Abstract
Physical activity intervention studies that focus on improving cognitive function in older adults have increasingly used magnetic resonance imaging (MRI) measures in addition to neurocognitive measures to assess effects on the brain. The purpose of this systematic review was to identify the effects of endurance-focused physical activity randomized controlled trial (RCT) interventions on the brain as measured by MRI in community-dwelling middle-aged or older adults without cognitive impairment. Five electronic databases were searched. The final sample included six studies. None of the studies reported racial or ethnic characteristics of the participants. All studies included neurocognitive measures in addition to MRI. Five of the six interventions included laboratory-based treadmill or supervised bike exercise sessions, while one included community-based physical activity. Physical activity measures were limited to assessment of cardiorespiratory fitness and, in one study, pedometer. Due to the lack of adequate data reported, effect sizes were calculated for only one study for MRI measures and two studies for neurocognitive measures. Effect sizes ranged from d = .2 to .3 for MRI measures and .2 to .32 for neurocognitive measures. Findings of the individual studies suggest that MRI measures may be more sensitive to the effects of physical activity than neurocognitive measures. Future studies are needed that include diverse, community-based participants, direct measures of physical activity, and complete reporting of MRI and neurocognitive findings.
Keywords: physical activity, exercise, cognition, brain, MRI, fMRI
Mild cognitive impairment, defined as decline in episodic memory, attention, and cognitive function beyond what is expected due to normal aging (Petersen et al., 2010), occurs in 22% of adults aged 71 years or older (Petersen et al., 2010; Plassman et al., 2008). Cognitive impairment due to dementia, a distressing chronic disease of cognitive dysfunction that impacts quality of life, independence, and family relations, occurs in 14% of adults aged over 71 years (Petersen et al., 2010; Plassman et al., 2007). Cognitive impairment (with or without dementia) is an increasing public health concern due to the rapidly growing older adult population. It is essential that health-care researchers and providers target modifiable health behaviors that can help offset or delay the onset of cognitive impairment.
Researchers have recently concentrated increased attention on the effect of physical activity, particularly endurance-focused physical activity (activity that raises heart rate and respiration; American College of Sports Medicine et al., 2009), on cognitive function in middle-aged and older adults (Behrman & Ebmeier, 2014). Most support for a beneficial effect of physical activity on cognition comes from clinical epidemiologic studies that suggest a small but protective effect of physical activity and aerobic fitness on risk of cognitive impairment and decline in healthy cognitive aging (Pignatti, Rozzini, & Trabucchi, 2002; Richards, Hardy, & Wadsworth, 2003; Weuve et al., 2004). In these studies, researchers used neurocognitive testing to examine specific cognitive functions and abilities, including memory and executive function.
Neurocognitive tests are widely used in physical activity and cognition research because they are relatively inexpensive to implement, have been validated in multiple age-groups and races, and can be implemented in clinical or community-based settings (McCarten, 2013; Zygouris & Tsolaki, 2015). Unfortunately, the sensitivity of neurocognitive measures in physical activity and cognition studies is questionable (Smith, Potter, McLaren, & Blumenthal, 2013). Not all neurocognitive tests are well validated in every ethnic or socioeconomic subgroup, and such tests may not be sensitive enough to detect early cognitive impairment (Holtzer et al., 2008). These limitations have led to investigation of more precise and sensitive measures of brain health.
Recently, investigators have used magnetic resonance imaging (MRI) methods to assess brain health because they enable more sensitive measurement of brain regions and neurophysiological functions than neurocognitive tests do (Smith et al., 2013). MRI methods may detect changes in brain structure or neurophysiological functions that are indicative of mild cognitive impairment or dementia disease processes prior to the time at which neurocognitive tests could detect them. Researchers have used MRI to assess the link between physical activity and brain structure (e.g., brain volume). Most MRI studies have used a physical activity correlational approach. More specifically, anatomical MRI studies have shown that physical activity is associated with reduced loss of brain tissue in the frontal, parietal, and temporal cortices (Kramer, Erickson, & Colcombe, 2006); greater preservation of hippocampal volume (Szabo et al., 2011); and lower volume of white matter lesions (Burzynska et al., 2014).
Functional MRI (fMRI) is used to assess brain activation. fMRI research has shown that, compared to less fit older adults, highly fit older adults performing a task with distracting stimuli had greater activation in prefrontal and parietal cortical regions involved in selective attention and inhibitory functioning and lower activation in an anterior cingulate region that monitors conflict in the central executive system (Colcombe et al., 2004). Furthermore, higher fitness levels in older adults have been associated with enhanced attentional function through increased recruitment of prefrontal cortical regions (Prakash et al., 2011).
Authors have recently published eight literature reviews examining physical activity interventions and cognitive function (Cumming, Tyedin, Churilov, Morris, & Bernhardt, 2012; Farina, Rusted, & Tabet, 2014; Gary & Brunn, 2014; Gates, Fiatarone Singh, Sachdev, & Valenzuela, 2013; Lautenschlager, Cox, & Kurz, 2010; Law, Barnett, Yau, & Gray, 2014; Littbrand, Stenvall, & Rosendahl, 2011; Smith et al., 2013; Snowden et al., 2011). However, most (n = 6) focused on participants with specific disease processes, while one examined combined cognitive and exercise interventions and did not examine the impact of physical activity separately (Law et al., 2014). All of these reviews focused on studies that used neurocognitive measures to assess cognitive function. Of the two reviews of physical activity interventions among healthy middle-aged and older adults, only Smith and colleagues’ (2013) narrative review addressed the use of MRI measures in two identified studies; however, they did not address the relationship between MRI measures and neurocognitive measures.
To our knowledge, no authors have systematically reviewed studies examining the effects of physical activity randomized controlled trials (RCTs) on cognition as measured by MRI methods in healthy, community-dwelling middle-aged or older adults. Further, no authors have investigated the relationship between MRI and neurocognitive measures in such studies. Therefore, the purpose of this review was to identify the effect of endurance-focused physical activity interventions on the brain as measured by MRI in community-dwelling middle-aged or older adults without cognitive impairment. We also aimed to determine whether there has been agreement between MRI and neurocognitive outcome measures in endurance-focused physical activity interventions.
Method
Design
We retrieved the existing literature on brain MRI outcomes in physical activity RCTs for middle-aged or older adults (Higgins & Green, 2011). We used the Preferred Reporting Items for Reviews and Meta-Analyses flow sheet and checklist to ensure complete reporting of the evidence-based minimum reporting items (Moher, Liberati, Tetzlaff, Altman, & Group, 2009).
Inclusion Criteria
Inclusion criteria for this review were that the article (a) report the results of an RCT, (b) involve implementation of an endurance-focused physical activity intervention (physical activity with an aerobic component that raises heart rate and respirations), (c) include a brain imaging outcome as measured by MRI, (d) include a healthy middle-aged or older adult sample without cognitive impairment at baseline, and (e) be written in English.
Search Methods
In May 2015, we conducted a search of five databases: PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), SciVerse Scopus, Health Source: Nursing/Academic Edition, and PsycInfo. Next, we searched the Cochrane Database of Systematic Reviews to obtain systematic reviews of physical activity interventions that aimed to improve or maintain cognition. Finally, we reviewed the reference lists of existing literature reviews that we had previously identified.
For the search strategy, we identified search terms according to three main categories: (a) physical activity, (b) cognition, and (c) MRI outcome measure. The search terms for the first category were physical activity (text word), walking (Medical Subject Headings [MeSH]), exercise (MeSH), leisure activities (MeSH), and lifestyle physical activity (text word). For the second category, the search terms were cognition, cognition disorder, memory, short-term memory, mental recall, recognition (psychology), retention (psychology), memory disorders, attention, and dementia (all MeSH). For the third category, the search terms were magnetic resonance imaging (MeSH), functional magnetic resonance imaging (MeSH), MRI (text word), and fMRI (text word). We applied the qualifier of randomized controlled trial (RCT) on the search to narrow results to studies with RCT designs. We also applied the age qualifiers of middle-aged and older adult to narrow results to those that included middle-aged and older adults. For example, the full electronic search strategy that we utilized for CINAHL was the following: (“Physical Activity” OR exercise OR “Leisure activities” OR “Walking” OR “lifestyle physical activity”) AND (cognition OR “Cognition disorder” OR “Auditory perceptual disorders” OR Memory OR “Memory, short term” OR “Mental recall” OR Recognition OR Retention OR “Memory disorders” OR Attention OR Dementia) AND (MRI OR “Magnetic resonance imaging” OR fMRI OR “Functional magnetic resonance imaging”), with the limits of RCT for design and middle-aged and older adult for age-group. To ensure retrieval of the complete possible selection of papers, we did not limit our search by publication date. A medical librarian verified the search strategy.
Search Outcome
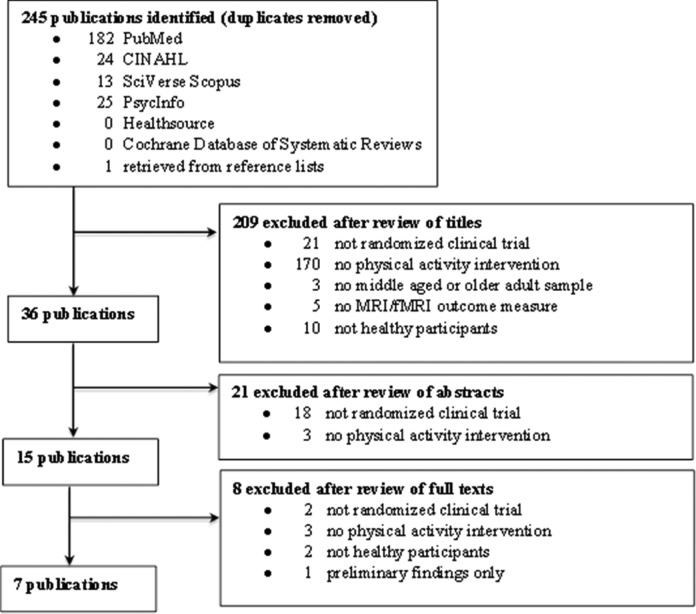
The initial search resulted in 245 unique titles (Figure 1). To evaluate titles for inclusion in the review, two of the authors (J.W. and S.H.) independently reviewed the retrieved titles followed by the abstracts and then the full-text articles. Based on title review, they excluded 209 papers, the majority of which (n = 170) because there was no physical activity intervention. Next, they evaluated the abstracts of the 36 remaining papers; of these, they excluded 21 papers, with most of these (n = 18) not having an RCT design. They then completed full-text review for the remaining 15 papers. The sample resulting from this search strategy was eight papers representing six independent studies (three papers for one study). Since there were three papers representing the same study, we excluded two of the three papers, both of which presented preliminary data or a subsample, from this review. J.W. and S.H. agreed on 95% of all decisions and reached a mutual consensus for decisions that were incongruent. The final sample included six papers representing six studies.
Figure 1.
Flowchart of search and retrieval process and results.
Measures and Analytic Strategy
Two of the authors (S.H. and J.W.) reviewed the six papers and abstracted them into narrative tables. They coded the results, checked them for inconsistencies, and discussed them for final consensus when necessary. They also assessed the six studies for potential risk of bias across studies as well as within the individual studies.
We first evaluated the six studies in regard to the country where the study took place, design and assessment points, setting, sample, physical activity intervention and control conditions, intervention duration, and intervention adherence (session attendance; Table 1). Next, we looked at outcome measures (physical activity, MRI, and neurocognitive) and outcome results (group effects and effect sizes; Table 2). We determined outcome results for MRI and neurocognitive measures by examining effect sizes (standardized mean differences from baseline to postintervention) based on available published results. We used calculated effect sizes according to standardized formulas with Cohen’s d, depending on the design of the study, and we categorized effect sizes by assigning them, respectively, Cohen’s d of .2, .5, and .8 for small, medium, and large effect, respectively (Cohen, 1988). We also examined the significant MRI and neurocognitive findings by comparing the MRI brain regions to the neurocognitive tests the authors assessed and reported in each paper (Table 3).
Table 1.
Sample and Intervention Characteristics of Studies Examining the Effects of Physical Activity Interventions on Brain Health With Middle-Aged and Older Adult Samples.
| Author (Year) Country | Sample |
Intervention |
|||||
|---|---|---|---|---|---|---|---|
| Design | Inclusion Criteria | Sample Characteristics | Setting | Intervention and Control | Intervention Duration | Attendance, Adherence | |
| Chapman et al. (2013) USA | RCT T1: baseline T2: 6 weeks T3: 12 weeks |
|
N = 37 (18 intervention, 19 control), age 57–75 years (M = 64.0 ± 3.9), 73% female | Lab |
Intervention
Control
|
12 weeks | Participants attended over 90% of sessions |
| Colcombe et al. (2004) USA | RCT T1: baseline T2: 25 weeks |
|
N = 29 (intervention and control ns not reported), age 58–77 years (M = 65.6 ± 5.7), 62.1% female, education M = 15.1 years | Not reported |
Intervention
Control
|
24 weeks | Not reported |
| Erickson et al. (2011) USA | RCT T1: within 4 weeks of intervention T2: 24 weeks T3: 1 year |
|
N = 120 (60 intervention, 60 control), age 60–79 years (M = 66.6 ± 5.6), 67% female | Not reported |
Intervention
Control
|
1 year | Participants attended 85% of sessions |
| Holzschneider et al. (2012) Germany | RCT T1: baseline T2: 24 weeks |
|
N = 33 (16 intervention [8 spatial, 8 perceptual], 17 control [9 spatial, 8 perceptual]), Age 40–55 years (M = 48.9 ± 3.8), 52% female | Not reported |
Intervention
Control
|
24 weeks | Not reported |
| Mortimer et al. (2012) China | RCT T1: baseline T2: 20 weeks T3: 40 weeks |
|
N = 120 (90 intervention [30 Tai Chi, 30 walking, 30 social interaction], 30 control), age 60–79 years (M = 67.9 ± 5.8), 67% female, education M = 11.7 years | Local park, gym, community center |
Intervention (3 groups):
Control
|
40 weeks | Not reported |
| Voelcker-Rehage et al. (2011) Germany | RCT T1: baseline T2: 24 weeks T3: 1 year |
|
N = 44 (17 cardiovascular, 16 coordination, 11 control), age 63–79 years (M = 69.6 ± 3.8), 64% female, education M = 12.4 years | Not reported |
Intervention (2 groups):
Control
|
1 year | Not reported |
Note. BMI = body mass index; IQ = intelligence quotient; MRI = magnetic resonance imaging; RCT = randomized controlled trial.
Table 2.
Description of Outcome Measures and Results of Studies Examining the Effects of Physical Activity Interventions on Brain Health With Middle-Aged and Older Adult Samples.
| Author (Year) | Measures | Outcome Results | |||
|---|---|---|---|---|---|
| PA | MRI | NC | Group Effects | Outcomes (Effect Sizes) | |
| Chapman et al. (2013) |
|
fMRI: cerebral blood flow |
|
|
Unable to calculate |
| Colcombe et al. (2004) |
|
fMRI: modified Eriksen flanker paradigm to assess cortical plasticity | Behavioral conflict: reaction time |
|
Unable to calculate |
| Erickson et al. (2011) | Maximal oxygen uptake (VO2max): treadmill protocol | MRI: brain volume | Spatial memory task: computer assessment |
|
|
| Holzschneider et al. (2012) | Maximal oxygen uptake (VO2peak): 3-min treadmill or cycle ergometer protocol | fMRI: virtual maze task with retrieval assessment to assess spatial learning capacity |
|
|
Unable to calculate |
| Mortimer et al. (2012) | Number of steps per week as measured by pedometer | MRI: brain volume | Neuropsychological battery: Digit Span, Bell Cancellation Test, Rey–Osterrieth Complex Figure (copying and recall), Stroop Test, Chinese Auditory Verbal Learning Test, Category Verbal Fluency Test, WAIS-R Similarities Test, Trail Making Test, Clock-Drawing Test, Boston Naming Test, and Mattis Dementia Rating Scale |
|
|
| Voelcker-Rehage et al. (2011) |
|
fMRI: Flanker task to assess brain activation patterns |
|
|
|
Note. fMRI = functional magnetic resonance imaging; L = left; MRI = magnetic resonance imaging; NC = neurocognitive; PA = physical activity; R = right.
aScales are reverse coded where a negative effect size indicates an improved score.
Table 3.
MRI and Neurocognitive Findings and Significance in Studies Examining the Effects of Physical Activity Interventions on Brain Health With Middle-Aged and Older Adult Samples.
| Study | MRI Brain Region | Significance | Neurocognitive Measures | Significance |
|---|---|---|---|---|
| Chapman et al. (2013) | Anterior cingulate region | + | Executive function: Trails A and B | NS |
| Memory: California Verbal Learning Test II, Wechsler Memory Scale IV | NS | |||
| Colcombe et al. (2004) | Anterior cingulate region | − | Behavioral conflict: reaction time | − |
| Middle, superior frontal gyrus | + | |||
| Superior parietal lobule | + | |||
| Erickson et al. (2011) | L/R anterior/posterior hippocampus | + | Spatial memory task: computer test | NS |
| L/R caudate nucleus | NS | |||
| Holzschneider et al. (2012) | Medial frontal gyrus | + | Spatial cognition: viewpoint, path integration task | NS |
| Cuneus | + | Spatial cognition: viewpoint, path integration task | NS | |
| Hippocampus, retrosplenial cortex, parahippocampal gyrus, frontal region, temporal region, cingulate region | NS | Spatial cognition: viewpoint, path integration task | NS | |
| Occipital region | NS | Perceptual cognition: visual discrimination task | NS | |
| Mortimer et al. (2012) | Whole brain volume | NS | ||
| Rey Figure (copying) | − | |||
| WAIS digit span, Bell cancellation test, Stroop Test, Auditory Verbal Learning Test, Category Verbal Fluency, WAIS Similarities, Trails A and B Time, Clock drawing test, Boston Naming Test, Mattis Dementia Rating Scale | NS | |||
| Voelcker-Rehage et al. (2011) | R medial frontal gyrus | NS | Executive control: modified Flanker task | + |
| Perceptual speed: Visual Search task | NS | |||
| L anterior cingulate | NS | Executive control: modified Flanker task | NS | |
| R posterior cingulate | NS | Executive control: modified Flanker task | NS | |
| R parahippocampal gyrus | NS | Executive control: modified Flanker task | NS | |
| Perceptual speed: Visual Search task | NS | |||
| R superior temporal gyrus | NS | |||
| R lentiform nucleus | NS | |||
| L parahippocampal gyrus | − | |||
| R superior, middle temporal gyrus | − | |||
| L anterior cingulate | − | Executive control: modified Flanker Task | NS | |
| IQ: neuropsychological battery (Digit-Symbol Substitution and Identical picture task, Figural Analogies and Letter Series, Paired Associate Test, Verbal Fluency, Vocabulary Test) | NS |
Note. Blank cells indicate that there was no corresponding MRI brain region or neurocognitive test. + = significant positive change in response to intervention; − = significant negative change in response to intervention; L = left; MRI = magnetic resonance imaging; NS = not significant; R = right.
Results
The six papers were published between 2004 and 2013 (see Table 1). Of these studies, three were conducted in the United States (Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011), two in Germany (Holzschneider, Wolbers, Röder, & Hötting, 2012; Voelcker-Rehage, Godde, & Staudinger, 2011), and one in China (Mortimer et al., 2012). There did not appear to be a risk of bias within the individual studies or across studies. We excluded two of the three papers from the same study prior to data extraction and analysis, preventing that particular risk of bias.
Sample Characteristics and Setting
Inclusion and exclusion criteria
All six studies included sedentary community-dwelling middle-aged (n = 1; Holzschneider et al., 2012) and/or older adults (n = 5; Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2012; Voelcker-Rehage et al., 2011). However, only three provided specific age parameters. Most studies included criteria to exclude those with neurological conditions, specifically those with cognitive impairment or dementia (n = 5; Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2012; Voelcker-Rehage et al., 2011) or stroke (n = 2; Mortimer et al., 2012; Voelcker-Rehage et al., 2011). Most (n = 5) also specified criteria regarding psychiatric status, including no history of psychiatric conditions (n = 1; Colcombe et al., 2004), no evidence of depressive symptoms (n = 2; Erickson et al., 2011; Mortimer et al., 2012), and no history of psychiatric conditions or depressive symptoms (n = 2; Chapman et al., 2013; Holzschneider et al., 2012). Mortimer et al. (2012) had the additional health-related exclusion criteria of no vascular disease related to cardiovascular disease or diabetes. As expected, all of the studies had standard exclusion criteria related to MRI procedures. Additional inclusion criteria were adequate visual acuity and right-handedness (Erickson et al., 2011).
Sample characteristics
The sample sizes reported in the six studies ranged from 37 to 120. The mean age for participants was 65.5 ± 5.1 years and ranged from 48.9 to 69.6 years. All six studies reported findings on men and women, and they all had more women than men (range = 55–72% women). Interestingly, none of the studies reported racial or ethnic characteristics of the participants. In the four studies that reported years of education, participants completed an average of 13.8 years. No study reported income level.
Setting
Of the two studies that reported the setting, interventions took place in a research laboratory (Chapman et al., 2013) and a variety of community sites, including a community center, park, and gym (Mortimer et al., 2012). All of the physical activity interventions across all of the studies appear to be have been supervised or monitored.
Intervention Characteristics
The interventions for all six studies involved physical activity training during which participants engaged in aerobic activity for 40- to 60-min sessions 2 or 3 times per week (Table 1). The aerobic activity included walking (n = 4; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2012; Voelcker-Rehage et al., 2011), cycling (Holzschneider et al., 2012), or a choice between walking and cycling (Chapman et al., 2013). One study had a second intervention condition involving coordination training designed to improve fine- and gross-motor coordination such as balance (Voelcker-Rehage et al., 2011). Another study provided two additional intervention conditions including tai chi and a social interaction group that self-selected discussion topics (Mortimer et al., 2012).
The attention control group most commonly received nonendurance stretching, flexibility, or toning training (n = 4; Colcombe et al., 2004; Erickson et al., 2011; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011). A fifth study provided an attention control group with periodic phone calls to keep them in the study (Mortimer et al., 2012) and another employed a wait-list control (Chapman et al., 2013). One study randomly assigned participants from both study conditions (aerobic endurance group and nonendurance control) to one of the two different cognitive training sessions (spatial or perceptual) during the last month of the intervention (Holzschneider et al., 2012).
Intervention duration ranged from 12 weeks to 1 year. Attendance was defined as the percentage of intervention sessions attended by participants. Only two of the six studies reported attendance: Chapman et al. (2013) reported that participants attended 90% of the sessions for their 12-week program, while Erickson et al. (2011) reported that participants had attended 85% of their sessions at 1 year.
Physical Activity, MRI, and Neurocognitive Measures
We examined study measures related to physical activity, MRI, and neurocognition (Table 2). Attendance was the only reported measure of dose of the physical activity intervention. None of the studies assessed the quantity or duration of the physical activity intervention through use of self-report questionnaires or accelerometers. Mortimer and colleagues (2012), however, used a pedometer to determine number of steps participants took weekly. The majority of studies included a measure of cardiorespiratory fitness, which is related to physical activity: four assessed VO2max or VO2peak with a graded treadmill test (Erickson et al., 2011; Voelcker-Rehage et al., 2011), Rockport 1-mile walk test (Colcombe et al., 2004), or choice of treadmill or cycle ergometer (Holzschneider et al., 2012), and one did not report the method for obtaining VO2max (Chapman et al., 2013). Researchers most commonly used cardiorespiratory fitness to determine intensity range for each participant during the physical activity intervention. Chapman and colleagues (2013) also assessed perceived exertion and Colcombe and colleagues (2004) assessed resting heart rate, both of which are related to cardiorespiratory fitness. One study also assessed motor fitness (i.e., feet tapping, one-leg stand; Voelcker-Rehage et al., 2011).
For MRI measures, two studies reported use of MRI to measure brain volume (Erickson et al., 2011; Mortimer et al., 2012). Of the others, one each measured cerebral blood flow (Chapman et al., 2013), cortical plasticity (Colcombe et al., 2004), spatial learning capacity (Holzschneider et al., 2012), and brain activation patterns (Voelcker-Rehage et al., 2011).
Neurocognitive tests were reported in all studies. Executive function (n = 3; Chapman et al., 2013; Mortimer et al., 2012; Voelcker-Rehage et al., 2011) was most frequently assessed, followed by spatial cognition or spatial memory (n = 3; Erickson et al., 2011; Holzschneider et al., 2012; Mortimer et al., 2012), verbal memory (n = 3; Chapman et al., 2013; Mortimer et al., 2012), and perceptual speed (n = 2; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011).
Outcome Results
All but one study reported significant improvements in MRI measures for the endurance-focused physical activity intervention groups. For the two studies that reported brain volume, one reported relative increases in brain volume for the endurance-focused physical activity intervention group compared to the control (Erickson et al., 2011). The other study did not find significant effects for the walking intervention (Mortimer et al., 2012). The other studies found significant differences between the endurance-focused physical activity intervention group and either the wait-list control group (Chapman et al., 2013) or the nonendurance toning/stretching group (Colcombe et al., 2004; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011). The significant changes associated with the endurance-focused physical activity interventions were (a) higher resting cerebral blood flow in the anterior cingulate region (Chapman et al., 2013), (b) greater level of task-related activities in attentional control areas of brain (middle frontal gyrus, superior frontal gyrus, superior parietal lobes) and reduced level of activity in the anterior cingulate cortex (Colcombe et al., 2004), (c) change in brain activation in the medial frontal gyrus and cuneus positively related to change in VO2peak (Holzschneider et al., 2012), and (d) reduced activity in the anterior cingulate cortex but lower task-related activity in the attentional control areas (Voelcker-Rehage et al., 2011).
We were able to calculate effect sizes of the physical activity intervention on MRI outcomes for only one study (Erickson et al., 2011). This study had positive but small effect sizes for four regions: left hippocampus (d = .21), right hippocampus (d = .20), left anterior hippocampus (d = .29), and right anterior hippocampus (d = .30). We were able to calculate the effect sizes to measure the impact of endurance-focused physical activity interventions on neurocognitive measures for two studies (Mortimer et al., 2012; Voelcker-Rehage et al., 2011). Both had small positive effects on neurocognitive measures, specifically in executive function (d = .32; Mortimer et al., 2012), perceptual speed (d = .20–24; Voelcker-Rehage et al., 2011), and delayed verbal memory (d = .23; Mortimer et al., 2012).
Agreement Between MRI and Neurocognitive Outcome Measures
We compared the significant findings of the MRI brain regions examined in each study with the corresponding neurocognitive tests assessed (Table 3). There was one concordant finding for significant effects of physical activity on the neurocognitive test of behavioral conflict (executive function) and on the anterior cingulate region (Colcombe et al., 2004). Most inconsistent findings were in the direction of physical activity having significant effects on MRI findings and nonsignificant effects on the neurocognitive tests. Most findings on the effects of physical activity were nonsignificant for both MRI and neurocognitive tests.
Discussion
In this review of six published RCTs, we examined the effect of endurance-focused physical activity interventions on the brain, as measured by MRI and neurocognitive measures, in community-dwelling middle-aged or older adults without cognitive impairment or dementia. The studies demonstrated modest effects of the physical activity interventions on MRI and neurocognitive measures (i.e., for those that could be calculated).
Despite the potential sensitivity of MRI measures, the nonspecific nature of such measures is a limitation. More research is necessary to identify the effects of physical activity interventions on specific brain changes, such as plasticity or neurogenesis (Thomas, Dennis, Bandettini, & Johansen-Berg, 2012). When comparing results for neurocognitive and MRI measures, we found overlap for only one significant finding (significant decreases in reaction time were associated with decreased activity in the anterior cingulated cortex). The absence of overlap in other areas seems largely due to the general lack of significant findings for both ways of evaluating intervention impact. In addition, there were no corresponding neurocognitive measures for two of the significant MRI findings. These findings may also support suggestions that MRI measures may be more sensitive to the effects of physical activity than neurocognitive measures (Smith et al., 2013); however, further research is needed to explore this hypothesis.
Our review has at least two limitations to keep in mind. First, we limited our review to RCTs only, which is the strongest study design. This strategy meant that we excluded studies of physical activity interventions that used other designs. Also, as mentioned earlier, we were only able to calculate effect sizes for three studies. This limitation precludes our ability to make broad statements regarding the effects of these endurance-focused physical activity interventions on the brain.
In spite of these limitations, this review shows that physical activity interventions that target brain health continue to be a promising area of research. These interventions have the potential to impact brain structure, and possibly cognitive function, in the middle-aged and older adult population; however, additional investigation is needed. Future RCTs should include larger community-based samples that represent diverse populations, and we encourage authors to provide effects sizes or adequate data to calculate effect sizes. In addition to including indirect measures of physical activity, such as cardiorespiratory fitness, studies should include direct measures of lifestyle physical activity, such as accelerometer data. To our knowledge, no study that compares endurance-focused physical activity to cognitive training has used MRI measures to assess impact on brain structure and function. Future research should address this gap. Moreover, given the time needed for behavioral changes to impact specific areas of brain function, interventions of greater duration and with longer follow-up may permit identification of consistent effects for specific brain regions and the corresponding neurocognitive tests (Smith et al., 2013).
Footnotes
Author Contribution: S. Halloway contributed to conception, design, data acquisition, data analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work ensuring integrity and accuracy. J. Wilbur contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work ensuring integrity and accuracy. M. Schoeny contributed to data analysis and interpretation, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work ensuring integrity and accuracy. K. Arfanakis contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute of Nursing Research, National Institutes of Health F31 NR015372, Jonas Nurse Leader Scholar, Jonas Center for Nursing and Veterans Healthcare, Midwest Nursing Research Society dissertation grant.
References
- American College of Sports Medicine, Chodzko-Zajko W. J., Proctor D. N., Fiatarone Singh M. A., Minson C. T., Nigg C. R.…Skinner J. S. (2009). American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise, 41, 1510–1530. doi:10.1249/MSS.0b013e3181a0c95c [DOI] [PubMed] [Google Scholar]
- Behrman S., Ebmeier K. P. (2014). Can exercise prevent cognitive decline? Practitioner, 258, 17–21, 2–3. [PubMed] [Google Scholar]
- Burzynska A. Z., Chaddock-Heyman L., Voss M. W., Wong C. N., Gothe N. P., Olson E. A.…Kramer A. F. (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PloS One, 9, e107413 doi:10.1371/journal.pone.0107413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. B., Aslan S., Spence J. S., DeFina L. F., Keebler M. W., Didehbani N., Lu H. (2013). Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience, 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. New York, NY: Lawrence Erlbaum. [Google Scholar]
- Colcombe S. J., Kramer A. F., Erickson K. I., Scalf P., McAuley E., Cohen N. J.…Elavsky S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101, 3316–3321. doi:10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming T. B., Tyedin K., Churilov L., Morris M. E., Bernhardt J. (2012). The effect of physical activity on cognitive function after stroke: A systematic review. International Psychogeriatrics, 24, 557–567. doi:10.1017/S1041610211001980 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L.…Kramer A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108, 3017–3022. doi:10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina N., Rusted J., Tabet N. (2014). The effect of exercise interventions on cognitive outcome in Alzheimer’s disease: A systematic review. International Psychogeriatrics, 26, 9–18. doi:10.1017/S1041610213001385 [DOI] [PubMed] [Google Scholar]
- Gary R. A., Brunn K. (2014). Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiology Research and Practice, 2014, 157508 doi:10.1155/2014/157508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates N., Fiatarone Singh M. A., Sachdev P. S., Valenzuela M. (2013). The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. American Journal of Geriatric Psychiatry, 21, 1086–1097. doi:10.1016/j.jagp.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Green S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions. Retrieved from http://handbook.cochrane.org/
- Holtzer R., Goldin Y., Zimmerman M., Katz M., Buschke H., Lipton R. B. (2008). Robust norms for selected neuropsychological tests in older adults. Archives of Clinical Neuropsychology, 23, 531–541. doi:10.1016/j.acn.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K., Wolbers T., Röder B., Hötting K. (2012). Cardiovascular fitness modulates brain activation associated with spatial learning. NeuroImage, 59, 3003–3014. doi:10.1016/j.neuroimage.2011.10.021 [DOI] [PubMed] [Google Scholar]
- Kramer A. F., Erickson K. I., Colcombe S. J. (2006). Exercise, cognition, and the aging brain. Journal of Applied Physiology, 101, 1237–1242. doi:10.1152/japplphysiol.00500.2006 [DOI] [PubMed] [Google Scholar]
- Lautenschlager N. T., Cox K., Kurz A. F. (2010). Physical activity and mild cognitive impairment and Alzheimer’s disease. Current Neurology and Neuroscience Reports, 10, 352–358. doi:10.1007/s11910-010-0121-7 [DOI] [PubMed] [Google Scholar]
- Law L. L. F., Barnett F., Yau M. K., Gray M. A. (2014). Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Research Reviews, 15, 61–75. doi:10.1016/j.arr.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Littbrand H., Stenvall M., Rosendahl E. (2011). Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: A systematic review. American Journal of Physical Medicine and Rehabilitation, 90, 495–518. doi:10.1097/PHM.0b013e318214de26 [DOI] [PubMed] [Google Scholar]
- McCarten J. R. (2013). Clinical evaluation of early cognitive symptoms. Clinics in Geriatric Medicine, 29, 791–807. doi:10.1016/j.cger.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group T. P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine, 6, e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J. A., Ding D., Borenstein A. R., DeCarli C., Guo Q., Wu Y.…Chu S. (2012). Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. Journal of Alzheimer’s Disease, 30, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Roberts R. O., Knopman D. S., Geda Y. E., Cha R. H., Pankratz V. S.…Rocca W. A. (2010). Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology, 75, 889–897. doi:10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti F., Rozzini R., Trabucchi M. (2002). Physical activity and cognitive decline in elderly persons. Archives of Internal Medicine, 162, 361–362. [DOI] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B.…Wallace R. B. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29, 125–132. doi:10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B. L., Langa K. M., Fisher G. G., Heeringa S. G., Weir D. R., Ofstedal M. B.…Wallace R. B. (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R. S., Voss M. W., Erickson K. I., Lewis J. M., Chaddock L., Malkowski E.…Kramer A. F. (2011). Cardiorespiratory fitness and attentional control in the aging brain. Frontiers in Human Neuroscience, 4, 229 doi:10.3389/fnhum.2010.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., Hardy R., Wadsworth M. E. J. (2003). Does active leisure protect cognition? Evidence from a national birth cohort. Social Science and Medicine, 56, 785–792. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Potter G. G., McLaren M. E., Blumenthal J. A. (2013). Impact of aerobic exercise on neurobehavioral outcomes. Mental Health and Physical Activity, 6, 139–153. doi:10.1016/j.mhpa.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M., Steinman L., Mochan K., Grodstein F., Prohaska T. R., Thurman D. J.…Anderson L. A. (2011). Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. Journal of the American Geriatrics Society, 59, 704–716. doi:10.1111/j.1532-5415.2011.03323.x [DOI] [PubMed] [Google Scholar]
- Szabo A. N., McAuley E., Erickson K. I., Voss M., Prakash R. S., Mailey E. L.…Kramer A. F. (2011). Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology, 25, 545–553. doi:10.1037/a0022733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. G., Dennis A., Bandettini P. A., Johansen-Berg H. (2012). The effects of aerobic activity on brain structure. Frontiers in Psychology, 3 doi:10.3389/fpsyg.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelcker-Rehage C., Godde B., Staudinger U. M. (2011). Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Frontiers in Human Neuroscience, 5 doi:10.3389/fnhum.2011.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Kang J. H., Manson J. E., Breteler M. M. B., Ware J. H., Grodstein F. (2004). Physical activity, including walking, and cognitive function in older women. Journal of the American Medical Association, 292, 1454–1461. doi:10.1001/jama.292.12.1454 [DOI] [PubMed] [Google Scholar]
- Zygouris S., Tsolaki M. (2015). Computerized cognitive testing for older adults: A review. American Journal of Alzheimer’s Disease and Other Dementias, 30, 13–28. doi:10.1177/1533317514522852 [DOI] [PMC free article] [PubMed] [Google Scholar]