Abstract
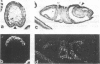
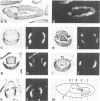
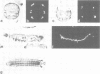
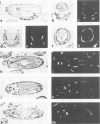
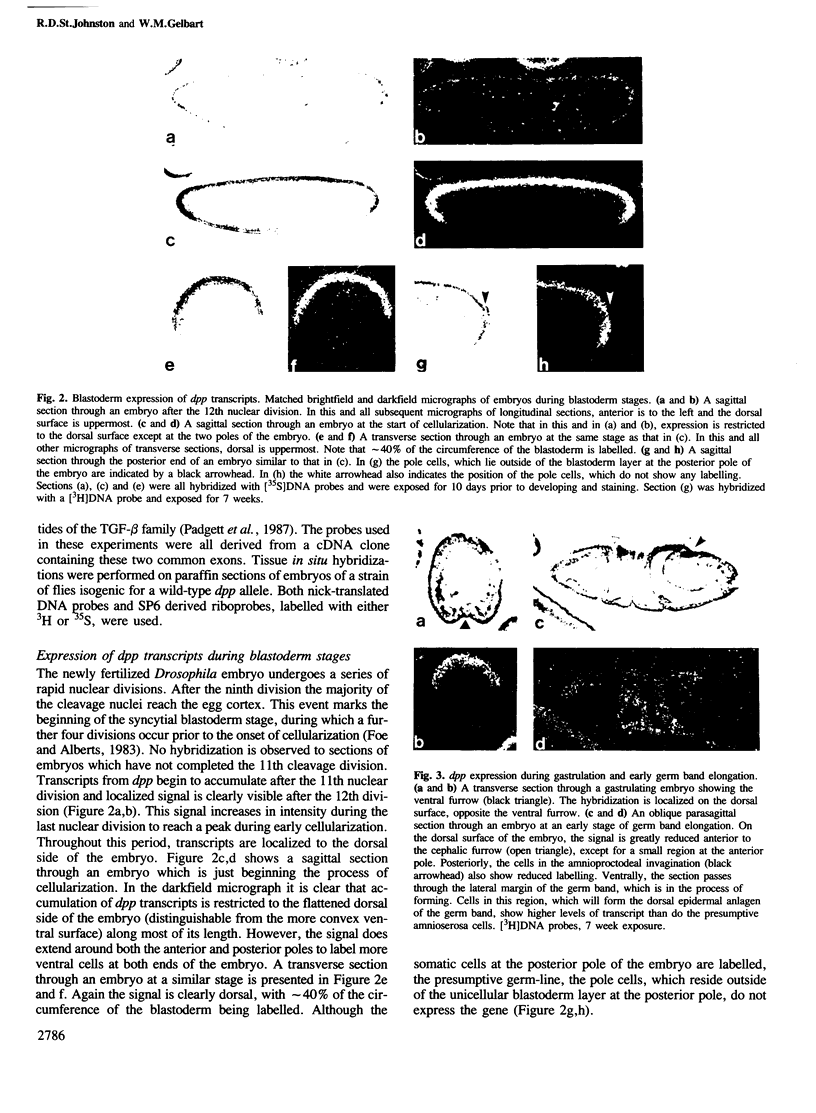
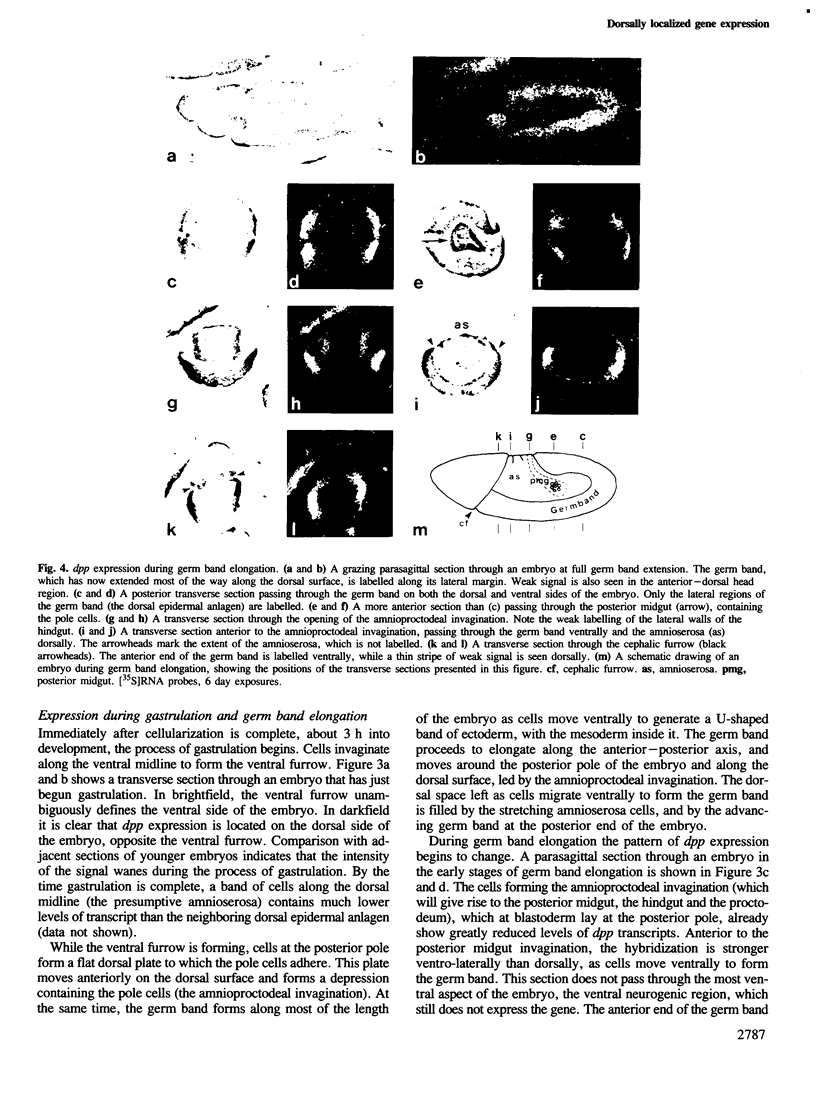
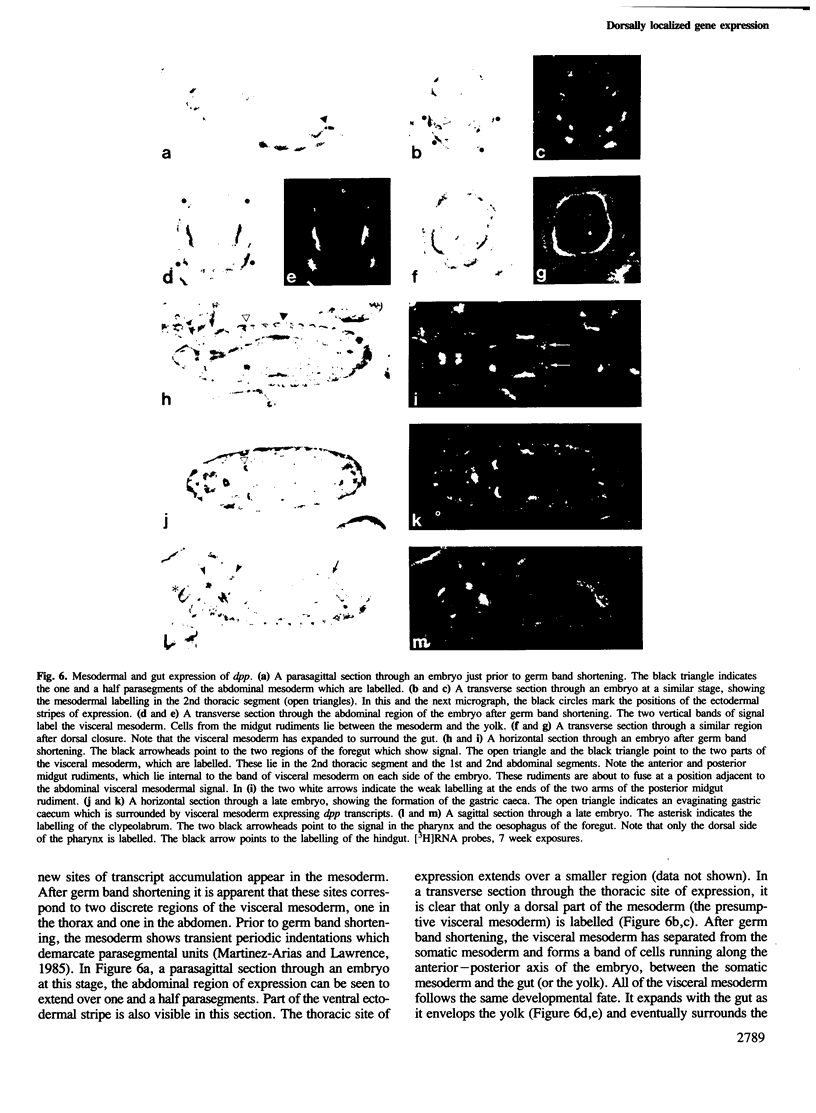
The decapentaplegic gene of Drosophila melanogaster (dpp) is related to the TGF-beta family of mammalian growth factors. In order to investigate the role of dpp during early development we examined the spatial pattern of expression of dpp transcripts in embryos. Transcription is first detected along the dorsal side of early syncytial blastoderm embryos. During germ band elongation, the gene is expressed in the region which will give rise to the dorsal epidermis. Since dppHin-embryos lack all derivatives of the dorsal epidermis, this suggests that dpp is required early in development for the determination of dorsal positional values. Later in embryogenesis, expression in the ectoderm changes to give two thin stripes running anterior-posteriorly along the length of the embryo. This pattern of expression may be involved in further subdivisions along the dorsal-ventral axis. At this stage, transcription can also be detected in discrete parts of the visceral mesoderm, foregut and hindgut.
Full text
PDF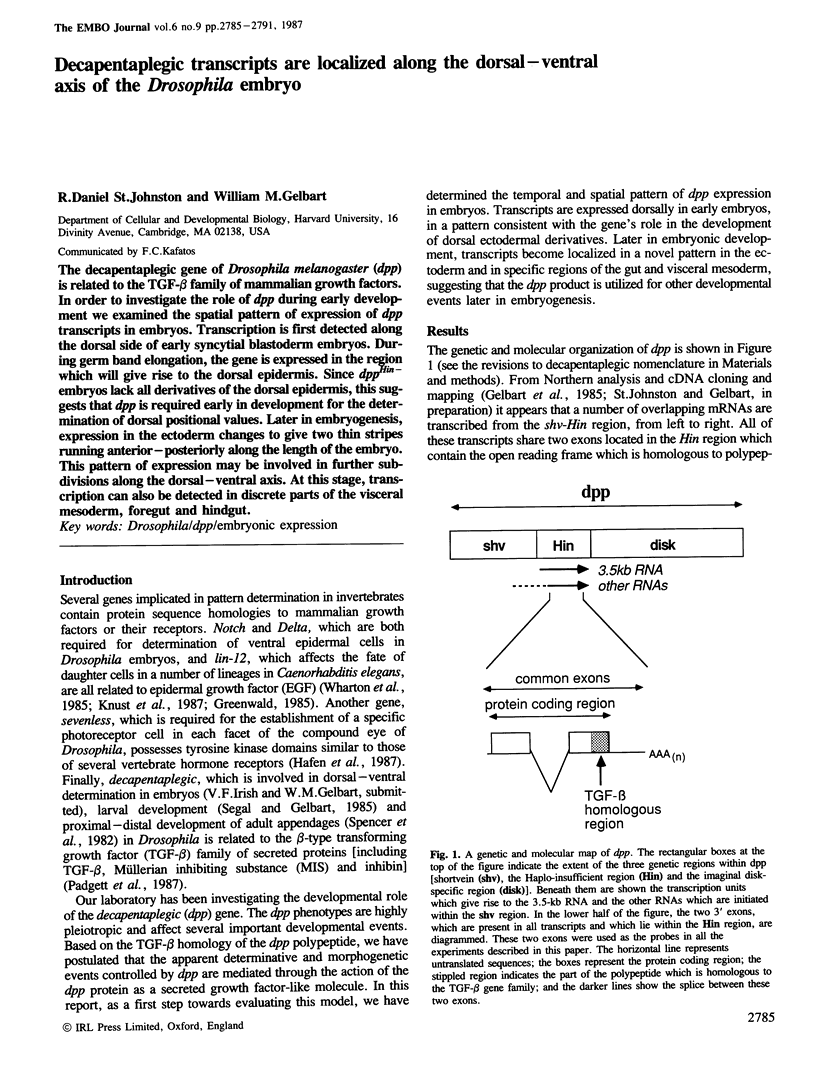



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll S. B., Scott M. P. Localization of the fushi tarazu protein during Drosophila embryogenesis. Cell. 1985 Nov;43(1):47–57. doi: 10.1016/0092-8674(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Chan L. N., Gehring W. Determination of blastoderm cells in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2217–2221. doi: 10.1073/pnas.68.9.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle H. J., Harding K., Hoey T., Levine M. Transcripts encoded by a homoeo box gene are restricted to dorsal tissues of Drosophila embryos. Nature. 1986 Sep 4;323(6083):76–79. doi: 10.1038/323076a0. [DOI] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983 May;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- Gelbart W. M., Irish V. F., St Johnston R. D., Hoffmann F. M., Blackman R. K., Segal D., Posakony L. M., Grimaila R. The decapentaplegic gene complex in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1985;50:119–125. [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Hafen E., Kuroiwa A., Gehring W. J. Spatial distribution of transcripts from the segmentation gene fushi tarazu during Drosophila embryonic development. Cell. 1984 Jul;37(3):833–841. doi: 10.1016/0092-8674(84)90418-5. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Knipple D. C., Seifert E., Rosenberg U. B., Preiss A., Jäckle H. Spatial and temporal patterns of Krüppel gene expression in early Drosophila embryos. Nature. 1985 Sep 5;317(6032):40–44. doi: 10.1038/317040a0. [DOI] [PubMed] [Google Scholar]
- Knust E., Dietrich U., Tepass U., Bremer K. A., Weigel D., Vässin H., Campos-Ortega J. A. EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J. 1987 Mar;6(3):761–766. doi: 10.1002/j.1460-2075.1987.tb04818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arias A., Lawrence P. A. Parasegments and compartments in the Drosophila embryo. Nature. 1985 Feb 21;313(6004):639–642. doi: 10.1038/313639a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Segal D., Gelbart W. M. Shortvein, a new component of the decapentaplegic gene complex in Drosophila melanogaster. Genetics. 1985 Jan;109(1):119–143. doi: 10.1093/genetics/109.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer F. A., Hoffmann F. M., Gelbart W. M. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell. 1982 Mar;28(3):451–461. doi: 10.1016/0092-8674(82)90199-4. [DOI] [PubMed] [Google Scholar]
- Wakimoto B. T., Turner F. R., Kaufman T. C. Defects in embryogenesis in mutants associated with the antennapedia gene complex of Drosophila melanogaster. Dev Biol. 1984 Mar;102(1):147–172. doi: 10.1016/0012-1606(84)90182-9. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]