Abstract
Objective
The aim of this investigation was to determine whether circulating inflammatory biomarkers c-reactive protein (CRP), interleukin-6 (IL6), and alpha 1-antichymotrypsin (ACT) were related to structural brain measures assessed by magnetic resonance imaging (MRI).
Methods
High-resolution structural MRI was collected on 680 non-demented elderly (mean age 80.1 years) participants of a community-based, multiethnic cohort. Approximately three quarters of these participants also had peripheral inflammatory biomarkers (CRP, IL6, and ACT) measured using ELISA. Structural measures including brain volumes and cortical thickness (with both global and regional measures) were derived from MRI scans, and repeated MRI measures were obtained after 4.5 years. Mean fractional anisotropy was used as the indicator of white matter integrity assessed with diffusion tensor imaging. We examined the association of inflammatory biomarkers with brain volume, cortical thickness, and white matter integrity using regression models adjusted for age, gender, ethnicity, education, APOE genotype, intracranial volume.
Results
A doubling in CRP (b= -2.48, p=0.002) was associated with a smaller total gray matter volume, equivalent to approximately 1.5 years of aging. A doubling in IL6 was associated with smaller total brain volume (b= -14.96, p<0.0001), equivalent to approximately 9 years of aging. Higher IL6 was also associated with smaller gray matter (b=-6.52, p=0.002) and white matter volumes (b=-7.47, p=0.004). The volumes of most cortical regions including frontal, occipital, parietal, temporal, as well as subcortical regions including pallidum and thalamus were associated with IL6. In a model additionally adjusted for depression, vascular factors, BMI, and smoking status, the association between IL6 and brain volumes remained, and a doubling in ACT was marginally associated with 0.054 (p=0.004) millimeter thinner mean cortical thickness, equivalent to that of approximately 2.7 years of aging. None of the biomarkers was associated with mean fractional anisotropy or longitudinal change of brain volumes and thickness.
Conclusions
Among older adults, increased circulating inflammatory biomarkers were associated with smaller brain volume and cortical thickness but not the white matter tract integrity. Our preliminary findings suggest that peripheral inflammatory processes may be involved in the brain atrophy in the elderly.
Keywords: C-reactive protein, interleukin-6, alpha 1-antichymotrypsin, magnetic resonance imaging, brain atrophy, cortical thickness, brain morphometry, neuroepidemiology
Introduction
Alzheimer's disease (AD) is the leading cause of dementia and the most common neurodegenerative disorder. As no effective cure is available for AD to date, there is a great need to understand its preclinical stage in order to prevent the disease from occurring, or at least prolong delay disease onset. Various pathological changes are believed to occur many years before the clinical manifestation of AD(1, 2). Among healthy aging individuals, brain morphological changes, including both macro-(3-7) and micro-(8, 9) structural changes, are found to be important predictors of cognitive decline and development of AD.
Therefore, understanding factors that are related or contribute to the brain atrophy or disruption of white matter integrity may have important implications for prevention of the disease and intervention at the early stages. Systemic inflammation has been increasingly recognized to play a critical role in AD and other neurodegenerative diseases(10). Increased peripheral levels of C-reactive protein (CRP), interleukin-6 (IL6), and alpha 1-antichymotrypsin (ACT) have been associated with increased risk of dementia, AD, or cognitive decline (11-30).
Inflammation may also be a key contributor to AD-related brain changes. However, only a few studies explored the relationship between peripheral inflammatory biomarkers in relation to brain measures among older adults without dementia, and the results remain inconclusive(31). Only three studies (32-34) investigated white matter microstructure and showed that systemic inflammation was associated with reduced fractional anisotropy (FA), an indicator of white matter integrity. CRP or IL6 was associated with reduced total brain volume (TBV)(35), total gray matter volume (TGMV)(36-38), total white matter volume (TWMV)(37), and hippocampal volume(36, 37, 39). However, CRP and IL6 were not associated with brain volumes in other studies (32, 33, 40-42). Only two studies examined whether inflammatory biomarkers were associated with cortical thickness (CT) and they found inconsistent results(37, 43). In one study of elderly adults, higher IL-6 was associated with accelerated annual rates of cortical thinning in the inferior temporal poles bilaterally(43), while another study found no cross-sectional associations of IL6 or CRP with CT in adults aged 30–54 years(37). Thus, inconsistency remains regarding the relationship between peripheral inflammatory biomarkers and brain volume; some brain measures such as microstructural white matter integrity(32-34), CT (37, 43), as well as regional brain volumes(37) were rarely examined. The role of ACT in brain structure is largely unknown despite its involvement in the Aβ-related pathogenesis in AD (44, 45). Longitudinal data are scarce. Additional studies are needed for older populations who are thought to manifest a systemic, progressive inflammatory state (46).
We examined whether circulating levels of inflammatory markers most likely to be important for neurodegeneration (i.e., CRP, IL6, and ACT)(20) were associated with macrostructural brain measures (i.e. TBV, TGMV, TWMV, mean CT, and regional brain volumes and CT) and microstructural white matter integrity (using mean FA as the indicator) among elderly participants of a community-based, multiethnic cohort, the Washington Heights/Hamilton Heights Inwood Columbia Aging Project (WHICAP).
Materials and Methods
Study Participants
The current study included participants from an ongoing prospective study of aging and dementia (WHICAP) who were identified from a probability sample of Medicare beneficiaries elderlies (≥65 years) residing in northern Manhattan(47). The original sample for this study included 2,776 participants. At baseline, a physician obtained each participant's medical and neurological history, and conducted a standardized physical and neurological examination. Participants also received assessments of health and function, and were assessed using a neuropsychological battery(48). Participants were followed every 18 months, repeating the baseline examinations. The diagnosis of dementia or its absence was based on standard research criteria Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R)(49), using all available information at a consensus conference. The type of dementia was subsequently determined using the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association for the diagnosis of probable or possible AD(50), and mild cognitive impairment (MCI) was diagnosed using Petersen(51) criteria as previously described(52).
The imaging sub-study was started in 2004 among ongoing dementia-free WHICAP participants(53). In total, 769 WHICAP participants received MRI scans, and they were slightly younger, and were more likely to be African Americans or male compared to those who were eligible but did not undergo MRI(53). Among them, a total of 508 subjects had CRP and 435 had IL6/ACT measured among those with baseline structural MRI scans, 228 had CRP and 195 had IL6/ACT among those received second structural MRI scans 4.5 (SD=0.8) years later, and 183 had CRP and 154 had IL6/ACT among those who received DTI scan (Figure 1). A total of 357 subjects were included in the baseline cross-sectional analysis after excluding 78 subjects whose IL6 level were out of the measurement range.
Figure 1.
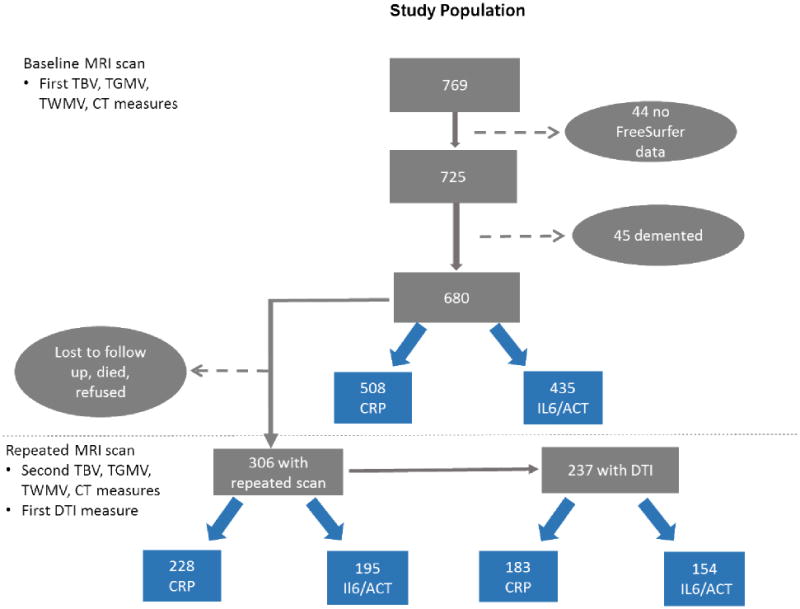
Selection of study subjects from the neuroimaging study of the Washington Heights/Hamilton Heights Inwood Columbia Aging Project (WHICAP).
The study subjects were those participants of the WHICAP imaging sub-study started in 2004. A total of 769 subjects received baseline MRI scans, and among whom we excluded subjects without FreeSurfer data (n=44) and had dementia (n=45). Among the 680 remaining non-demented participants who had baseline MRI data, 306 had repeated structural MRI scan on average 4.5 (SD=0.8) years later. In addition, we also added DTI modality during the follow-up scan for white matter integrity measurement and 237 participants received DTI. For the current study, a total of 508 subjects had CRP, 435 had IL6/ACT measured among those with baseline structural MRI scans, 228 had CRP and 195 had IL6/ACT among those received second structural MRI scans, and 183 had CRP and 154 had IL6/ACT among those who received DTI scan. A total of 357 subjects were included in the analysis after excluding 78 subjects whose IL6 level were out of the measurement range.
Standard protocol approvals, registrations, and patient consents
The Columbia University Institutional Review Board has reviewed and approved this project. All individuals provided written informed consent.
MRI protocol
Scans were acquired on a 1.5T Philips Intera scanner at Columbia University. T1-weighted images were acquired with the following parameters: repetition time, 20 milliseconds; echo time, 2.1 milliseconds; field of view, 240 cm; 256×160–pixel matrix with 1.3-mm section thickness, and voxel size 1 × 1 × 1.3 mm. All the T1 images were analyzed using Freesurfer (V.5.1) (http://surfer.nmr.mgh.harvard.edu/). Freesurfer output underwent visual quality control and manual correction whenever necessary, and then Freesurfer steps were repeated. Regional cortical thicknesses and volumetric measures were obtained in 34 regions of interest (ROI) in each hemisphere through a series of steps including removal of non-brain tissue using a hybrid watershed/surface deformation procedure(54), followed by automated Talairach transformation, segmentation of the subcortical white matter (WM) and deep gray matter (GM) volumetric structures(55) intensity normalization(56), tessellation of the GM-WM boundary, automated topology correction(57), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class(58, 59).
We first explored global brain measures such as TBV, TGMV, TWMV. To adjust for differences in head size across participants, regression models were run with intra-cranial volume (ICV) as the independent variable and brain volume as the outcome variable, and the regression residuals were then used in the analyses. We calculated mean CT across all ROIs within each subject.
Diffusion tensor imaging (DTI)
Whole brain diffusion imaging (field of view=224 × 224, contiguous slices, slice thickness = 2mm, TR=10586, TE=70) were acquired along 15 directions with a maximum b-factor of 800 s/mm2, complemented by two scans with b=0 s/mm2. Fractional anisotropy maps were constructed for each participant with software implemented in MATLAB® (R2013b, the MathWorks, the FMRIB Software Library (FSL) and Inc., Natick, Massachusetts, USA). ROIs were derived with the JHU-ICBM-DTI-81 white matter labels atlas(60). Each subject's FA map was first registered to the FA atlas template with the nonlinear transformation tool (FNIRT) in FSL using a linear initialization (default FSL parameters) and the predefined configuration file for FA registration provided by FSL. By applying the inverse non-linear registration transformation to the atlas, the atlas was warped, allowing for ROI analysis in subject space. Twenty-six ROIs in left and right hemispheres, and midline structures, were considered for analysis. A mean FA value of the 26 tracts was calculated and used in the analysis(61). FA ranges from 0 to 1, with higher values indicating greater orientation of diffusion and preserved microstructure.
Biomarker Measurement
Non-fasting plasma (CRP) and serum (IL6 and ACT) samples were used in all analyses. Blood was collected in BD vacutainers containing EDTA and centrifuged at 2,000×g for 15 minutes at room temperature. Plasma is then stored at -80°C in polypropylene cryotubes until analysis. Serum was obtained by allowing the blood to clog for 30 minutes after collection; subsequently, it is centrifuged, aliquot and stored at -80°C as previously described for plasma. High sensitivity CRP levels were measured using ELISA (Diagnostic systems laboratories, INC, Webster, Texas), with a sensitivity of 1.6 ng/ml, and the intra-assay and inter-assay coefficient of variations (CVs) of 4.6% and 11.7%, respectively. The IL-6 levels were measured in duplicate using high sensitivity quantitative sandwich enzyme immunoassay kit (R&D Systems, Minneapolis, MN), with a sensitivity of 0.11 pg/ml and the average intra- and inter-assay CVs of 7.4% and 7.8%, respectively. The ACT levels were measured in duplicate using an immunoperoxidase assay kit (Immunology Consultants Laboratory, Inc. Newberg, USA, catalogue number E-80CYT), with a sensitivity of 2.518 ng/ml and both the intra-assay and inter-assay CVs <10%. Laboratory personnel were blinded as to the demographic and clinical status of the subjects.
Covariates
We considered continuous variables including age (years), education (years), and body mass index (BMI; kg/m2). Ethnicity, including African American (Black non-Hispanic), Hispanic, White (non-Hispanic) or Other based on self-report using the format of the 2000 US census, was used as a dummy variable with non-Hispanic White as the reference. Sex (female vs. male), and smoking status (ever smoked vs. never smoked) were used as dichotomous variables. Apolipoprotein (APOE) genotyping employed standard PCR-RFLP methods using Hha1 (CfoI) digestion of an APOE genomic PCR product spanning the polymorphic (cys/arg) sites at codons 112 and 158. Acrylamide gel electrophoresis was used to assess and document the restriction fragment sizes(62). APOE-ε4 genotype (presence of either 1 or 2 vs. absence of ε4 alleles) was also used as a dichotomous variable. Stroke information was self-reported by participant or relatives as well as neurological examination or medical records review. Presence or absence of heart disease, diabetes mellitus, and hypertension were based on self-report or use of medications. These four vascular comorbidities were used as dichotomous variables with absence of the condition used as the reference.
Statistical analyses
Characteristics of the subjects
To reduce the right skewness of the distributions of the cytokine concentrations, circulating levels of inflammatory biomarkers were transformed using logarithm with a base of 2. Thus, a unit increase in log2 transformed biomarker level will indicate a doubling on the original scale. Pearson correlation tests were run to examine the relationship between the log-transformed biomarkers and brain measures. Demographic, clinical, and brain morphological characteristics of participants by biomarker quartile were compared using t-test for continuous variables and χ2 test for categorical variables.
Cross-sectional associations between the inflammatory biomarkers and brain measures
Generalized Linear Models were used to test the associations between the inflammatory biomarkers and baseline brain measures. A series of models was used, first adjusted for age at time of scan, sex, ethnicity/race (Model 1), and then additionally adjusted for APOE status and ICV (Model 2), and finally adjusted for all the Model 2 variables and BMI, smoking status, and vascular factors (Model 3). All covariates were treated as time independent variables.
Longitudinal data analysis
The mean yearly percent changes of brain measures were calculated as ((second measure – first measure) / first measure)/ years between the two measurements. Generalized estimating equation (GEE) models were used to test whether inflammatory biomarkers were associated with differential rates of brain measures change, with brain measure (i.e. TBV, TGMV, TWMV, or CT) as the outcome variable and time from baseline scan to the follow-up scan as the main predictor variable, and biomarker quartile level as the main predictor variable. A significant effect of the biomarker would indicate a difference in brain measure at the initial visit. A significant time effect would indicate a brain volume change over time. A significant interaction term between the inflammatory biomarker and time would indicate differential rates of change in brain measures as a function of inflammatory biomarker level.
Supplementary analyses
We performed a few supplementary analyses.
First, we excluded 161 subjects with MCI and repeated the analyses on global brain measures among cognitively normal subjects only.
Second, we examined whether age (younger-old vs. old-old by median age), sex, APOE, or ethnicity modified the relationship between inflammatory biomarkers and brain measures. Each of the potential modifier was dichotomized (female vs. male, APOE e4 positive vs. negative, Black vs. Whites or Hispanics vs. Whites). Stratified analysis was then run for each category of the modifier.
Finally, for the inflammatory biomarkers that were associated with global brain measured identified in the main analysis, we examined whether the inflammatory biomarker was independently associated with certain regional brain measures. We used multivariate analysis of variance (MANOVA) to see whether the linear combination of the 12 cortical and subcortical regional volumes (see supplementary data)(63) was associated with inflammatory biomarkers. We then examined univariate F tests for individual ROIs. A similar analysis was conducted for 12 thickness ROIs that have been shown to reflect AD-associated neurodegeneration (63, 64) (see supplementary data), and for 26 white matter tracts (61). Because individual ROI analyses were guided by the MANOVA results, and were essentially exploratory, no correction for multiple comparisons has been performed.
All analyses were performed using PASW Statistics 17.0 (formerly SPSS Inc., Chicago, IL USA). All p-values were based on two-sided tests with the significance level set at 0.003 (=0.05/15) using the Bonferroni correction method after taking into account of 15 (5 brain measures × 3 biomarkers) multiple comparisons.
Results
Missing data analysis
Among the 680 participants of WHICAP neuroimaging sub-study, 508 (75%) and 435 (66%) subjects had CRP and IL6/ACT measured, respectively. Participants who had measured biomarkers were younger and had fewer vascular risk factors than those did not have biomarker data sample (Table 1). African-Americans were less likely to have had biomarkers measured than Whites and Hispanics (Table 1). Those with follow-up MRI scans were younger at baseline than those did not come back for repeated MRI (79.3 vs. 80.6, p=0.002) but were similar otherwise.
Table 1.
Demographic, clinical, and brain morphology characteristics of the WHICAP neuroimaging study participants.
| Number (%) | CRP not measured 172 | CRP measured 508 | Total | p | IL6 or ACT not measured 245 | IL6 or ACT measured 435 | Total | p |
|---|---|---|---|---|---|---|---|---|
| (25%) | (75%) | 680 | (34%) | (66%) | 680 | |||
| Age at scan visit, years, mean(SD) | 81.06 (5.52) | 79.69 (5.56) | 80.04 (5.58) | 0.01 | 80.56 (5.63) | 79.75 (5.54) | 80.04 (5.58) | 0.07 |
| Female, N (%) | 126(73) | 334(66) | 460(68) | 0.07 | 170(69) | 290(67) | 460(68) | 0.47 |
| Race/ethnicity | 0.01 | 0.05 | ||||||
| White | 36(21) | 156(31) | 192(28) | 57(23) | 135(31) | 192(28) | ||
| Blacks | 76(44) | 159(31) | 235(35) | 94(38) | 141(32) | 235(35) | ||
| Hispanics | 56(33) | 184(36) | 240(35) | 92(38) | 148(34) | 240(35) | ||
| Others | 4(2) | 9(2) | 13(2) | 2(0) | 11(0) | 13(0) | ||
| Education, years, mean(SD) | 10.19 (5.03) | 10.93 (4.69) | 10.74 (4.79) | 0.08 | 10.44 (5.08) | 10.92 (4.61) | 10.74 (4.79) | 0.21 |
| Ever smoked, N (%) | 65(41) | 219(47) | 284(46) | 0.22 | 99(45) | 185(46) | 284(46) | 0.93 |
| APOE e4+, N (%) | 40(25) | 127(25) | 167(25) | 0.94 | 66(29) | 101(23) | 167(25) | 0.13 |
| Vascular score, mean(SD) | 1.94 (1.02) | 1.76 (1.00) | 1.81 (1.01) | 0.05 | 1.93 (1.01) | 1.74 (1.00) | 1.81 (1.01) | 0.02 |
| BMI, kg/m2, mean(SD) | 27.29 (5.17) | 27.83 (5.67) | 27.69 (5.55) | 0.28 | 28.12 (5.63) | 27.45 (5.49) | 27.69 (5.55) | 0.14 |
| ICV, mm3, mean(SD) | 1294.61 (149.81) | 1308.97 (158.33) | 1305.34 (156.23) | 0.30 | 1291.58 (159.23) | 1313.09 (154.16) | 1305.34 (156.23) | 0.08 |
| TBV, mm3, mean(SD) | 860.11 (93.31) | 874.53 (102.85) | 870.88 (100.65) | 0.10 | 858.74 (95.76) | 877.72 (102.78) | 870.88 (100.65) | 0.02 |
| Mean CT, mm, mean(SD) | 2.45 (0.12) | 2.46 (0.11) | 2.46 (0.11) | 0.11 | 2.45 (0.12) | 2.46 (0.11) | 2.46 (0.11) | 0.30 |
| Mean FA, mean(SD) | 0.45 (0.02) | 0.45 (0.02) | 0.45 (0.02) | 0.99 | 2.45 (0.12) | 2.46 (0.11) | 2.46 (0.11) | 0.30 |
Relation of inflammatory biomarkers to brain and participants characteristics
The three inflammatory markers positively correlated with each other (Table 2), and in general, they were negatively correlated with brain volumetric measures (Table 2 and Figure A.1) and with cortical thickness (Table 2 and Figure A.2).
Table 2. Pearson correlations between inflammatory biomarkers and demographics and brain characteristics.
| Unadjusted correlation | Adjusted partial correlation† | |||||
|---|---|---|---|---|---|---|
| CRP | IL6 | ACT | CRP | IL6 | ACT | |
| CRP | 1 | 0.488** | 0.383** | 1 | 0.492** | 0.383** |
| IL6 | 0.488** | 1 | 0.185** | 0.492** | 1 | 0.182** |
| ACT | 0.383** | 0.185** | 1 | 0.383** | 0.182** | 1 |
| Age | -0.068 | 0.037 | -0.004 | / | / | / |
| Education | -0.005 | -0.020 | 0.151** | / | / | / |
| ICV | -0.045 | -0.003 | 0.013 | 0.009 | 0.026 | 0.038 |
| TBV | -0.105* | -0.169** | -0.027 | -0.137* | -0.159** | -0.03 |
| TGMV | -0.118** | -0.150** | -0.047 | -0.192** | -0.162** | -0.062 |
| TWMV | -0.079 | -0.126* | -0.065 | -0.127* | -0.12* | -0.075 |
| Mean CT | -0.115* | -0.053 | -0.106* | -0.136* | -0.088 | -0.149** |
| Mean FA | -0.075 | -0.102 | -0.006 | -0.118 | -0.108 | -0.110 |
Partial correlation adjusted for age, sex, education, and race/ethnicity. The log-transformed inflammatory markers were used in the analysis.
Abbreviation: C-reactive protein (CRP); cortical thickness (CT); fractional anisotropy (FA); hippocampal volume (HipVol); interleukin-6 (IL6); intra-cranial volume (ICV); total brain volume (TBV); total gray matter volume (TGMV); total white matter volume (TWMV).
<0.01;
<0.05
CRP negatively correlated with TBV, TGMV, as well as CT, even after controlling for age at scan, sex, race/ethnicity, and education as seen in partial correlation tests (Table 2). Compared to those in the lowest CRP quartile, those in the highest CRP quartile had more smokers, more vascular risk factors, higher BMI, and smaller brain volumes and thinner cortex, and they were less likely to be APOE ε4 positive and more likely to be Blacks (Table 3). IL6 negatively correlated with TBV, TGMV, and TWMV (Table 2). Subjects who had the highest IL6 quartile had more smokers, higher BMI, and smaller brain volumes (Table 3). ACT did not correlate with any of the brain volume measures, but negatively correlated with CT (Table 2). Subjects in the highest ACT quartile had higher education and more vascular comorbidities, and are more likely to be females and Whites (Table 3).
Table 3.
Demographic, clinical, and dietary characteristics in relation to CRP, IL6, and ACT.
| CRP | IL6 | ACT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q4 | P | Q1 | Q4 | P | Q1 | Q4 | P | |
| Number (%) | 127 | 127 | 89 | 89 | 108 | 109 | |||
| Age at scan visit, years, mean (SD) | 80.5 (5.5) | 79.1 (5.2) | 0.045 | 79.1 (5.4) | 79.9 (5) | 0.304 | 79.4 (5.9) | 79.8 (5.4) | 0.647 |
| Female, N (%) | 82 (65) | 91 (72) | 0.226 | 53 (60) | 61 (69) | 0.211 | 30 (28) | 40 (37) | 0.043 |
| Race/ethnicity | 0.012 | 0.121 | 0.025 | ||||||
| White | 49 (39) | 31 (24) | 35 (39) | 21 (24) | 30 (28) | 40 (37) | |||
| Blacks | 29 (23) | 46 (36) | 24 (27) | 34 (38) | 35 (32) | 40 (37) | |||
| Hispanics | 49 (39) | 47 (37) | 26 (29) | 31 (35) | 42 (39) | 24 (22) | |||
| Others | 0 (0) | 3 (2) | 4 (4) | 3 (3) | 1 (1) | 5 (5) | |||
| Education, years, mean (SD) | 10.7 (4.8) | 10.9 (4.4) | 0.655 | 11.8 (4.5) | 10.7 (4.4) | 0.100 | 10.1 (4.9) | 12.1 (4.1) | 0.001 |
| Ever smoked, N (%) | 46 (38) | 63 (55) | 0.010 | 29 (35) | 44 (52) | 0.033 | 49 (49) | 52 (53) | 0.670 |
| APOE e4+, N (%) | 41 (32) | 25 (20) | 0.022 | 20 (22) | 25 (28) | 0.389 | 32 (30) | 31 (28) | 0.847 |
| Vascular score, mean (SD) | 1.5 (0.9) | 2 (1) | 0.000 | 1.7 (1) | 1.7 (1) | 0.940 | 1.6 (0.9) | 1.8 (1) | 0.038 |
| BMI, kg/m2, mean (SD) | 25.6 (4.8) | 30.8 (6.7) | 0.000 | 26.4 (4.6) | 28.3 (5.5) | 0.014 | 28.2 (5.3) | 27.6 (6.6) | 0.490 |
| CRP, mg/L, median (IQR)+ | 1.6 (0.7) | 35.8 (22.8) | <0.001 | 4.9 (7.8) | 22.4(22.6) | <0.001 | 7.4(8.2) | 26.2 (28.0) | <0.001 |
| IL6,pg/ml, median (IQR)+ | 2.2 (1.5) | 4.4 (2.2) | <0.001 | 1.3 (0.3) | 5.8 (1.7) | <0.001 | 2.8 (1.9) | 3.7 (2.2) | 0.003 |
| ACT, mg/L, median (IQR)+ | 179.6 (32.5) | 229 (72.8) | <0.001 | 186.2 (36.3) | 213.8 (54.7) | <0.001 | 149.2 (14) | 262.5 (53.8) | <0.001 |
| ICV, mm3, mean (SD) | 1320.6 (155.6) | 1292.8 (160.2) | 0.162 | 1332.6 (150.6) | 1319.3 (168.9) | 0.581 | 1315.6 (156.9) | 1317.4 (160.8) | 0.932 |
| TBV, mm3, mean (SD) | 883.2 (108.3) | 852.1 (94.4) | 0.016 | 906.4 (104.4) | 867.6 (104.2) | 0.014 | 876.3 (112.5) | 877.1 (97) | 0.951 |
| TGMV, mm3, mean (SD) | 525.6 (50.2) | 508.8 (47.6) | 0.007 | 532.7 (48.9) | 516.6 (51.2) | 0.033 | 522.8 (53.8) | 521.4 (50.6) | 0.848 |
| TWMV, mm3, mean (SD) | 380.9 (60.7) | 367.6 (50.1) | 0.058 | 392.6 (59.6) | 372.3 (54.6) | 0.018 | 378.0 (61.5) | 376.5 (52.3) | 0.837 |
| Mean CT, mm, mean (SD) | 2.473 (0.101) | 2.442 (0.112) | 0.020 | 2.457 (0.101) | 2.446 (0.111) | 0.481 | 2.476 (0.111) | 2.451 (0.113) | 0.114 |
| Mean FA, mean (SD) + | 0.452 (0.021) | 0.447 (0.022) | 0.296 | 0.454 (0.022) | 0.445 (0.025) | 0.101 | 0.448 (0.024) | 0.451 (0.022) | 0.692 |
p values were from χ2 test for categorical variables, or one-way ANOVA test for continuous variables.
Cross-sectional association between inflammatory biomarkers and brain structural measures
A doubling in CRP (b= -2.48, p=0.002) was associated with a smaller TGMV , equivalent to approximately 1.5 years of aging (b associated with TGMV for one year increase in age = -1.74, p<0.001) after adjusted for age at time of scan, gender, ethnicity/race, APOE status, and ICV (Model 2 in Table 4). CRP showed a trend of being negatively associated with smaller TBV and TWMV, as well as thinner cortex (Model 2 in Table 4). The associations between CRP and brain measures were attenuated and no longer significant after adjusting for BMI, depression, smoking status, and vascular comorbidities (Model 3 in Table 4). CRP was not associated with mean FA.
Table 4.
Association between inflammatory biomarkers and brain structural MRI measures.
| Brain MRI measure | Biomarker continuous or quartiles | CRP | IL6 | ACT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | b | p | N | b | p | N | b | p | ||
|
| ||||||||||
| Model 1: adjusted for age at time of scan, gender, ethnicity/race | ||||||||||
|
| ||||||||||
| TBV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -4.13 | 0.008 | 357 | -15.01 | <0.0001 | 435 | -6.83 | 0.454 |
| -19.91 | 0.010 | -31.18 | 0.001 | -0.36 | 0.970 | |||||
| -5.89 | 0.016 | -11.93 | <0.0001 | -2.00 | 0.454 | |||||
|
| ||||||||||
| TGMV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -2.42 | 0.003 | 357 | -6.61 | 0.001 | 435 | -5.97 | 0.218 |
| -11.02 | 0.006 | -10.56 | 0.034 | -1.99 | 0.658 | |||||
| -3.82 | 0.003 | -4.63 | 0.004 | -1.75 | 0.220 | |||||
|
| ||||||||||
| TWMV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -2.36 | 0.023 | 357 | -7.58 | 0.004 | 435 | -8.94 | 0.142 |
| -9.87 | 0.056 | -16.17 | 0.011 | -1.21 | 0.830 | |||||
| -2.86 | 0.081 | -5.70 | 0.005 | -1.88 | 0.293 | |||||
|
| ||||||||||
| CT | Continuous Q4 vs. Q1 | 508 | -0.008 | 0.004 | 357 | -0.006 | 0.333 | 435 | -0.042 | 0.007 |
| -0.034 | 0.010 | -0.007 | 0.655 | -0.030 | 0.030 | |||||
| -0.012 | 0.005 | -0.004 | 0.390 | -0.010 | 0.021 | |||||
|
| ||||||||||
| mean FA | Continuous Q4 vs. Q1 trend of quartiles | 183 | -0.001 | 0.328 | 129 | -0.004 | 0.119 | 154 | -0.003 | 0.663 |
| -0.004 | 0.374 | -0.007 | 0.210 | 0.001 | 0.822 | |||||
| -0.001 | 0.497 | -0.002 | 0.260 | 0.001 | 0.684 | |||||
|
| ||||||||||
| Model 2: adjusted for age at time of scan, gender, ethnicity/race, APOE status, and ICV | ||||||||||
|
| ||||||||||
| TBV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -4.31 | 0.006 | 357 | -14.96 | <0.0001 | 435 | -6.99 | 0.443 |
| -20.49 | 0.008 | -31.02 | 0.001 | -0.54 | 0.949 | |||||
| -6.14 | 0.014 | -11.89 | <0.0001 | -2.04 | 0.441 | |||||
|
| ||||||||||
| TGMV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -2.48 | 0.002 | 357 | -6.52 | 0.002 | 435 | -5.89 | 0.224 |
| -11.97 | 0.003 | -10.35 | 0.038 | -1.95 | 0.66 | |||||
| -3.91 | 0.002 | -4.57 | 0.004 | -1.73 | 0.226 | |||||
|
| ||||||||||
| TWMV | Continuous Q4 vs. Q1 trend of quartiles | 508 | -2.39 | 0.023 | 357 | -7.47 | 0.004 | 435 | -8.93 | 0.143 |
| -9.92 | 0.056 | -16.04 | 0.012 | -1.20 | 0.831 | |||||
| -2.83 | 0.082 | -5.63 | 0.005 | -1.88 | 0.295 | |||||
|
| ||||||||||
| CT | Continuous Q4 vs. Q1 trend of quartiles | 508 | -0.007 | 0.007 | 357 | -0.006 | 0.361 | 435 | -0.041 | 0.007 |
| -0.033 | 0.013 | -0.004 | 0.413 | -0.031 | 0.033 | |||||
| -0.011 | 0.008 | -0.004 | 0.462 | -0.010 | 0.023 | |||||
|
| ||||||||||
| mean FA | Continuous Q4 vs. Q1 trend of quartiles | 183 | -0.001 | 0.310 | 129 | -0.004 | 0.125 | 154 | -0.003 | 0.642 |
| -0.005 | 0.365 | -0.007 | 0.218 | 0.001 | 0.835 | |||||
| -0.001 | 0.476 | -0.002 | 0.268 | 0.001 | 0.671 | |||||
|
| ||||||||||
| Model 3: adjusted for age at time of scan, gender, ethnicity/race, APOE status, ICV, depression, BMI, smoking status, and vascular factors | ||||||||||
|
| ||||||||||
| TBV | Continuous Q4 vs. Q1 trend of quartiles | 447 | -2.17 | 0.229 | 324 | -14.33 | 0.001 | 393 | 1.44 | 0.832 |
| -8.99 | 0.308 | -32.46 | 0.001 | 8.32 | 0.349 | |||||
| -2.73 | 0.331 | -11.77 | <0.0001 | 0.39 | 0.891 | |||||
|
| ||||||||||
| TGMV | Continuous Q4 vs. Q1 trend of quartiles | 447 | -1.35 | 0.142 | 324 | -6.69 | 0.002 | 393 | -3.71 | 0.460 |
| -6.12 | 0.172 | -12.39 | 0.015 | 0.79 | 0.864 | |||||
| -2.11 | 0.140 | -4.93 | 0.002 | -1.13 | 0.441 | |||||
|
| ||||||||||
| TWMV | Continuous Q4 vs. Q1 trend of quartiles | 447 | -1.87 | 0.122 | 324 | -8.13 | 0.003 | 393 | -4.79 | 0.460 |
| -5.73 | 0.335 | -18.49 | 0.006 | 2.87 | 0.629 | |||||
| -1.75 | 0.352 | -6.37 | 0.003 | -0.88 | 0.641 | |||||
|
| ||||||||||
| CT | Continuous Q4 vs. Q1 trend of quartiles | 447 | -0.006 | 0.064 | 324 | -0.005 | 0.456 | 393 | -0.054 | 0.001 |
| -0.027 | 0.077 | -0.007 | 0.676 | -0.038 | 0.011 | |||||
| -0.010 | 0.045 | -0.004 | 0.515 | -0.013 | 0.006 | |||||
|
| ||||||||||
| mean FA | Continuous Q4 vs. Q1 trend of quartiles | 164 | 0.0001 | 0.880 | 118 | -0.003 | 0.294 | 141 | 0.000 | 0.985 |
| -0.0002 | 0.978 | -0.005 | 0.402 | 0.002 | 0.708 | |||||
| -0.0001 | 0.917 | -0.001 | 0.541 | -0.0001 | 0.827 | |||||
Results from regression models adjusted for age at time of scan, gender, ethnicity/race (Model 1), and then additionally adjusted for APOE status and ICV (Model 2), and finally adjusted for all the Model 2 variables and BMI, smoking status, and vascular factors (Model 3). Abbreviation: Apolipoprotein (APOE); C-reactive protein (CRP); cortical thickness (CT); fractional anisotropy (FA); interleukin-6 (IL6); intra-cranial volume (ICV); total brain volume (TBV); total gray matter volume (TGMV); total white matter volume (TWMV).
Q4 is the 4th (the highest) quartile, and Q1 the first (the lowest) quartile.
The trend tests were analyzed by entering the quartiles as an ordinal variable into the linear models.
A doubling in IL6 (b= -14.96, p=0.001) was associated with a smaller TBV, equivalent to approximately 9 years of aging (b associated with TBV for one year increase in age = -1.70, p<0.001) (Model 2 in Table 4), and the association remained significant after additionally adjusted for BMI, depression, smoking status, and vascular comorbidities (Model 3 in Table 4). Higher IL6 was also associated with smaller gray matter (b=-6.52, p=0.002) and marginally with white matter volumes (b=-7.47, p=0.004), but was not associated with mean CT or mean FA (Table 2).
In the fully adjusted model (Model 3 in Table 4), a doubling in ACT was associated with 0.054 (p=0.001) millimeter thinner mean CT, equivalent to that of approximately 2.7 years of aging (b associated with CT for one year increase in age = -0.002, p=0.046). ACT was not associated with brain volumes or mean FA (Table 4).
Longitudinal association between inflammatory biomarkers and brain structural measures
Repeated MRI scans were collected after an average of 4.5 (SD=0.8, range 2.3-6.9) years. The mean yearly percent changes for TBV, TGMV, TWMV, Hippocampal volume, and CT were -0.42%, -0.40%, -0.75%, -1.29%, and 0.16%, respectively. GEE models including o nly the time variable showed that TBV, TGMV, TWMV, and mean CT had non-significant decrease of 2.51 mm3 (p=0.38), 0.36 mm3 (p=0.86), 3.7 mm3 (p=0.09), and 0.003 mm (p=0.66) annually, respectively. However, there was a significant decrease of 0.15 mm3 (p=0.007) per year in hippocampal volume. Therefore, we focused on hippocampal volume in the following analyses. We found that inflammatory biomarkers did not modify the rate of hippocampal volume change during the observed years: b=0.04 (p=0.36) for the interaction between CRP and time, 0.0017 (p=0.98) for IL6 × time interaction, -0.122 (p=0.52) for ACT × time interaction, after adjusted for age, sex, education, race/ethnicity, and APOE.
Supplementary Analyses
After excluding MCI subjects and adjusted for Model 2 covariates, a doubling in CRP was associated with a smaller TBV (b= -4.58, p=0.009) and CT (b=-0.009, p=0.002), and marginally with TGMV (b=-2.31, p =0.01) and TWMV (b=-2.91, p=0.01); a doubling in IL6 was associated with a smaller TBV (b= -17.64, p<0.0001), TGMV (b=-7.03, p =0.003), and marginally with TWMV (b=-8.23, p=0.007); and a doubling in ACT was associated with 0.054 (p=0.003) millimeter thinner mean CT.
We used the association between IL6 and TBV, the strongest association we found in the main analysis, to explore the potential interaction of inflammatory biomarkers and several variables. There was no interaction between IL6 and age groups, sex, ethnicity, or APOE status. Stratified analyses adjusted for Model 2 covariates showed a doubling in IL6 was associated with larger TBV in both age groups (b=-14.2, p=0.015 for younger group, and b=15.2, p=0.003 for older group), in both men and women (b= -22.0, p=0.003 in men, and b=-11.3, p=0.01 in women), and in both APOE-ε4 negative (b=-14.9, p=0.002) and ε4 positive (b=-15.4, p=0.02). The association between IL6 and TBV was seen in Whites (b= -19.4, p=0.004) and in African-Americans (b=-16.9, p=0.03), but not in Hispanics (b=-9.12, p=0.12).
Multivariate analysis adjusted for the Model 2 variables indicated significant differences between IL6 quartiles on a linear combination of the volumes in 12 ROIs (Wilks' Lambda = 0.84, p=0.01). The volumes of cortical regions including frontal (univariate p=0.05), occipital (p=0.01), parietal (p=0.02), temporal (p=0.003), as well as subcortical regions including pallidum (p=0.02) and thalamus (p=0.003) contributed most to distinguish the different IL6 levels, while cingulate (p=0.27), caudate (p=0.18), putamen (p=0.12), accumbens (p=0.62), amygdala (p=0.88), hippocampus (p=0.36) did not. Regarding CT, no association of CRP (Wilks' Lambda = 0.92, p=0.23) or ACT (Wilks' Lambda = 0.91, p=0.73) quartiles with the 12 regions was found, although CRP might be associated with temporal region CT (p=0.001).
Discussion
In a multiethnic, non-demented, elderly population, we found higher IL6 circulating levels was associated with smaller TBV, TGMV and TWMV. ACT was not associated with brain volume but was associated with smaller cortical thickness. To put the magnitude of the association into context, doubling in IL6 and ACT levels were associated with smaller total brain volume and cortical thickness, comparable with the effect of 9 and 2.7 years of aging, respectively. The associations were independent of age at time of scan, gender, ethnicity/race, APOE status, ICV, BMI, depression, smoking status, and vascular comorbidities. CRP also showed a similar trend of being negatively associated with smaller gray matter volume. None of the biomarkers was significantly associated with white matter integrity. The inflammatory biomarkers did not modify the change of brain measures over 4.5 years.
Cytokines in relation with MRI measures
Our findings of inflammatory biomarkers on global brain volume are consistent with previous studies. While no association was found between CRP or IL6 levels and brain volumes (TBV, TGMV, or TWMV) in some studies (32, 33, 41, 42), others have found CRP and IL6 are related to brain volumes(35-40). In one study, hsCRP level was negatively correlated with regional gray matter volumes (38). In a study of 1,926 Framingham Offspring participants, IL6, but not CRP, was inversely associated with TBV(35). Data from Adult Health and Behavior Project – Phase 2 also showed that higher IL6 and CRP were associated with smaller lower TGMV and TWMV(37, 39). Among the 268 elderly community participants, a cytokine-chemokine combination including IL-6 was associated with severe cortical atrophy (40). More recently, a study on 1316 healthy elderly adults from the Three City-Dijon cohort found that higher concentration of IL-6, and, to a lesser degree, of CRP, was associated with lower TGMV (36). Overall, our study confirmed previous findings that pro-inflammatory cytokines, especially IL6, were associated with smaller global brain volume.
It is also interesting to examine which brain regions are involved in relation to inflammatory biomarkers. Given the prominent role of the hippocampus in memory and dementia(65) and its tendency to decline in volume early in aging(14), it is not surprising that hippocampal volume is the most commonly examined region. However, findings from previous studies have produced inconsistent results. Several studies found inverse associations between CRP or IL6 and hippocampal volume (37, 45, 46) or left medial temporal lobe(38, 66), others did not(41, 42). In our study, we found IL6 was negatively associated with temporal region and thalamus, but not hippocampus volume. The inconsistency might be due to population differences in key factors such as age, and whether potential confounders such as APOE status and depression were controlled. Nevertheless, the possibility of cytokines being involved in hippocampal atrophy may not be completely ruled out, as other inflammatory cytokines such as tumor necrosis factor-α or its receptors were reported to be associated with hippocampal volume(41, 42), so an evaluation of a more comprehensive panel of inflammatory biomarkers may be needed.
Regarding cortical thickness, few data exist to draw concrete conclusions. Similar to our study, no cross-sectional associations of IL6 or CRP with cortical thickness was found in adults aged 30–54 years in one study(37). However, in a study of old adults, higher IL6 was associated with thinner gray matter in certain brain regions, as well as accelerated annual rates of cortical thinning in the inferior temporal poles bilaterally (43). We also found ACT and possibly CRP was associated with thinner cortex. It is unclear why in our study IL6 was associated with brain vlume but not CT, while ACT was associated with CT but not brain volume is unclear. While CT may contribute to cortical brain volume, cortical surface area, which is genetically independent from CT(67), may also have influence on brain volume. Thus, it is possible that different inflammatory markers may have effects on selective brain structures. Overall, data are scarce to draw conclusions on the role of inflammatory markers on cortical thickness and further studies are warranted.
With regard to the microstructural integrity of brain white matter, previous studies showed that systemic inflammation was associated with reduced FA (32-34). We did not find white matter integrity linked with circulating inflammatory biomarkers using our method. While data are scarce, the current overall evidence suggests that inflammatory process might be involved in altered microstructural integrity.
Mechanisms for the association
There are at least three potential interpretations for our findings. First, peripheral inflammation may contribute to brain atrophy and degeneration, as there is increasing evidence to suggest that an inflammatory response may be involved in driving the AD neurodegenerative cascade(68). The mechanisms by which peripheral IL-6 contributes to brain atrophy remain unclear, but most likely are manifold. The peripheral inflammatory response can contribute to neurotoxicity through the β-amyloid pathway. In a murine model, circulating IL-6 levels were associated with increased influx and decreased efflux of β-amyloid across the blood brain barrier (BBB), as well as increased neuronal synthesis of β-amyloid directly in the brain(69). In human neuronal cell cultures, a combination of proinflammatory cytokines induces the production of β-amyloid in these neuronal cells, while pretreatment with ibuprofen, a nonsteroidal anti-inflammatory drug, was associated with decreased β-amyloid secretion and accumulation in the cytokine-stimulated neurons(70). Alternatively, inflammation may also directly contribute to the neurodegenerative pathways such as inducing neuronal apoptosis(71), inhibiting hippocampal neurogenesis(72), and influencing synaptic plasticity(73, 74).
Second, the cross-sectional results might be due to the existence of common factors contributing to both the elevation of inflammatory biomarkers and brain atrophy. For example, consistent evidence indicates that peripheral inflammatory markers are associated with risk of cardiovascular diseases(75, 76), which can contribute to the cerebrovascular pathology seen in dementia (77). Nevertheless, the association between IL6 and brain volume persisted following adjustment for many potential confounders including several vascular factors, depression, and smoking, suggesting an independent contribution of IL6 to brain volume.
Finally, the possibility that increased concentrations of proinflammatory cytokines may be a consequence of AD pathology cannot be completely ruled out. It has been shown that brain cholinesterase activity plays a role in regulating the release of cytokines from peripheral tissues through ‘cholinergic anti-inflammatory pathway’(78). In rat models, an inflammatory response in the brain results in an increased peripheral IL6 concentration(79). It is also possible that at early stages of neurodegenerative diseases, neurodegenerative factors may activate glial cells (microglia and astrocytes), triggering abnormal production and releasing of pro-inflammatory cytokines which in turn accelerates neurodegeneration(80). In the longitudinal analysis of the current study, we did not find baseline IL6 to be associated with further decline in brain volume, which seem to support this possibility. However, the longitudinal analysis of our study was done over a limited period of time (4.5 years on average) among a group of subjects with advanced age, which might not be the ideal setting to confirm our cross-sectional findings. It has been proposed that the phenomenon of elevated inflammatory markers in older adults is the consequence of accumulative damaging effects of lifelong immune response(81). In addition, it is thought that brain volume loss can start as early as age 35 years (82). Thus, it is reasonable to assume that the cross-sectional relationship between IL6 and brain volumes we saw in the study to some extent reflects the accumulative change in the brain due to elevated IL6 over many years, even decades. Thus, future studies may need to test whether inflammatory biomarkers indeed contribute to longitudinal brain volume change among healthy, middle-age to younger-old subjects, with longer follow-up time.
Limitations and strengths
A few limitations of the present study need to be noted. Although we adjusted for several key factors, we cannot completely rule out the possibility of residual confounding. We only examined three of the many cytokines or inflammatory markers (20) in our study. As mentioned earlier, other cytokines might also be important (41, 42). In addition, cytokines are regulated and influenced by each other. Therefore, it would be also interesting to examine a more comprehensive panel of inflammatory markers to adequately describe changes in the cytokine network and examine its association with brain measures. Second, while cytokines can pass through BBB, peripheral cytokines may not specifically reflect inflammatory activity within the central nervous system. However, it has been shown cerebrospinal fluid and plasma IL-6 levels were highly correlated (83). We only measured the inflammatory markers once and they may not represent the long-term average level of the subject. However, it has been shown that many circulating cytokines are quite stable within individuals (84). We also recognize that, with the ever-improving technology, the 1.5T MRI with limited direction DTI is no longer state-of-the-art. Nonetheless, this is what we had at the time of data collection, and the method has been deployed in numerous meaningful studies and are still considered valid and reliable. Finally, despite a relatively large overall sample size, our study might be underpowered to examine interactions, stratified analyses in subgroups, and the longitudinal relationship.
Our study has many strengths. Our study added new evidence to this line of research by including several key inflammatory markers, both cross-sectional and longitudinal analyses, an elderly population with advanced age, and different features of brain and regional brain measures. In addition, our study population is composed of multiple ethnic groups, including not only White non-Hispanics but also African Americans and Hispanics, and thus the results are likely to be more generalizable to the increasingly diverse US population(53). Finally, several key potential confounding factors were adjusted in the analyses, thus giving us an estimate of the relationship between cytokines and brain measures independent of these covariates.
Among old adults, a higher level of peripheral pro-inflammatory status, indicated by higher level of IL-6, was associated with smaller brain volume but not the white matter tract integrity. Our findings suggest that inflammatory process might be involved in the brain volume loss among the elderly.
Supplementary Material
Highlights for the study.
Cross-sectional and longitudinal associations between inflammatory biomarkers brain structural measures were examined among elderly individuals without dementia.
Higher interleukin-6 level was associated with smaller brain volume in the cross-sectional analysis.
Higher alpha 1-antichymotrypsin level tended to be associated with thinner cortical thickness in the cross-sectional analysis.
The results suggest peripheral inflammatory processes may be involved in the brain atrophy in the elderly.
Acknowledgments
Data collection and sharing for this project was supported by the Washington Heights-Inwood Columbia Aging Project (WHICAP, PO1AG07232, R01AG037212, RF1AG054023) funded by the National Institute on Aging (NIA) and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. This work was also supported by National Institutes of Health grants AG042483, AG034189, and AG045334. This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study.
Non-standard abbreviations
- ACT
alpha 1-antichymotrypsin
- APOE
Apolipoprotein
- CDR
Clinical Dementia Rating
- CRP
C-reactive protein
- CT
cortical thickness
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- IL6
interleukin-6
- ICV
intra-cranial volume
- MRI
magnetic resonance imaging
- ROI
region of interest
- TBV
total brain volume
- TGMV
total gray matter volume
- TWMV
total white matter volume
- WHICAP
Washington Heights/Hamilton Heights Inwood Columbia Aging Project
Footnotes
Conflicts of interest: The authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Raskin J, Cummings J, Hardy J, Schuh K, Dean RA. Neurobiology of Alzheimer's Disease: Integrated Molecular, Physiological, Anatomical, Biomarker, and Cognitive Dimensions. Current Alzheimer research. 2015;12(8):712–22. doi: 10.2174/1567205012666150701103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. Journal of Neuroscience. 2003;23(8):3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: Prediction of Alzheimer disease. Neurology. 2007;68(11):828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–13. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V, et al. Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain. 2009;132(Pt 8):2036–47. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerch JP, Pruessner J, Zijdenbos AP, Collins DL, Teipel SJ, Hampel H, et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer's patients from normal elderly controls. Neurobiology of aging. 2008;29(1):23–30. doi: 10.1016/j.neurobiolaging.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Archives of general psychiatry. 2009;66(5):545–53. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 9.Rosano C, Aizenstein HJ, Newman AB, Venkatraman V, Harris T, Ding J, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. NeuroImage. 2012;62(1):307–13. doi: 10.1016/j.neuroimage.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta neuropathologica. 2010;120(3):277–86. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 11.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Archives of neurology. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunstad J, Bausserman L, Paul RH, Tate DF, Hoth K, Poppas A, et al. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2006;13(5):540–6. doi: 10.1016/j.jocn.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 14.Dlugaj M, Gerwig M, Wege N, Siegrist J, Mann K, Brocker-Preuss M, et al. Elevated levels of high-sensitivity C-reactive protein are associated with mild cognitive impairment and its subtypes: results of a population-based case-control study. Journal of Alzheimer's disease : JAD. 2012;28(3):503–14. doi: 10.3233/JAD-2011-111352. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals of neurology. 2002;52(2):168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 16.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology. 2004;61(5):668–72. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 17.Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiology of aging. 2007;28(12):1810–20. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson UK, Pedersen NL, Reynolds CA, Hong MG, Prince JA, Gatz M, et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer's disease and dementia. Journal of Alzheimer's disease : JAD. 2011;23(2):361–9. doi: 10.3233/JAD-2010-101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Archives of neurology. 2012;69(5):594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(4):433–40. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83(6):486–93. doi: 10.1212/WNL.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC. Interleukin-6 and C-Reactive Protein Levels and 9-Year Cognitive Decline in Community-Dwelling Older Women: The Women's Health and Aging Study II. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70(7):873–8. doi: 10.1093/gerona/glu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reale M, Greig NH, Kamal MA. Peripheral chemo-cytokine profiles in Alzheimer's and Parkinson's diseases. Mini reviews in medicinal chemistry. 2009;9(10):1229–41. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooijaart SP, Sattar N, Trompet S, Lucke J, Stott DJ, Ford I, et al. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. Journal of internal medicine. 2013;274(1):77–85. doi: 10.1111/joim.12052. [DOI] [PubMed] [Google Scholar]
- 25.Economos A, Wright CB, Moon YP, Rundek T, Rabbani L, Paik MC, et al. Interleukin 6 plasma concentration associates with cognitive decline: the northern Manhattan study. Neuroepidemiology. 2013;40(4):253–9. doi: 10.1159/000343276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 27.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371–7. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 28.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–8. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 29.Licastro F, Parnetti L, Morini MC, Davis LJ, Cucinotta D, Gaiti A, et al. Acute phase reactant alpha 1-antichymotrypsin is increased in cerebrospinal fluid and serum of patients with probable Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9(2):112–8. doi: 10.1097/00002093-199509020-00009. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara E, Hirai S, Amari M, Shoji M, Yamaguchi H, Okamoto K, et al. Alpha 1-antichymotrypsin as a possible biochemical marker for Alzheimer-type dementia. Annals of neurology. 1990;28(4):561–7. doi: 10.1002/ana.410280414. [DOI] [PubMed] [Google Scholar]
- 31.Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in neuro-psychopharmacology & biological psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–9. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 33.Miralbell J, Soriano JJ, Spulber G, Lopez-Cancio E, Arenillas JF, Bargallo N, et al. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiology of aging. 2012;33(5):1003.e9–17. doi: 10.1016/j.neurobiolaging.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Arfanakis K, Fleischman DA, Grisot G, Barth CM, Varentsova A, Morris MC, et al. Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PloS one. 2013;8(8):e73107. doi: 10.1371/journal.pone.0073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferson AL, Massaro JM, Wolf PA, Seshadri S, Au R, Vasan RS, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032–8. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78(10):720–7. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 37.Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain, behavior, and immunity. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Wu K, et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Human brain mapping. 2013;34(10):2418–24. doi: 10.1002/hbm.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biological psychiatry. 2008;64(6):484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baune BT, Ponath G, Rothermundt M, Roesler A, Berger K. Association between cytokines and cerebral MRI changes in the aging brain. J Geriatr Psychiatry Neurol. 2009;22(1):23–34. doi: 10.1177/0891988708328216. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H, Sachdev PS, Wen W, Crawford JD, Brodaty H, Baune BT, et al. The relationship between inflammatory markers and voxel-based gray matter volumes in nondemented older adults. Neurobiology of aging. 2016;37:138–46. doi: 10.1016/j.neurobiolaging.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt MF, Freeman KB, Windham BG, Griswold ME, Kullo IJ, Turner ST, et al. Associations Between Serum Inflammatory Markers and Hippocampal Volume in a Community Sample. J Am Geriatr Soc. 2016;64(9):1823–9. doi: 10.1111/jgs.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarrey AC, Pacheco J, Carlson OD, Egan JM, Thambisetty M, An Y, et al. Interleukin-6 is linked to longitudinal rates of cortical thinning in aging. Translational neuroscience. 2014;5(1):1–7. doi: 10.2478/s13380-014-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham CR, Shirahama T, Potter H. Alpha 1-antichymotrypsin is associated solely with amyloid deposits containing the beta-protein. Amyloid and cell localization of alpha 1-antichymotrypsin. Neurobiology of aging. 1990;11(2):123–9. doi: 10.1016/0197-4580(90)90045-2. [DOI] [PubMed] [Google Scholar]
- 45.Porcellini E, Davis EJ, Chiappelli M, Ianni E, Di Stefano G, Forti P, et al. Elevated plasma levels of alpha-1-anti-chymotrypsin in age-related cognitive decline and Alzheimer's disease: a potential therapeutic target. Current pharmaceutical design. 2008;14(26):2659–64. doi: 10.2174/138161208786264151. [DOI] [PubMed] [Google Scholar]
- 46.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 47.Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 48.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Archives of neurology. 1992;49(5):453–60. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 49.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 50.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 51.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine. 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 52.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Archives of neurology. 2005;62(11):1739–46. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 53.Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Archives of neurology. 2008;65(8):1053–61. doi: 10.1001/archneur.65.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 56.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 57.Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE transactions on medical imaging. 2007;26(4):518–29. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- 58.Dale AM. Optimal experimental design for event-related fMRI. Human brain mapping. 1999;8(2-3):109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40(2):570–82. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Y, Vorburger RS, Gazes Y, Habeck CG, Stern Y, Luchsinger JA, et al. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Annals of neurology. 2016;79(6):1014–25. doi: 10.1002/ana.24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of lipid research. 1990;31(3):545–8. [PubMed] [Google Scholar]
- 63.Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology. 2015;85(20):1744–51. doi: 10.1212/WNL.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual review of neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 66.Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain, behavior, and immunity. 2012;26(1):103–8. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain, behavior, and immunity. 2009;23(4):507–17. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blasko I, Apochal A, Boeck G, Hartmann T, Grubeck-Loebenstein B, Ransmayr G. Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiology of disease. 2001;8(6):1094–101. doi: 10.1006/nbdi.2001.0451. [DOI] [PubMed] [Google Scholar]
- 71.Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. Journal of chemical neuroanatomy. 2005;30(2-3):144–57. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science (New York, NY) 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 73.Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. Journal of neurochemistry. 2000;75(2):634–43. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- 74.Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, et al. Interleukin-6: a cytokine to forget. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(14):1788–90. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 75.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 76.McCarty MF. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Medical hypotheses. 1999;52(5):465–77. doi: 10.1054/mehy.1997.0684. [DOI] [PubMed] [Google Scholar]
- 77.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, behavior, and immunity. 2009;23(1):41–5. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Simoni MG, De Luigi A, Gemma L, Sironi M, Manfridi A, Ghezzi P. Modulation of systemic interleukin-6 induction by central interleukin-1. The American journal of physiology. 1993;265(4 Pt 2):R739–42. doi: 10.1152/ajpregu.1993.265.4.R739. [DOI] [PubMed] [Google Scholar]
- 80.Sochocka M, Diniz BS, Leszek J. Inflammatory Response in the CNS: Friend or Foe? Molecular neurobiology. 2016 doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing research reviews. 2011;10(3):319–29. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Human brain mapping. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer's disease. Dementia and geriatric cognitive disorders. 2003;16(3):136–44. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- 84.Gu Y, Zeleniuch-Jacquotte A, Linkov F, Koenig KL, Liu M, Velikokhatnaya L, et al. Reproducibility of serum cytokines and growth factors. Cytokine. 2009;45(1):44–9. doi: 10.1016/j.cyto.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


