversal of respiratory depression cannot be accomplished quickly, intubation may be required.
Naloxone is a competitive μ-opioid receptor antagonist, which serves as an effective antidote for opioid overdose. The recommended initial dose of naloxone is 0.4 to 2 mg (Zimmerman, 2014; Kim and Nelson, 2015). However, it is known that doses of 10 to 20 mg may be required to reverse the effects of potent synthetic opioids. Emergency department data from a fentanyl outbreak in Chicago during 2005 to 2006 revealed that the standard 0.4 mg naloxone dose was only successful in reversing 15% of cases, and the mean naloxone dose required for rescue was 3.36 mg (Schumann et al., 2008). Despite initial naloxone doses exceeding 2 mg, no withdrawal symptoms or other adverse effects were noted in this study. Given these data and the increasing prevalence of IMF and NSO, it seems logical to increase the standard initial naloxone dose from 0.4 to 2 mg. Currently, there are 2 forms of naloxone available for emergency use in community settings—an intramuscular autoinjector manufactured by Kaléo Pharma and a 4 mg nasal spray manufactured by Adapt Pharma (Traynor, 2016b). Kaléo received US FDA approval on October 19, 2016, to begin manufacturing a 2 mg autoinjector, and this has recently replaced its 0.4 mg formulation (Traynor, 2016a). Concerns have been raised regarding the efficacy of intranasal naloxone in reversing overdose from synthetic opioids (Zuckerman et al., 2014). However, the basis for such concerns involved a 2 mg dosage, and the current formulation for intranasal delivery is 4 mg. A randomized controlled trial comparing intranasal and intramuscular naloxone of the same dosage for suspected heroin overdose concluded similar efficacy, and both routes could be used as first-line treatment (Kerr et al., 2009).
FORENSIC DETECTION
The detection of NSOs presents challenges for clinical toxicologists and forensic scientists. When new substances first appear in the recreational drug marketplace, they must be identified and quantified in confiscated drug products, and in biological specimens from patients exposed to the drugs (Smith et al., 2015). Standard urine toxicology screens use antibody-based methods, such as enzyme-mediated immunoassays, to detect misuse of heroin (Tenore, 2010; Rogers et al., 2016). Such methods recognize morphine, its metabolites, and related semisynthetic analogs, but do not detect structurally distinct opioids such as fentanyl. On the contrary, fentanyl can be detected using a separate enzyme-linked immunosorbent assay (ELISA) (eg, Ruangyuttikarn et al., 1990), but distinguishing fentanyl from its various structural analogs requires more sophisticated analytical methods such as gas chromatography–mass spectrometry (GC-MS). Because of the cross-reactivity between fentanyl and its analogs in ELISA tests, the presence of fentanyl analogs often goes unnoticed (Stogner, 2014). Numerous case reports describe opioid overdose patients who displayed positive ELISA results for fentanyl, but did not have fentanyl present when specimens were assayed by GC-MS (McIntyre et al., 2015; Fort et al., 2016; McIntyre et al., 2016). Acetylfentanyl, butyrylfentanyl, and possibly other analogs cross-react with fentanyl ELISA, so the presence of these fentanyl-related NSOs requires additional analytical confirmation.
To date, no antibody-based methods are commercially available to detect non-fentanyl analogs such as AH-7921, U-47700, or MT-45. Given the rapid increase in number and variety of NSOs, the cumbersome process of developing immunoassays probably cannot keep pace with the appearance of new substances. The lack of specificity for immunoassays can be problematic as well. In 1 case report, U-47700 caused a false-positive for the presence of benzodiazepines using immunoassay methods (Schneir et al., 2017). Consequently, alternative analytical methods such as GC-MS, liquid chromatography–mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FTIR), or nuclear magnetic resonance (NMR) spectrometry are required to definitively verify the presence of many NSOs (UNODC, 2013; Elliott et al., 2016; Mohr et al., 2016; Papsun et al., 2016). Since analytical methods like GC-MS and LC-MS are not routinely available in many clinical settings, the true prevalence of use for NSOs is difficult to ascertain and most likely under-reported. Even when sophisticated instrumentation is available, forensic specialists face serious impediments to method development and validation, because there is a time lag between drug identification and availability of reference material from commercial suppliers (Brandt et al., 2014).
LEGAL AND REGULATORY ISSUES
The rapid emergence of NPS, combined with the internet as an efficient mechanism for global marketing and sales, is creating a challenge for regulators (Seddon, 2014). As mentioned previously, the current IMF and NSO crisis is being fueled by drug and precursor supplies coming from Asian laboratories, especially those located in China (DEA, 2016b). China is actively collaborating with US partners like the DEA, but addressing the issue of NSO is complicated by the high potency of the substances, which engenders small masses to intercept and the use of freight forwarding by traffickers. The problem of NPS has led to a call for action by 2 general strategies. The first strategy aims to find faster and more efficient mechanisms for banning those NPS that are dangerous enough to merit prohibition, whereas the second seeks to leverage the NPS problem to revisit the possibility for creating alternatives to criminalization, and rekindling old debates about drug policy reform. The former topic will be reviewed briefly below; regarding the latter topic, the reader may refer to the 2014 London School of Economics review entitled “Ending the Drug Wars” for a detailed examination of this stance (Collins, 2014).
Three UN treaties, the oldest from 1961, seek to “advance the health and welfare of mankind” by prohibiting the nonmedical use of certain drugs (Godlee and Hurley, 2016). If the UN decides to schedule a substance, each Member State must regulate that substance with at least as much stringency (Coulson and Caulkins, 2012). Many governments, including those of China, Russia, Sweden, and the United States, are strongly in favor of tougher law enforcement (Farrell, 2014). In 2016, there was an unprecedented degree of collaboration between the US DEA and the Chinese counterpart—the Narcotics Control Bureau, part of the Ministry of Public Security. In personal communications with Russell Baer of the DEA's National Media Affairs, it was relayed that China is collaborating with the United States to stem the flow of fentanyl and its analogs. He reports that this collaboration reaches to the highest levels of international government, with the topic being discussed by President Obama and Chinese President Xi Jinping during the September 2016 G20 summit in Hangzhou. Within the United States, the DEA is expanding its 360 Strategy which leverages partnerships at federal, state, and local levels on 3 different fronts: law enforcement, diversion control, and demand reduction (DEA, 2016c).
With regard to US government expenditures for drug policy, it should be noted that two-thirds of expenditures are currently spent on law enforcement and supply reduction (Farrell, 2014). Recently, there has been a call for a more equitable distribution of resources to fund prevention, treatment, and harm reduction. Multiple strategies, including targeted education interventions for primary prevention, greater access to medication-assisted therapies, and increased availability of naloxone to prevent overdose deaths, are currently being explored (Hawk et al., 2015; Wolfe et al., 2016).
OTHER EMERGING THREATS
Carfentanil (IUPAC name: 4-[{1-oxopropyl}-phenylamino]-1-[2-phenylethyl]-4-piperidinecarboxylic acid methyl ester) is a fentanyl analog which was first synthesized by Janssen in 1974, and introduced as a veterinary anesthetic for large animals in 1986 (Stanley et al., 2008). It is reportedly 10,000 times more potent than morphine, and 100 times stronger than fentanyl. On September 22, 2016, the DEA issued a warning to police and the public about carfentanil being present in the recreational drug markets in multiple communities, often disguised as heroin (DEA, 2016a). Whereas the lethal dose of carfentanil in humans is not known, relative potency estimates suggest that 20 μg of carfentanil could cause death. The chief medical examiner in Broward County, FL, reported on November 3, 2016, that carfentanil was the suspected culprit in at least 53 overdose deaths during the year. Carfentanil was also reported as an adulterant in the heroin supply in several regions of Ohio (Samet, 2016). In Canada, carfentanil has been linked to 15 deaths in Alberta, and the drug was found in blotter form in Manitoba (CBC News, 2016; Ziegler and Laville, 2016). For the purposes of the present review, we decided not to include carfentanil along with other NSOs, because the drug is not novel, being used in veterinary medicine since 1986. Nevertheless, the reported misuse of carfentanil as an adulterant in heroin, and the possibility of its presence in other drug products or counterfeit pills, presents a serious public health threat.
W-18 (IUPAC name: 4-chloro-N-[{2Z}-1-[2-{4-nitrophenyl}ethyl]piperidin-2-ylidene]benzene-1-sulfonamide) was developed in 1981 at the University of Alberta and is part of a class of compounds referred to as the W-series (Knaus et al., 1984). Despite data from the 1984 patent showing analgesic potency 10,000 times greater than morphine, recent studies reveal no activity for W-18 or any of its metabolites at opioid receptors, or any other target of psychoactive drugs (Knaus et al., 1984; Huang et al., 2016; Kroll, 2016). However, W-18 is still considered an emerging threat as it has been seized by traffickers in Florida and Alberta (CCENDU, 2016). W-18 is schedule I in Canada as of June 1, 2016, and in personal communication with Russell Baer of the DEA, the United States is considering temporary scheduling of W-18.
Finally, starting in the second quarter of 2016, valerylfentanyl (IUPAC name: N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl]pentanamide) has been reported in NFLIS seizure data (DEA, 2016d). At present, there are no data available on possible fatalities associated with valerylfentanyl, or descriptions of pharmacological effects in humans or animals. Extrapolating from known SAR for fentanyl compounds, which show analgesic potency decreases with increasing length of carboxamide substituents, it can be inferred that valerylfentanyl would be less potent than butyrylfentanyl (Vucković et al., 2009).
CONCLUSIONS
Novel synthetic opioids have been responsible for hundreds of analytically confirmed deaths in the past 2 years, and this number is likely an underestimate. If deaths from IMF are included, the total number of fatalities over the 2-year period exceeds 15,000. As deaths from natural and semisynthetic opiates are stabilizing, deaths from synthetic opioids are rising at an alarming rate (Rudd et al., 2016). This scourge of opioid-related fatalities affects a demographic that is traditionally associated with use of heroin and other illicit opioids, but also impacts those who misuse prescription pain medications. The latter demographic is now being targeted by unscrupulous counterfeiters who are creating pain medications containing IMF and NSO. Many of the drugs discussed here are several times more potent than morphine, approaching the strength of fentanyl. Based on the increasing prevalence of synthetic opioids of unknown origin and potency, we recommend foregoing the 0.4 mg naloxone dose in cases of suspected opioid overdose and proceeding directly to 2 mg. Some NSOs, such as MT-45, have unique clinical features—bilateral hearing loss and low miotic effect, whereas others such as U-47700 are short-acting and lead to a strong urge for re-dosing. No controlled clinical studies have been carried out to examine the pharmacology of NSOs, and few animal studies have examined the biological effects of NSOs using in vitro receptor assays or in vivo paradigms. Clearly, more basic research on the pharmacology and toxicology of these compounds is warranted.
The true prevalence of NSO misuse is difficult to determine. In most cases of opioid overdose, naloxone is administered to ameliorate symptoms, and confirmatory analytical testing is not performed. When the presence of fentanyl analogs was examined in overdose death cases, analogs were implicated as the cause of death in 17% of cases that were initially thought to be due to fentanyl (Peterson et al., 2016). Thus, there should be increased efforts by clinical toxicologists and forensic scientists to detect the presence of NSOs in heroin and fentanyl-related intoxications and deaths, to determine the precise role of novel substances in the growing overdose epidemic (Mohr et al., 2016). Like other NPS, synthetic opioids present extraordinary challenges for regulators and law enforcement. In recent times, most of the NSOs mentioned in this review were placed into schedule I control as a means to reduce demand. We suggest that expenditures on regulation and law enforcement be balanced by efforts to decrease demand though education, medication-assisted treatment, and easier access to naloxone (Farrell, 2014; Hawk et al., 2015; Wolfe et al., 2016). Overall, IMF and NSO represent a significant threat to public health. As great progress has been made in stabilizing deaths from prescription opioids, much work is now needed to address the rise in deaths caused by illicitly manufactured synthetic opioids.
Acknowledgments
The authors wish to thank medical illustrator Jessica Holland, MS, CMI, for the creation of the illustrations used in this article. This work is supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Amlani A, McKee G, Khamis N, et al. Why the FUSS (Fentanyl Urine Screen Study)? A cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm Reduct J 2015; 12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian P, Olson A, Anaya A, et al. Fentanyl and a novel synthetic opioid U-47700 masquerading as street “Norco” in Central California: a case report. Ann Emerg Med 2017; 69:87–90. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol 2013; 698:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, King LA, Evans-Brown M. The new drug phenomenon. Drug Test Anal 2014; 6:587–597. [DOI] [PubMed] [Google Scholar]
- Brittain RT, Kellett DN, Neat ML, et al. Proceedings: anti-nociceptive effects in N-substituted cyclohexylmethylbenzamides. Br J Pharmacol 1973; 49:158–159. [PMC free article] [PubMed] [Google Scholar]
- CBC News. ’It's scary stuff’: deadly drug carfentanil now in Winnipeg [CBC News web site]. September 29, 2016. Available at: http://www.cbc.ca/news/canada/manitoba/carfentanil-drug-confirm-winnipeg-1.3783861. Accessed January 24, 2016.
- CCENDU. Novel Synthetic Opioids in Counterfeit Pharmaceuticals and Other Illicit Street Drugs [CCENDU Bulletin]. June, 2016. Available at: http://www.ccsa.ca/Resource%20Library/CCSA-CCENDU-Novel-Synthetic-Opioids-Bulletin-2016-en.pdf. Accessed January 26, 2017.
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 2014; 76 (Pt B):204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney BV, Szmuszkovicz J, Lahti RA, et al. Factors affecting binding of trans-N-[2-(methylamino)cyclohexyl]benzamides at the primary morphine receptor. J Med Chem 1985; 28:1853–1864. [DOI] [PubMed] [Google Scholar]
- Collins J, ed. Ending the drug wars. [Report of the London School of Economics Expert Group on the Economics of Drug Policy]. May 2014. Available at: http://www.lse.ac.uk/IDEAS/publications/reports/pdf/LSE-IDEAS-DRUGS-REPORT-FINAL-WEB01.pdf. Accessed January 20, 2016.
- Coppola M, Mondola R. MT-45: a new, dangerous legal high. J Opioid Manag 2014; 10:301–302. [PubMed] [Google Scholar]
- Coulson C, Caulkins JP. Scheduling of newly emerging drugs: a critical review of decisions over 40 years. Addiction 2012; 107:766–773. [DOI] [PubMed] [Google Scholar]
- Cox BM. Pasternak GW. Pharmacology of opioid drugs. The Opiate Receptors 2nd ed.New York: Springer; 2011. 23–57. [Google Scholar]
- Cunningham SM, Haikal NA, Kraner JC. Fatal Intoxication with Acetyl Fentanyl. J Forensic Sci 2016; 61 suppl 1:S276–S280. [DOI] [PubMed] [Google Scholar]
- DEA. Schedules of controlled substances: temporary placement of acetyl fentanyl Into Schedule I [Docket No. DEA-413F]. July 17, 2015. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2015/fr0717_7.htm. Accessed January 17, 2017.
- DEA. Carfentanil: a dangerous new factor in the U.S. Opioid crisis [Officer Safety Alert]. September 22, 2016. Available at: https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed January 22, 2016.
- DEA. Counterfeit prescription pills containing fentanyls: a global threat [DEA Intelligence Brief]. July 16, 2016. Available at: https://www.dea.gov/docs/Counterfeit%20Prescription%20Pills.pdf. Accessed January 17, 2017.
- DEA. DEA 360 strategy [Factsheet]. July 14, 2016. Available at: https://www.dea.gov/docs/360_Fact_Sheet071416.pdf. Accessed January 21, 2016.
- DEA. Emerging threat report mid-year 2016 [DEA Emerging Trends Report]. September 21, 2016. Available at: https://ndews.umd.edu/sites/ndews.umd.edu/files/pubs/emergingthreatreport2016mid-year.pdf. Accessed January 27, 2017.
- DEA. National heroin threat assessment summary: updated [DEA Intelligence Brief]. June 27, 2016. Available at: https://www.dea.gov/divisions/hq/2016/hq062716_attach.pdf. Accessed January 17, 2017.
- DEA. Schedules of controlled substances: temporary placement of butyryl fentanyl and beta-hydroxythiofentanyl Into Schedule I [Docket No. DEA-434F]. May 12, 2016. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr0512_2.htm. Accessed January 17, 2017. [PubMed]
- DEA. Schedules of controlled substances: temporary placement of furanyl fentanyl Into Schedule I [Docket No. DEA-448]. September 27, 2016. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr0927.htm. Accessed January 17, 2017.
- DEA. Schedules of controlled substances: temporary placement of U-47700 Into Schedule I [Docket No. DEA-440]. November 14, 2016. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr1114.htm. Accessed January 18, 2017.
- Elliott SP, Brandt SD, Smith C. The first reported fatality associated with the synthetic opioid 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700) and implications for forensic analysis. Drug Test Anal 2016; 8:875–879. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Negus SS, et al. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain 1998; 75:129–140. [DOI] [PubMed] [Google Scholar]
- EMCDDA. EMCDDA: Europol Joint Report on a new psychoactive substance: 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (‘MT-45’) [Risk assessment report]. September 2014. Available at: http://www.emcdda.europa.eu/publications/joint-reports/MT-45. Accessed January 19, 2017.
- Fareed A, Stout S, Casarella J, et al. Illicit opioid intoxication: diagnosis and treatment. Subst Abuse 2011; 5:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M. Drug legalisation. BMJ 2014; 349:g5233. [DOI] [PubMed] [Google Scholar]
- Fort C, Curtis B, Nichols C, et al. Acetyl fentanyl toxicity: two case reports. J Anal Toxicol 2016; 40:754–757. [DOI] [PubMed] [Google Scholar]
- Fujimura H, Tsurumi K, Nozaki M, et al. Analgesic activity and opiate receptor binding of 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine. Jpn J Pharmacol 1978; 28:505–506. [DOI] [PubMed] [Google Scholar]
- Fujita W, Gomes I, Devi LA. Heteromers of μ-δ opioid receptors: new pharmacology and novel therapeutic possibilities. Br J Pharmacol 2015; 172:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths: 27 States, 2013–2014. MMWR Morb Mortal Wkly Rep 2016; 65:837–843. [DOI] [PubMed] [Google Scholar]
- Godlee F, Hurley R. The war on drugs has failed: doctors should lead calls for drug policy reform. BMJ 2016; 355:i6067. [Google Scholar]
- Green TC, Gilbert M. Counterfeit medications and fentanyl. JAMA Intern Med 2016; 176:1555–1557. [DOI] [PubMed] [Google Scholar]
- Harper NJ, Veitch GB, inventors; Allen & Hanburys Ltd, assignee. 1-(3,4-dichlorobenzamidomethyl)-cyclohexyldimethylamine. US patent 3,975,443. August 17, 1976.
- Harper NJ, Veitch GB, Wibberley DG. 1-(3,4-Dichlorobenzamidomethyl)cyclohexyldimethylamine and related compounds as potential analgesics. J Med Chem 1974; 17:1188–1193. [DOI] [PubMed] [Google Scholar]
- Hawk KF, Vaca FE, D’Onofrio G. Reducing fatal opioid overdose: prevention, treatment and harm reduction strategies. Yale J Biol Med 2015; 88:235–245. [PMC free article] [PubMed] [Google Scholar]
- Hayes AG, Tyers MB. Determination of receptors that mediate opiate side effects in the mouse. Br J Pharmacol 1983; 79:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Bäckberg M, Beck O. MT-45, a new psychoactive substance associated with hearing loss and unconsciousness. Clin Toxicol (Phila) 2014; 52:901–904. [DOI] [PubMed] [Google Scholar]
- Higashikawa Y, Suzuki S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure–analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol 2008; 26:1–5. [Google Scholar]
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin 2012; 28:479–498. [DOI] [PubMed] [Google Scholar]
- Huang BS et al., inventors; The BOC Group Inc, assignee. N-aryl-N-(4-piperidinyl)amides and pharmaceutical compositions and method employing such compounds. US patent 4,584,303. April 22, 1986.
- Huang XP, Che T, Mangano TJ et al. Pharmacology of W-18 and W-15. bioRxiv 2016. 10.1101/065623
- Janssen PAJ, Gardocki JF, inventors; Research Laboratorium Dr. C. Janssen N.V., assignee. Method for producing analgesia. US patent 3,141,823. July 21, 1964.
- Jones MJ, Hernandez BS, Janis GC, et al. A case of U-47700 overdose with laboratory confirmation and metabolite identification. Clin Toxicol (Phila) 2017; 55:55–59. [DOI] [PubMed] [Google Scholar]
- Karinen R, Tuv SS, Rogde S, et al. Lethal poisonings with AH-7921 in combination with other substances. Forensic Sci Int 2014; 244:e21–e24. [DOI] [PubMed] [Google Scholar]
- Kerr D, Kelly AM, Dietze P, et al. Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction 2009; 104:2067–2074. [DOI] [PubMed] [Google Scholar]
- Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf 2015; 14:1137–1146. [DOI] [PubMed] [Google Scholar]
- Knaus EE, Warren BK, Ondrus TA, inventors; Canadian Patents & Development Ltd., Assignee. Analgesic substituted piperidylidene-2-sulfon(cyan)amide derivatives. US patent 4,468,403. August 28, 1984.
- Kroll D. W-18 Is Not A Super-Potent Designer Opioid As Originally Believed [Forbes Pharma & Healthcare website]. July 28, 2016. Available at: http://www.forbes.com/sites/davidkroll/2016/07/28/w-18-is-not-a-super-potent-designer-opioid-as-originally-believed. Accessed January 25, 2017.
- Kronstrand R, Thelander G, Lindstedt D, et al. Fatal intoxications associated with the designer opioid AH-7921. J Anal Toxicol 2014; 38:599–604. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 2000; 40:389–430. [DOI] [PubMed] [Google Scholar]
- Law PY. Pasternak GW. Opioid receptor signal transduction mechanisms. The Opiate Receptors 2nd ed.New York: Springer; 2011. 195–238. [Google Scholar]
- Loew G, Lawson J, Toll L, et al. Structure activity studies of two classes of beta-amino-amides: the search for kappa-selective opioids. NIDA Res Monogr 1988; 90:144–151. [PubMed] [Google Scholar]
- Lozier MJ, Boyd M, Stanley C, et al. Acetyl fentanyl, a novel fentanyl analog causes 14 overdose deaths in Rhode Island, March-May 2013. J Med Toxicol 2015; 11:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie M, Zed PJ, Ensom MH. Opioid pharmacokinetics-pharmacodynamics: clinical implications in acute pain management in trauma. Ann Pharmacother 2016; 50:209–218. [DOI] [PubMed] [Google Scholar]
- Madras BK. The growing problem of new psychoactive substances (NPS). Curr Top Behav Neurosci 2017; 32:1–18. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Trochta A, Gary RD, et al. An acute acetyl fentanyl fatality: a case report with post mortem concentrations. J Anal Toxicol 2015; 39:490–494. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Trochta A, Gary RD, et al. An acute butyr-fentanyl fatality: a case report with post mortem concentrations. J Anal Toxicol 2016; 40:162–166. [DOI] [PubMed] [Google Scholar]
- Mohr AL, Friscia M, Papsun D, et al. Analysis of novel synthetic opioids U-47700, U-50488 and furanyl fentanyl by LC-MS/MS in post mortem casework. J Anal Toxicol 2016; 40:709–717. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Shimizu M. Comparative study of 1-cyclohexyl-4-(1,2-diphenylethyl)-piperazine and its enantiomorphs on analgesic and othe pharmacological activities in experimental animals. Arch Int Pharmacodyn Ther 1976; 221:105–121. [PubMed] [Google Scholar]
- Narita M, Imai S, Itou Y, et al. Possible involvement of mu1-opioid receptors in the fentanyl- or morphine-induced antinociception at supraspinal and spinal sites. Life Sci 2002; 70:2341–2354. [DOI] [PubMed] [Google Scholar]
- Natsuka K, Nakamura H, Nishikawa Y, et al. Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity. J Med Chem 1987; 30:1779–1787. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Uno H, Shimokawa N, et al, inventors; Dainippon Pharmaceutical Co. Ltd., assignee. 1-substituted-4-(1,2-diphenylethyl)-piperazine derivatives and their salts and the preparation thereof. US patent 3,957,788. May 18, 1976.
- Papsun D, Krywanczyk A, Vose JC, et al. Analysis of MT-45, a novel synthetic opioid, in human whole blood by LC-MS-MS and its identification in a drug-related death. J Anal Toxicol 2016; 40:313–317. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Opioids and their receptors: are we there yet? Neuropharmacology 2014; 76 (Pt B):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 2013; 65:1257–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AB, Gladden RM, Delcher C, et al. Increases in fentanyl-related overdose deaths: Florida and Ohio, 2013-2015. MMWR Morb Mortal Wkly Rep 2016; 65:844–849. [DOI] [PubMed] [Google Scholar]
- Poklis J, Poklis A, Wolf C, et al. Postmortem tissue distribution of acetyl fentanyl, fentanyl and their respective nor-metabolites analyzed by ultrahigh performance liquid chromatography with tandem mass spectrometry. Forensic Sci Int 2015; 257:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis J, Poklis A, Wolf C, et al. Two fatal intoxications involving butyryl fentanyl. J Anal Toxicol 2016; 40:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JS, Rehrer SJ, Hoot NR. Acetylfentanyl: an emerging drug of abuse. J Emerg Med 2016; 50:433–436. [DOI] [PubMed] [Google Scholar]
- Ruan X, Chiravuri S, Kaye AD. Comparing fatal cases involving U-47700. Forensic Sci Med Pathol 2016; 12:369–371. [DOI] [PubMed] [Google Scholar]
- Ruangyuttikarn W, Law MY, Rollins DE, et al. Detection of fentanyl and its analogs by enzyme-linked immunosorbent assay. J Anal Toxicol 1990; 14:160–164. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths: United States, 2010-2015. MMWR Morb Mortal Wkly Rep 2016; 65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Samet M. Heroin adulterant creating deadly combination [Hamilton County Heroin Coalition news release]. July 15, 2016. Available at: http://www.hamiltoncountyhealth.org/files/files/Press%20Releases/Carfentanil_7_15_2016.pdf. Accessed January 22, 2016.
- SAMHSA. Prescription drug use and misuse in the United States: results from the 2015 National Survey on Drug Use and Health [NSDUH Data Review]. September, 2016. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm. Accessed January 17, 2017.
- Schneir A, Metushi IG, Sloane C, et al. Near death from a novel synthetic opioid labeled U-47700: emergence of a new opioid class. Clin Toxicol (Phila) 2017; 55:51–54. [DOI] [PubMed] [Google Scholar]
- Schumacher MA. Katzung BG, Trevor AJ, et al. Opioid agonists and antagonists. Basic and Clinical Pharmacology 13th ed.New York: McGraw Hill Education; 2015. 531–551. [Google Scholar]
- Schumann H, Erickson T, Thompson TM, et al. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila) 2008; 46:501–506. [DOI] [PubMed] [Google Scholar]
- Seddon T. Drug policy and global regulatory capitalism: the case of new psychoactive substances (NPS). Int J Drug Policy 2014; 25:1019–1024. [DOI] [PubMed] [Google Scholar]
- Siddiqi S, Verney C, Dargan P, et al. Understanding the availability, prevalence of use, desired effects, acute toxicity and dependence potential of the novel opioid MT-45. Clin Toxicol (Phila) 2015; 53:54–59. [DOI] [PubMed] [Google Scholar]
- Smith JP, Sutcliffe OB, Banks CE. An overview of recent developments in the analytical detection of new psychoactive substances (NPSs). Analyst 2015; 140:4932–4948. [DOI] [PubMed] [Google Scholar]
- Stanley TH, Egan TD, Van Aken H. A tribute to Dr. Paul A. J. Janssen: entrepreneur extraordinaire, innovative scientist, and significant contributor to anesthesiology. Anesth Analg 2008; 106:451–462. [DOI] [PubMed] [Google Scholar]
- Stogner JM. The potential threat of acetyl fentanyl: legal issues, contaminated heroin, and acetyl fentanyl “disguised” as other opioids. Ann Emerg Med 2014; 64:637–639. [DOI] [PubMed] [Google Scholar]
- Szmuszkovicz J. U-50,488 and the kappa receptor: a personalized account covering the period 1973 to 1990. Prog Drug Res 1999; 52:167–195. [DOI] [PubMed] [Google Scholar]
- Szmuszkovicz J, inventor; The Upjohn Company, assignee. Analgesic N-(2-aminocycloaliphatic)benzamides. US patent 4,098,904. July 4, 1978.
- Tenore PL. Advanced urine toxicology testing. J Addict Dis 2010; 29:436–448. [DOI] [PubMed] [Google Scholar]
- Traynor K. Experts weigh minimum naloxone dose as opioid crisis evolves. Am J Health Syst Pharm 2016; 73:1892–1894. [DOI] [PubMed] [Google Scholar]
- Traynor K. FDA approves first intranasal naloxone product. Am J Health Syst Pharm 2016; 73:e2–e3. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. The challenge of new psychoactive substances [A report of the global SMART programme]. March 2013. Available at: https://www.unodc.org/documents/scientific/NPS_2013_SMART.pdf. Accessed January 17, 2017.
- Vardanyan RS, Hruby VJ. Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med Chem 2014; 6:385–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucković S, Prostran M, Ivanović M, et al. Fentanyl analogs: structure-activity-relationship study. Curr Med Chem 2009; 16:2468–2474. [DOI] [PubMed] [Google Scholar]
- WHO. Acetylfentanyl [Critical Review Report]. November 2015. Available at: http://www.who.int/medicines/access/controlled-substances/5.2_Acetylfentanyl_CRev.pdf. Accessed January 19, 2017.
- WHO. U-47700 [Critical Review Report]. November 2016. Available at: http://www.who.int/medicines/access/controlled-substances/4.1_U-47700_CritReview.pdf. Accessed January 19, 2017.
- Williams DA, Roche VF, Roche EB. Lemke TL, Williams DA, Roche VF, Zito SW. Central analgesics. Foye's Principles of Medicinal Chemistry 7th ed.Philadelphia: Lippincott Williams & Wilkins; 2013. 658–699. [Google Scholar]
- Wolfe S, Bouffard DL, Modesto-Lowe V. The opioid crisis and the physician's role in contributing to its resolution: step one: prevention of overdoses. Conn Med 2016; 80:325–334. [PubMed] [Google Scholar]
- Woods J, Medzihradsky F, Smith C, et al. Evaluation of new compounds for opioid activity: 1987 annual report. NIDA Res Monogr 1988; 81:543–590. [PubMed] [Google Scholar]
- Ziegler C, Laville D. Toxic opioid carfentanil linked to 15 deaths [Alberta Government Announcement]. December 5, 2016. Available at: https://www.alberta.ca/release.cfm?xID=449592B2D1813-CB6E-055D-0800F5C807A854D8. Accessed January 23, 2016.
- Zimmerman JL. Parrillo JE, Dellinger RP. Poisonings. Critical Care Medicine Principles of Diagnosis and Management in the Adult 4th ed.Philadelphia: Elsevier; 2014. 1199–1219. [Google Scholar]
- Zuckerman M, Weisberg SN, Boyer EW. Pitfalls of intranasal naloxone. Prehosp Emerg Care 2014; 18:550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]


