Abstract
The primate visual system contains two major cortical pathways: a ventral-temporal pathway that has been associated with object processing and recognition, and a dorsal-parietal pathway that has been associated with spatial processing and action guidance. Our understanding of the role of the dorsal pathway, in particular, has greatly evolved within the framework of the two-pathway hypothesis since its original conception. Here, we present a comparative review of the primate dorsal pathway in humans and monkeys based on electrophysiological, neuroimaging, neuropsychological, and neuroanatomical studies. We consider similarities and differences across species in terms of the topographic representation of visual space; specificity for eye, reaching, or grasping movements; multi-modal response properties; and the representation of objects and tools. We also review the relative anatomical location of functionally- and topographically-defined regions of the posterior parietal cortex. An emerging theme from this comparative analysis is that non-spatial information is represented to a greater degree, and with increased complexity, in the human dorsal visual system. We propose that non-spatial information in the primate parietal cortex contributes to the perception-to-action system aimed at manipulating objects in peripersonal space. In humans, this network has expanded in multiple ways, including the development of a dorsal object vision system mirroring the complexity of the ventral stream, the integration of object information with parietal working memory systems, and the emergence of tool-specific object representations in the anterior intraparietal sulcus and regions of the inferior parietal lobe. We propose that these evolutionary changes have enabled the emergence of human-specific behaviors, such as the sophisticated use of tools.
Keywords: object vision, tools, dorsal pathway, neuroimaging, electrophysiology, visual-motor transformation
Introduction
The notion of two functionally segregated visual pathways has significantly shaped our conception of primate vision over the last few decades. The initial conception of the two pathway hypothesis, based largely on empirical studies of the macaque monkey, stated that the ventral-temporal pathway serves object vision, whereas the dorsal-parietal pathway serves spatial vision (Mishkin & Ungerleider, 1982). In accordance with this hypothesis, early monkey physiology studies showed shape- and object-selective responses in neurons of inferior temporal (IT) cortex (Desimone, Albright, Gross, & Bruce, 1984; Gross, Rocha-Miranda, & Bender, 1972; Logothetis & Sheinberg, 1996). In contrast, neurons in the posterior parietal cortex (PPC) transform visual information into different spatial reference frames with respect to the eyes, body, or world (Andersen, Snyder, Bradley, & Xing, 1997), and this information is further integrated with spatial information from the auditory and somatosensory systems.
Observations of the human visual system are also consistent with the two-pathway hypothesis. For example, a large swath of ventral temporal and lateral occipital cortex, referred to as the lateral occipital complex (LOC), responds more strongly to pictures of objects than to their scrambled counterparts (Grill-Spector, et al., 1999; Kourtzi & Kanwisher, 2001; Malach, et al., 1995). Both IT and LOC represent objects independent of the precise physical cues that define them (Grill-Spector, et al., 1998) and regardless of changes in external viewing conditions that affect an object’s appearance, but not its identity (Grill-Spector, et al., 1999; James, et al., 2002; Sawamura, et al., 2005; Vuilleumier, et al., 2002). Hence, IT and LOC display response properties that characterize an effective object recognition system subserving perceptual object constancy. With respect to spatial cognition, lesions of human PPC can lead to the neglect of contralateral space with respect to different spatial reference frames (e.g., spatial neglect, Corbetta & Shulman, 2011) or visuomotor deficits (e.g., optic ataxia, Perenin & Vighetto, 1988). PPC is also an important node in the spatial attention network, which underlines its more general role in spatial cognition (Szczepanski, Konen, & Kastner, 2010).
However, other observations from human subjects suggested that this original framework was incomplete. For example, some patients (most notably patient D.F.) with ventral stream lesions show severe forms of visual agnosia, yet can perform visually-guided reaching and grasping movements towards those same objects (Goodale, Milner, Jakobson, & Carey, 1991; James, et al., 2002). Such observations led to a reformulation of the two-pathway hypothesis, with an emphasis on vision for perception for the ventral pathway versus vision for action for the dorsal pathway (Goodale & Milner, 1992). In this influential framework, spatial cognition is one among other aspects of the dorsal visual representation. Notably, there is an assumption that there is also a representation of object information in the dorsal stream, which is specifically utilized for visually-guided movement.
In the past few decades, continued study of the human and macaque visual systems (reviewed below) has provided support for the broad framework of the action-perception two-pathway hypothesis. However, recent studies have revealed important differences across the two species, in particular with respect to dorsal object representations. As we review in detail below, the object vision system of the human dorsal pathway is much more developed and refined that that in the macaque. Indeed, the complexity of the human dorsal object representations appears to mirror the complexity of those found in ventral representations (Konen & Kastner, 2008a).
Here, we review and compare some of the literature on the dorsal pathway in humans and monkeys with particular emphasis on PPC. We show that many of the disparate findings are due to species differences in the functional organization of the dorsal pathway. Finally, we propose a novel hypothesis as to why the human dorsal pathway may have undergone significant functional transformations relative to other species in the primate lineage.
Topographic organization
Phase-encoded retinotopic mapping along the polar angle and eccentricity dimensions using functional magnetic resonance imaging (fMRI) has been widely used to reveal topographic organization within the human (Arcaro, et al., 2009; Brewer, Liu, Wade, & Wandell, 2005; Engel, Glover, & Wandell, 1997; Hagler & Sereno, 2006; Kastner, et al., 2007; Konen & Kastner, 2008a; Larsson & Heeger, 2006; Schneider, Richter, & Kastner, 2004; Sereno, Dale, Reppas, & Kwong, 1995; Swisher, Halko, Merabet, McMains, & Somers, 2007) and also the macaque monkey visual system (Brewer, Press, Logothetis, & Wandell, 2002; Kolster, et al., 2009). Specifically, such topographic mapping enables the simultaneous investigation of visuotopic organization across a large region of cortex, thereby allowing the visuospatial map of an individual area to be anchored in the framework of the topographic organization across multiple surrounding areas. Thus, comparing the topographic large-scale organization of primate PPC at the level of maps serves as a first useful approach in our comparative review.
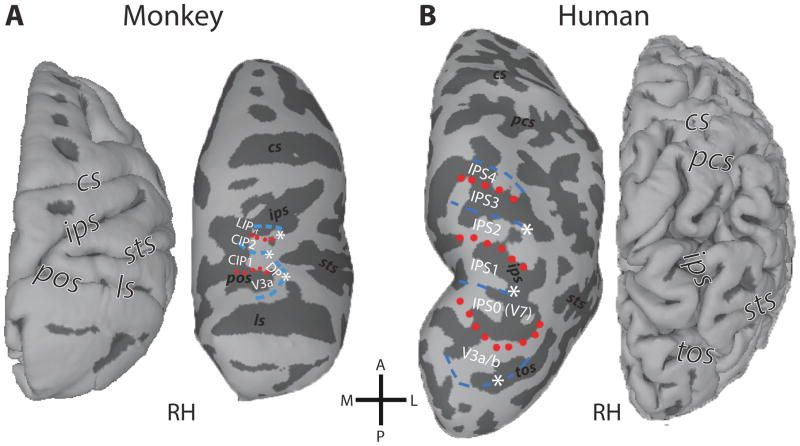
In macaque monkeys, multiple visual areas within and adjacent to the intraparietal sulcus (IPS) have been identified in PPC (Andersen, Bracewell, Barash, Gnadt, & Fogassi, 1990; Pandya & Seltzer, 1982; Van Essen, 2004). Visual areas within PPC have been distinguished based on their cyto- and myeloarchitecture, as well as their connectivity patterns, including the ventral and dorsal lateral intraparietal areas (LIPv/d), and two cortical zones, dorsal prelunate (DP) and lateral occipital parietal (LOP), also referred to as caudal intraparietal (CIP). Arcaro et al. (2011) studied the visuotopic organization of these regions. They identified two visual field maps anterior to area V3A within caudal PPC (named CIP-1 and CIP-2). In the polar angle dimension, the representation in CIP-1 extended from regions near the upper vertical meridian (that is the shared border with V3A) to those within the lower visual field (that is the shared border with CIP-2), thus spanning a contralateral hemifield. The polar angle representation in CIP-2 was found to be a mirror reversal of the CIP-1 representation. Anterior to CIP-2, a third polar angle representation was found within LIP (visuotopic LIP, LIPvt), thereby corroborating previous findings (Ben Hamed, Duhamel, Bremmer, & Graf, 2001; Blatt, Andersen, & Stoner, 1990; Patel, et al., 2010). The polar angle representation in LIP extended from regions near the upper vertical meridian (that is the shared border with CIP-2) to those near the lower vertical meridian (Fig. 1A).
Figure 1. Topographic organization of primate PPC.
Comparison of the fMRI-defined retinotopic organization of dorsal occipital and parietal cortex in both monkeys (A) and humans (B). Lines denote areal boundaries formed by phase angles at or close to the upper (red, dotted) or lower (blue, dashed) vertical meridian. Surfaces are rendered with anterior (A) up, posterior (P) down, medial (M) left, and lateral (L) right. Major sulci are labeled in each view: cs, central sulcus; pcs, postcentral sulcus; ips, intraparietal sulcus; ls, lunate sulcus; pos, parieto-occipital sulcus; tos, transverse occipital sulcus. (After: Arcaro, et al., 2011)
In humans, seven topographically organized parietal areas have been described: six of these areas form a contiguous band along the intraparietal sulcus, and one area branches off into the superior parietal lobule (SPL) (Konen & Kastner, 2008b; Schluppeck, Glimcher, & Heeger, 2005; Sereno, Pitzalis, & Martinez, 2001; Silver, Ress, & Heeger, 2005; Swisher, et al., 2007). Each of these topographic areas contains a continuous representation of the contralateral visual field, and boundaries between these areas correspond to alternating representations of the upper and lower vertical meridian. The most posterior area, referred to as IPS0 (sometimes also as V7), is located anterior to area V3A at the intersection of the transverse occipital sulcus and the IPS. IPS1 and IPS2 are in the posterior part of the IPS. More anterior, IPS3 and IPS4 are located in the anterior/lateral branch of the IPS (Fig. 1B), whereas the most anterior area, IPS5, typically extends into the intersection between the IPS and the postcentral sulcus (not shown in Fig. 1B, see Fig. 2B). An additional representation of the contralateral visual field, referred to as SPL1, typically branches off the most superior IPS areas and extends medially into the SPL (not shown in Fig. 1B, see Fig. 2B).
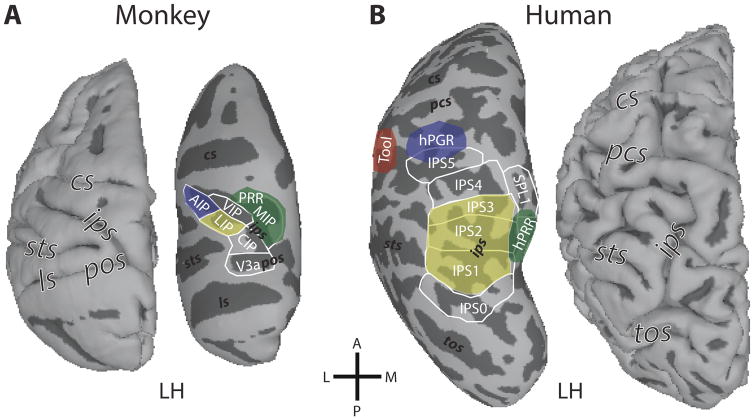
Figure 2. Functional organization of primate PPC.
Schematic outline of functional regions with respect to effector responses within monkey (A) and human (B) parietal cortex. In monkeys, anatomically-defined areas CIP, LIP, AIP, VIP, and MIP are located along the IPS. The functionally-defined PRR (parietal reach region) overlaps with and extends beyond MIP. In humans, topographically-defined areas IPS0–5 are located along the IPS, with SPL1 extending into the superior parietal lobe. In both species, preferences for different effectors are indicated by the yellow (saccade-related responses), green (reaching-related responses), and blue (grasping-related responses) color patches. In monkeys, these regions are largely confined within the IPS. In humans, reaching-related responses (hPRR, human parietal reach region) are typically found outside the IPS, medially in SPL, and grasping-related responses (hPGR, human parietal grasp region) are located laterally at the intersection of the IPS and the postcentral sulcus. A human-specific region in the left hemisphere in the inferior parietal lobule shows tool-related responses (red). Labels for surface orientation and major sulci are as in Fig. 1.
The topographic organization of PPC in the macaque and human can be compared based on polar angle (Konen & Kastner, 2008b) and representations of central space (Konen & Kastner 2008, unpublished observations; also see Swisher, et al., 2007). The topographic organization of V3A in the macaque is similar to area V3A in the human (Brewer, et al., 2002; Fize, et al., 2003; Tsao, et al., 2003; Van Essen, et al., 2001). The topographic organization and location in relation to surrounding topographic regions of CIP-1 and CIP-2 in the macaque is similar to areas IPS0 and IPS1 in the human. For both monkey and human topographies, the representations of central space are located on the lateral border (denoted by asterisks in Fig. 1), with more peripheral representations located medially. From a topographic perspective, LIPvt in the macaque appears similar to IPS2 in the human. In both species, there is a posterior-to-anterior progression from the upper to the lower vertical meridian representations with a representation of central space on the lateral border (Fig. 1).
Topographic organization within the IPS in humans consists of additional areas, IPS3/4/5, extending further anterior. The IPS also extends anterior from LIPvt in the macaque. However, consistent topographic organization has not been identified beyond LIPvt. It is important to note that our comparison of the topographic organization of the dorsal pathway in humans and monkeys is not aimed at establishing homology between areas, but instead, it is aimed at revealing similarities and differences in large-scale brain topography as a first starting point to ultimately identify regions that show similar functional response profiles across primate species. We review functional response properties of IPS areas in the following sections.
Functional response properties: Effector specificity and multi-modal responses
A different way to look at the functional parcellation of primate PPC is based on the specificity of responses related to motor effectors for eye, grasping, or reaching movements. Macaque area LIP, which is located in the lateral bank of macaque IPS, has been shown to be involved in the encoding of saccadic eye movements (Andersen, et al., 1990). About half of LIP neurons exhibit pre- and peri-saccadic responses (Barash, Bracewell, Fogassi, Gnadt, & Andersen, 1991). In contrast, little evidence for saccade-related activity has been found in the ventral intraparietal area (VIP), which is located anterior to LIP in the fundus of the IPS (Schaafsma & Duysens, 1996); but see (Thier & Andersen, 1998). The majority of VIP neurons respond during smooth pursuit eye movements (53%) (Schlack, Hoffmann, & Bremmer, 2003), which is a higher proportion than has been reported in LIP (39%) (Bremmer, Distler, & Hoffmann, 1997; Colby, Duhamel, & Goldberg, 1993b). In humans, preferential responses for saccadic or smooth pursuit eye movements change gradually across areas of the IPS with IPS1/2 and the medial SPL1 preferring saccadic eye movements and IPS3/4/5 preferring smooth pursuit eye movements (Konen & Kastner, 2008b, schematically depicted in Fig. 2B). Interestingly, areas in close anatomical proximity such as IPS1/2 and SPL1 in the posterior/medial PPC and IPS3/4/5 in the anterior/lateral PPC show similar response characteristics with respect to effector specificity.
In macaque monkeys (Fig. 2A), neurons in the anterior intraparietal area (AIP), which is located in the anterior-lateral part of the IPS, play a key role in preshaping of the hand for grasping (Murata, Gallese, Luppino, Kaseda, & Sakata, 2000; Sakata, Taira, Kusunoki, Murata, & Tanaka, 1997; Sakata, Taira, Mine, & Murata, 1992; Taira, Mine, Georgopoulos, Murata, & Sakata, 1990). These neurons exhibit selectivity for specific grasping movements, as well as for the shape, size and orientation of three-dimensional visual objects, regardless of the object’s position in visual space (Murata, et al., 2000). Moreover, inactivation of AIP disrupts a monkey’s ability to utilize visual information for preshaping the contralateral hand (Gallese, Murata, Kaseda, Niki, & Sakata, 1994). In humans, grasping-specific activation is typically found in the left anterior IPS, partially overlapping with IPS5, and extending into the postcentral sulcus (Fig. 2B, schematically outlined in blue; Konen, Mruczek, Montoya, & Kastner, 2013). We refer to this area as the human parietal grasp region (hPGR) to emphasize the functional definition of these areas, while implying that there may be functional homology, or at least strong functional similarity between hPGR and macaque AIP. This area appears to be critical for grasping movements, since patients with lesions in the anterior-lateral IPS show deficits in finger coordination (Binkofski, et al., 1998; Jeannerod, Decety, & Michel, 1994), and transcranial magnetic stimulation of the anterior IPS, but not more posterior regions, disrupts grasping movements (Rice, Tunik, & Grafton, 2006; Tunik, Frey, & Grafton, 2005).
In macaques, neurons in the parietal reach region (PRR) have been associated with the planning and execution of reaching movements. PRR is located on the posterior-medial bank of the IPS (Fig. 2A). It is a functionally defined area (and thus may extend medially beyond the borders of the IPS) and likely overlaps with the medial intraparietal area (MIP) and area V6A (Andersen & Buneo, 2002; Snyder, Batista, & Andersen, 1997). PRR appears to code the targets for a reach in visual coordinates relative to the direction of gaze (Buneo & Andersen, 2006; Buneo, Jarvis, Batista, & Andersen, 2002; Pesaran, Nelson, & Andersen, 2006). Moreover, PRR exhibits intention-related activation reflecting the plan for a reaching movement (for review see Andersen & Buneo, 2002). In humans, reaching-specific activation is typically found medially in the left precuneus and SPL, partially overlapping with IPS1/2 and SPL1, in an area that we refer to as the human parietal reach region (hPRR) (Fig. 2B, schematically outlined in green).
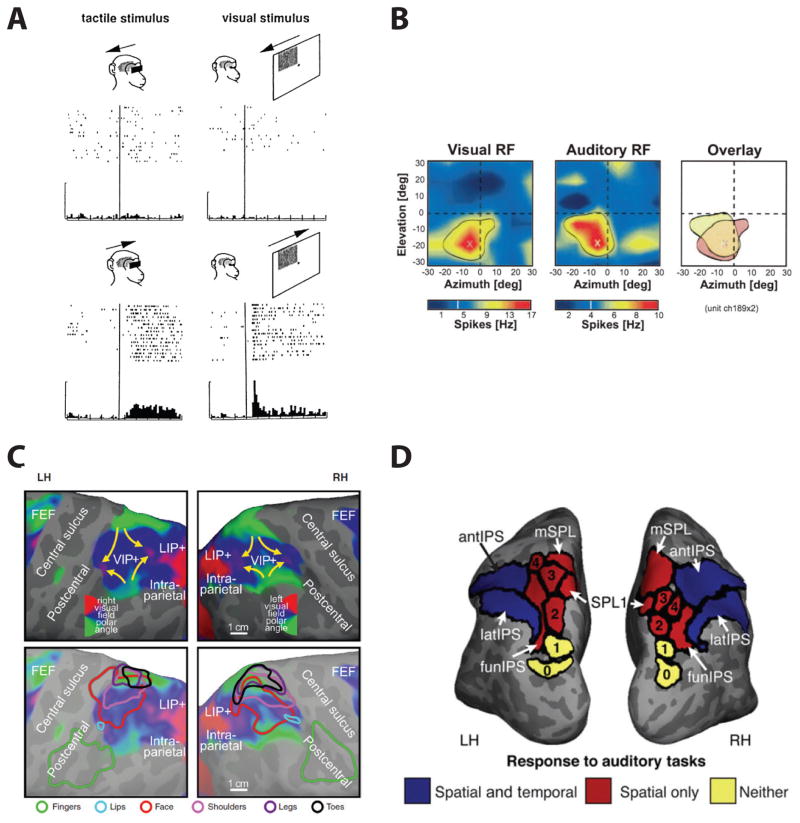
In monkeys, neurons in VIP, LIP and PRR have also been shown to have bi- or even tri-modal sensory responses, to visual, auditory, and/or somatosensory stimuli (Fig. 3) (Mullette-Gillman, Cohen, & Groh, 2005, 2009; Schlack, Sterbing-D’Angelo, Hartung, Hoffmann, & Bremmer, 2005) suggesting an important function in multisensory integration. In that respect, a particularly critical area appears to be VIP. Colby and colleagues were the first to show tactile responses in VIP: multisensory VIP neurons showed large visual receptive fields with spatially restricted, aligned somatosensory receptive fields on the face and shoulders (Colby, Duhamel, & Goldberg, 1993a; Duhamel, Colby, & Goldberg, 1998). For example, a VIP neuron with a visual receptive field in the upper right quadrant of the visual field also typically has a somatosensory receptive field located on the upper right part of the forehead (Fig. 3A). In addition to the tactile responses, bimodal auditory and visual neurons were found to code comparable regions of space (Mullette-Gillman, et al., 2005, 2009; Schlack, et al., 2005). In most cases, the auditory responses were spatially tuned, and the auditory and visual receptive field locations were typically found to be spatially congruent (Fig. 3B; Schlack, et al., 2005). Similar results were obtained in LIP and PRR. Given their multi-modal response properties, regions in the macaque PPC likely integrate sensory information from multiple modalities. The finding that different regions of primate PPC are also closely linked to specific motor effectors indicates their functional significance in integrating sensory information with motor plans.
Figure 3. Multimodal responses in primate PPC.
(A) Tactile (left) and visual (right) stimuli converge onto similar directional selectivity and receptive field location in a multisensory neuron of area VIP. Upper row: non-preferred direction. Lower row: preferred direction. Reproduced with permission from Duhamel, et al. (1998). (B) Spatially congruent visual (left) and auditory (middle) receptive fields (RFs) in a VIP neuron. The RFs are superimposed in the right panel. Color bars indicate spike rates relative to spontaneous activity. Reproduced with permission from Schlack, et al. (2005). (C) Overlapping retinotopic (upper panels) and somatotopic (lower panels) maps in human anterior parietal cortex. The color contours in the lower panels show spherically aligned somatotopic whole body mapping data superimposed on the visual maps. Reproduced with permission from Sereno and Huang (2014). (D) Activation in the human parietal lobule during spatial and temporal auditory processing. Reproduced with permission from Michalka, et al. (2016).
In humans, a multisensory area that has a representation of retinotopic visual space as well as a somatotopic map has been revealed by somatosensory stimulation of the face in the form of air puffs (Sereno & Huang, 2006, 2014). This area is located in the anterior IPS and overlaps or is identical with IPS5. Furthermore, by combining full body air-puff mapping with wide field visual stimulation, bimodal (somatosensory and visual) fMRI mapping experiments revealed that visual and somatosensory responses overlapped in the parietal cortex extending beyond IPS5 to include a medial region concentrating on the lower body and peripheral lower visual field (Fig. 3C; Huang, Chen, Tran, Holstein, & Sereno, 2012). In contrast to visual and somatosensory responses, the evidence for auditory responses in human PPC is sparse. A recent fMRI study suggests that, depending on auditory task demands (spatial vs. temporal processing), auditory stimulation may recruit visuotopic regions within the PPC (Fig. 3D; Michalka, Rosen, Kong, Shinn-Cunningham, & Somers, 2016). Furthermore, transcranial magnetic stimulation (TMS) of PPC near the junction of the anterior intraparietal sulcus and the postcentral sulcus eliminates the facilitatory influence of vision (Pasalar, Ro, & Beauchamp, 2010) or audition (Serino, Canzoneri, & Avenanti, 2011) on tactile perception near the hand, suggesting a causal role of these parts of human PPC in multisensory integration.
The location of hPGR and hPRR relative to topographically organized cortex indicates that over the course of evolution, functionally similar areas that are now located within the IPS in non-human primates were partially relocated outside of the IPS in humans (Fig. 2), possibly in order to accommodate human-specific areas in PPC. The macaque IPS contains areas LIP, VIP, AIP, and portions of PRR. A number of studies have indicated functional similarities between human IPS5 and area VIP in monkeys. Both contain a co-registered, multimodal representation of tactile and visual space, especially near the face (Duhamel, et al., 1998; Sereno & Huang, 2006). Additionally, both show a preference for smooth pursuit eye movements compared to saccades (Konen & Kastner, 2008b; Schlack, et al., 2003), as well as selectivity for motion including optic flow patterns (Konen & Kastner, 2008b; Schaafsma, Duysens, & Gielen, 1997). In monkeys, area AIP is located anterior to VIP (Colby, et al., 1993b; Taira, et al., 1990). Similarly, in humans, hPGR is located anterior to IPS5 (Konen, et al., 2013; Mruczek, von Loga, & Kastner, 2013), suggesting that the spatial relationship known in monkeys between AIP and VIP is preserved. However, hPGR is located outside of the IPS, at the junction of the IPS and the post-central sulcus.
Previous studies have also suggested a functional correspondence between macaque LIP and human IPS1/2. For example, both in humans and monkeys, these IPS regions show activity related to spatial attention (Colby & Goldberg, 1999; Lauritzen, D’Esposito, Heeger, & Silver, 2009; Silver, et al., 2005; Szczepanski, et al., 2010), saccadic eye movements (Konen & Kastner, 2008b; Snyder, Batista, & Andersen, 2000), working memory (Gnadt & Andersen, 1988; Killebrew, Mruczek, & Berryhill, 2015; Schluppeck, Curtis, Glimcher, & Heeger, 2006; Sheremata, Bettencourt, & Somers, 2010), and object stimuli (Janssen, Srivastava, Ombelet, & Orban, 2008; Konen & Kastner, 2008a; Mruczek, et al., 2013; Sereno & Maunsell, 1998), as will be elaborated in the next section. Additionally, human IPS2 and the ventral portion of macaque LIP show similar topographic representations of visual space relative to more posterior regions (Arcaro, et al., 2011; Ben Hamed, et al., 2001). In humans, IPS1/2 span across both banks of the IPS, whereas monkey LIP is confined to the lateral bank of the IPS (see also, Koyama, et al., 2004). In monkeys, area MIP and V6A form the functionally defined PRR and are located posterior and medial to LIP (Andersen & Buneo, 2002). In humans, the relative position of PRR to LIP is preserved, but hPRR has been relocated outside of the IPS and into the SPL. Consequently, hPRR, similar to hPGR, appears to be relatively distinct from the topographic regions in the IPS (Konen, et al., 2013).
The comparison of macaque and human IPS suggests that IPS3/4 may be candidate areas that contribute to the large increase in human parietal cortex relative to the macaque (Brodmann, 1909; Van Essen, et al., 2001). These areas may be human-specific; that is, there may be no functional equivalent of these areas in the macaque monkey. Little is known about their functional response properties. Simon et al. (2002) found that calculation and language-related tasks activated the lateral bank of the IPS and that this activation was independent from that observed for grasping, pointing, saccades or attentional shifts. Thus, these IPS areas may support advanced visuo-spatial skills that are associated with human-specific abilities such as abstract number representation and calculation (Dastjerdi, Ozker, Foster, Rangarajan, & Parvizi, 2013; Dehaene, Dehaene-Lambertz, & Cohen, 1998; Nieder & Dehaene, 2009; Piazza, Pinel, Le Bihan, & Dehaene, 2007). However, it is not yet clear to what extent regions for number representation and calculation overlap with the topographic maps, leaving open the possibility that IPS3/4 are not newly evolved areas, but instead that during human evolution other regions of the IPS split into multiple subregions with different functions. These alternative possibilities need to be tested in future studies.
In summary, it appears that, over the course of evolution, the organization and functional contributions of the IPS has diverged in macaques and humans since their last common ancestor. Specifically, putatively homologous regions in macaque posterior-medial (LIP, PRR/MIP/V6A) and anterior-lateral (AIP, VIP) IPS are spatially separated in the human PPC, presumably to accommodate expanses in parietal cortex that support human-specific functions. Notably, in the human, unique networks have emerged that are associated with human-specific abilities, such as the tool network (Mruczek, et al., 2013), that do not have counterparts in macaques. However, before discussing the tool network, we first review the representation of object information in primate PPC more generally.
Representation of object information
In monkeys, object-related activity has been typically found in the context of visually-guided actions such as grasping, specifically in AIP (Murata, et al., 2000; Tsutsui, Jiang, Sakata, & Taira, 2003). However, object-related activity has also been reported under passive viewing conditions, thus independent of action planning. For example, Sereno and Maunsell (1998) were the first to report 2D shape selectivity in area LIP (Sereno & Maunsell, 1998). However, shape-selective neurons in LIP rarely exhibited size- and position-invariance (Janssen, et al., 2008), suggesting that the shape representation in the dorsal stream is fundamentally distinct from that in the ventral visual stream. In addition to LIP, neurons in AIP also show significant selectivity for the contours of 2D objects (Romero, Van Dromme, & Janssen, 2012) and to simple shape features or fragments of objects (Romero, Pani, & Janssen, 2014; Romero, Van Dromme, & Janssen, 2013). Similar to LIP, the fragment selectivity in AIP does not show tolerance to stimulus position (Romero, et al., 2014).
In addition to 2D shape selectivity in LIP and AIP, a series of single-cell and fMRI studies in monkeys (Durand, et al., 2007; Joly, Vanduffel, & Orban, 2009) as well as imaging experiments in humans (Georgieva, Peeters, Kolster, Todd, & Orban, 2009) have suggested that CIP (see Fig. 1A) is sensitive to the 3D depth structure of objects. In monkeys, CIP neurons can signal the 3D orientation of large planar surfaces when either disparity or texture gradients are used as depth cues (Tsutsui, Sakata, Naganuma, & Taira, 2002). CIP neurons can also be selective for concave and convex surfaces defined by disparity (Katsuyama, et al., 2010), and jointly encode the tilt and slant of large planar surfaces (Rosenberg, Cowan, & Angelaki, 2013). In addition to CIP, AIP neurons in monkeys also respond selectively to concave and convex surfaces that have identical contours (Srivastava, Orban, De Maziere, & Janssen, 2009). It has been proposed that depth structure information of both objects and the environment is first extracted from disparity and texture in CIP, and is then transmitted to AIP (Nakamura, et al., 2001; Orban, 2011; Sakata, Tsutsui, & Taira, 2005; Tsutsui, Taira, & Sakata, 2005). In humans, fMRI experiments using identical stimuli and paradigms as those used in the monkey studies revealed that the 3D shape network is very similar to that in monkeys (Georgieva, et al., 2009). Depth perception in humans consistently evokes strong BOLD responses in dorsal visual areas, particularly V3A, IPS0/V7, and PPC.
Taken together, the functions of 2D and 3D object information in the monkey dorsal pathway are still poorly understood. One might argue that LIP serves as a relay conveying mainly 2D object information to AIP, whereas CIP serves as a source relaying 3D object information to AIP. This object information then is utilized to mediate visuo-motor transformations of AIP neurons involved with grasping movements.
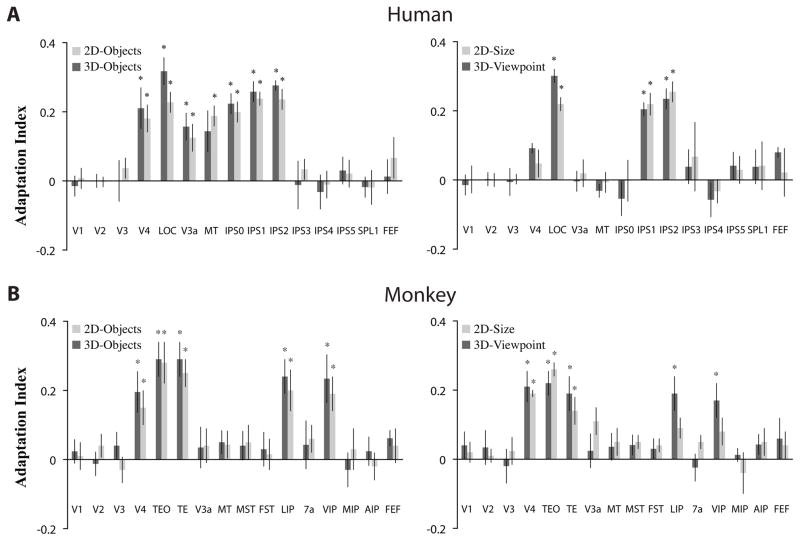
In humans, there is mounting evidence for the representation of considerably more complex object information in dorsal extrastriate cortex and PPC (Jeong & Xu, 2016; Konen & Kastner, 2008a; Xu & Jeong, 2015). Konen et al. (2008a) used a parametric fMRI adaptation paradigm to investigate neural representations of different types of object stimuli such as 2D- and 3D-objects, line drawings of objects and tools, as well as size- and viewpoint-invariance of these object categories in areas of the dorsal pathway. Furthermore, they compared responses directly across both the ventral and dorsal pathways in the same study participants. Adaptation is a robust phenomenon in which repeated presentations of the same visual stimulus leads to gradual response reductions as a function of repetition frequency, thereby providing an indirect measure of an area’s selectivity for certain visual stimuli. This measure can then be quantified by an adaptation index, with positive values indicating selectivity for a given stimulus (see Fig. 4 for a replication of these results from a follow-up study, which is described in detail below).
Figure 4. Object selectivity in the human and monkey visual systems.
In a cross-species comparison study (Kastner, et al., 2009), an adaptation index was used to quantify the degree of object selectivity in each area. Positive index values indicate stronger adaptation and thus greater object selectivity; values around zero indicate the absence of adaptation effects/object selectivity. Error bars denote SEM. Asterisks (*) indicates that the adaptation index value significantly different from zero. For both humans (A), replicating the results of Konen and Kastner (2008a) and monkeys (B), left panels show results from experiments probing object selectivity and right panels show results from experiments probing size- and viewpoint-invariance. Human ROIs were topographically and/or functionally defined using standard criteria. Monkey ROIs were anatomically defined using the Saleem and Logothetis (2007) atlas. Both species showed significant object-selective and viewpoint-invariant responses in ventral stream regions (e.g., LOC in humans; TEO and TE in monkeys). Both species also showed significant object-selective responses in dorsal stream regions (e.g., IPS0–2 in humans; LIP and VIP in monkeys). However, high-level, viewpoint-invariance responses in the dorsal stream were only observed in humans (e.g., IPS1–2 in humans).
In the human ventral pathway, Konen et al. (2008a) found evidence corroborating earlier reports showing object-selectivity in LOC for stimulus types such as photographs, line drawings, and 3D-objects (see also Fig. 4A, left; Grill-Spector, et al., 1999; Kourtzi, Erb, Grodd, & Bulthoff, 2003; Kourtzi & Kanwisher, 2001; Malach, et al., 1995). They were also able to replicate the finding that the LOC response is invariant to image transformations such as size, position, and viewpoint (see also Fig. 4A, right; Grill-Spector, et al., 1999; Kourtzi, et al., 2003; Sawamura, et al., 2005; Vuilleumier, et al., 2002) suggesting that this area is part of a recognition system that subserves perceptual object constancy (Riesenhuber & Poggio, 2002). In contrast, at intermediate stages of processing within the ventral pathway, in area V4, a different response pattern was observed. V4 responded selectively to a variety of object stimuli, but these responses depended specifically on object size and viewpoint (see also Fig. 4A). The dissociation of neural responses with respect to viewpoint and size between V4 and LOC is in agreement with studies in monkeys showing that neurons at successive stages of the ventral pathway encode progressively more complex attributes of an object, and exhibit response properties invariant to image transformations in IT (Booth & Rolls, 1998; Desimone, et al., 1984; Gross, et al., 1972; Sawamura, et al., 2005). These findings therefore provided further evidence to support the notion of a hierarchical organization of neural representations related to object information in the ventral pathway of primates (Riesenhuber & Poggio, 2002).
Surprisingly, Konen et al. (2008a) found a similar hierarchically organized system of object representations in the human dorsal pathway. As in the ventral pathway, areas at intermediate stages of cortical processing such as V3A, MT, and IPS0/V7 showed size- and viewpoint-specific responses, whereas high-level areas in PPC such as IPS1/2 showed size- and viewpoint-invariant responses (see also Fig. 4A). These object-related neural representations appeared to generalize across multiple types of stimuli including 2D- and 3D-objects, and line drawings of objects and tools. Additionally, these areas did not distinguish general objects from tools, but responded similarly to them (see also Mruczek, et al., 2013). No object-selective responses were found in the anterior/lateral IPS (IPS3/4) implicating functional differences across topographically organized areas of PPC. Overall, object representations in the dorsal system appeared to mirror those of the well-known ventral system in terms of basic response properties such as category selectivity and invariance to image transformations.
A number of characteristics of object representations in the dorsal pathway are worth noting. First, the object responses were found for abstract 2D images, under passive viewing conditions, and even when attention was drawn away from the stimuli. Thus, these object representations are active even when the behavioral context does not require concurrent action planning. Rather, these data provide strong evidence that object information is represented in the human dorsal pathway independent of action planning, although this information would likely be utilized for such purposes, when required. Second, the dorsal object-related activity appeared indistinguishable from that in the ventral pathway and was not specific to changes of viewpoint and size but rather generalized across changing viewing conditions. While it is widely agreed upon that a successful object recognition system requires generalization across changing viewing conditions (Riesenhuber & Poggio, 2002), one would predict that a system that uses object information for the control of skilled actions would preserve the specificity of object appearance with regard to shape, size and orientation (Craighero, Fadiga, Umilta, & Rizzolatti, 1996). For example, the same object when presented from different viewpoints will require different hand postures during grasping (James, et al., 2002). The finding that object-related activity generalizes across image transformations, therefore, does not support this view. Alternatively, view-independent object information in the dorsal pathway may serve more sophisticated forms of object manipulation than simple sensory-motor transformations in visually-guided behavior. For example, such representations may be critical in guiding the placement of the hand and fingers on occluded regions of an object or predicting how an object could be reoriented during manipulation.
The finding of an elaborate second dorsal object vision system in humans did not seem compatible with existing monkey physiology literature, as reviewed above. These findings were therefore met with considerable skepticism. One possibility is that the results were not compatible due to differences in approach. For example, studies in humans and monkeys typically use different methods – mainly intracranial electrophysiology in monkeys and functional brain imaging in humans – and experimental designs that vary considerably. Furthermore, monkey neurophysiology often focuses on differences within a small number of subjects, whereas human neuroimaging often focuses on group-averaged data. Finally, studies of object vision often utilize stimuli drawn from object categories that carry considerable meaning to humans (e.g., tools, clothing, vehicles), even if the specific exemplars and images used are novel.
To address these concerns, Kastner, Konen and colleagues performed a follow-up study in which an identical experimental approach and design was used in the two primate species (Kastner, Pinsk, Arcaro, Li, & Konen, 2009). Specifically, they used the same fMRI adaptation paradigm as in Konen et al. (2008a) while acquiring fMRI data in a second group of human participants and in two monkeys trained to maintain fixation over extended periods of times (i.e., for several minutes) in the scanner environment. In addition to using a similar data collection technique for both species, the study used a region-of-interest analysis that focuses on effects in single-subjects. Finally, they used a subset of the stimulus types previously used in Konen et al. (2008a) that had no semantic or categorical attributes, specifically the abstract 2D and 3D objects..
In humans, Kastner, Konen and colleagues replicated the pattern of results regarding the neural representation of object information in the ventral and dorsal pathway described above (Fig. 4A; Konen & Kastner, 2008a). In contrast, in monkeys (Fig. 4B), they found that while the ventral pathway was similarly organized in representing 2D- and 3D-objects independent of viewing conditions, the organization of the dorsal pathway was species-specific: areas at intermediate levels of visual processing (e.g., MT, V3A) did not carry object information, whereas areas at advanced levels of processing such as LIP and VIP showed shape selectivity that reflected viewpoint-specific, low-level features of objects. These studies are among the very few that demonstrate a difference in the functional organization of a particular response property in the primate brain. Furthermore, these results not only reconcile a wealth of knowledge from monkey electrophysiology, but also indicate that from an evolutionary perspective, the human dorsal object vision system appears to be a recent development and may have evolved to enable human-specific behaviors related to PPC such as sophisticated tool use. We elaborate on this hypothesis in the final section of this review. But first, we take a closer look at the human tool network.
Uniquely human functions: The tool network
Thus far, we have reviewed the neural representation of object information in the dorsal visual pathway and PPC in general terms. However, with respect to human parietal cortex function, there is one object category that deserves special consideration: tools. While tool use and even tool making are not unique to humans – apes and crows use sticks to forage for insects, to give just two examples (see Shumaker, Walkup, & Beck, 2011 for many more) – humans appear to be the only sophisticated tool users and makers in the animal kingdom. One important distinction is that humans thoroughly understand the causal impact that an object used as a tool has on a different object or on the environment. Remarkably, humans also seamlessly transform ordinary objects into tools. For example, when a ball rolls under a cabinet, thereby getting out of reach for a two year old child, he or she will use a stick or any other elongated object to extend the radius of the arm to reach the ball. This is a remarkable ability that illustrates the potential and behavioral flexibility of the ‘human toolmaker’ from early development onwards.
Human tool use ability is reflected in the evolution of a dedicated network that represents information about tools. In parietal cortex, neuroimaging studies have shown that viewing pictures of tools, which have strong and specific action associations, activates a left-lateralized network of brain regions including the anterior IPS and regions in the inferior parietal lobe, along with regions in frontal and temporal cortex (Chao & Martin, 2000; Chouinard & Goodale, 2012; Lewis, 2006; Valyear, Cavina-Pratesi, Stiglick, & Culham, 2007). The same left-lateralized network has been associated with viewing, hearing, executing, planning and pantomiming tool use (Lewis, 2006). Importantly, a functionally equivalent tool region is not found in macaques, even after extensive training with tool usage (Peeters, et al., 2009).
Tool-related activity has been consistently found lateral, anterior, and inferior to IPS5 and lateral and inferior to hPGR, with some partial overlap of all three regions (Mruczek, et al., 2013; Valyear, et al., 2007). Importantly, this activity appears to be specific to tools; graspable objects other than tools do not activate these regions as strongly. Tool-evoked activity is found throughout most regions of the IPS, especially in the left hemisphere. However, the response evoked by the presentation of graspable objects and tools is similar in posterior regions (Konen & Kastner, 2008a; Mruczek, et al., 2013), thus not discriminating between these two categories of objects. Posterior IPS regions also do not discriminate between action-related or non-action related objects. In contrast, responses to non-action related objects are absent in anterior IPS (Fig. 4A), and graspable objects evoke a weaker response than tools in anterior regions (Mruczek, et al., 2013). One interpretation of this pattern of results is that the posterior IPS encodes basic visual features common to any graspable object (of which tools form a subset), whereas the anterior IPS integrates this information with stored knowledge of action associations, affordances, and goals based on one’s personal and evolutionary experience with tools.
Such an account is also corroborated by studies in patients with lesions in regions of the parietal tool network. Lesions of the left posterior parietal cortex can lead to ideomotor apraxia (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005; Buxbaum, Kyle, & Menon, 2005; Johnson-Frey, 2004), in which patients maintain the ability to grasp and manipulate objects, but fail to grasp tools in a manner that is consistent with their function (Buxbaum, Sirigu, Schwartz, & Klatzky, 2003; Goldenberg & Hagmann, 1998; Sirigu, et al., 1995). Consistent with the presence of the tool-specific region identified by neuroimaging studies, this indicates a dissociation between the neural mechanisms that support grasping an object (i.e., sensorimotor transformations) and those that support using an object (Binkofski & Buxbaum, 2013; Johnson-Frey, 2004). In some cases, patients fail to demonstrate (i.e., pantomime) appropriate tool usage, even though they maintain the ability to recognize tools and describe their function (Daprati & Sirigu, 2006; Johnson-Frey, 2004). Based on a quantitative analysis of lesion sites and behavioral deficits, Goldenberg and Spatt (2009) concluded that left posterior parietal cortex is necessary for understanding general principles of tool usage and mechanical interactions between objects, rather than prototypical uses or specific grips or movements associated with tools.
A hypothesis on brain adaptations for the ‘human toolmaker’
Based on a comparative review of the response properties of monkey and human PPC, as detailed above, in this final section we propose a hypothesis regarding the evolution of the human-specific tool network. The most extensively studied primate brain model that we used as a basis for discussion in this review, the macaque monkey, is not a tool user in natural environments. Although monkeys can be trained on tool use (Ishibashi, Hihara, & Iriki, 2000; Ishibashi, et al., 2002), they will not transfer or generalize the learned use to similar object manipulations. However, it is conceivable to reason that the non-spatial and viewpoint-specific object information in monkey PPC is utilized for informing visually-guided object manipulations. The circuit that subserves such function in monkey PPC can be outlined as follows. Both area LIP, which carries information for 2D aspects of objects, and area CIP, which carries information about 3D aspects of objects, project to area AIP. The object representations in these areas may provide necessary information for the manipulation and grasping of objects executed by a parieto-frontal motor network that includes AIP. Importantly, area LIP has also been implicated in a wide variety of cognitive functions including spatial attention, decision making, categorization, and working memory (e.g., Colby & Goldberg, 1999; Freedman & Assad, 2006; Kusunoki, Gottlieb, & Goldberg, 2000; Shadlen & Newsome, 1996, 2001). In this respect, LIP can be considered a cognitive hub that may integrate object information with other cognitive processes relevant to ongoing behavior and provide such integrated object information to area AIP in order to be implemented with motor plans and ultimately to mediate visually-guided behavior. Importantly, LIP also projects directly to VIP, where sensory responses have a bias for objects that are near or approaching the body and stimulation of VIP results in defensive-like movements (Cooke, Taylor, Moore, & Graziano, 2003; Graziano & Cooke, 2006). A major function of area VIP is to maintain a margin of safety around the body and to coordinate action that defend the body surface (Graziano & Cooke, 2006). In this respect, object information in VIP may be useful to represent objects in peripersonal space and to integrate them into the body schema (Ishibashi, et al., 2002; Obayashi, et al., 2001). For example, physiology studies have shown that VIP neurons may expand their receptive fields to include manipulable objects extending from the hand (Obayashi, et al., 2001). Taken together, we propose that non-spatial object information in areas LIP, CIP and VIP of the macaque constitutes an integral part of a fronto-parietal perception-to-action system aimed at manipulating objects in peripersonal space.
This account is consistent with the role of the dorsal pathway in the framework of the perception-action hypothesis of primate vision. How did this network for perception-to-action become transformed in humans in order to support the human toolmaker? We propose four adaptations, as detailed below.
1. Formation of a second dorsal object vision system
While viewpoint-specific representations of 2D- and 3D-aspects of objects may be sufficient for object manipulations involving the fronto-parietal motor system such as grasping a cup or a piece of food, they may not be sufficient to support the ability to manipulate tools flexibly and, importantly, to build them. The sophistication that is observed in human tool use may require highly flexible object representations that can get transformed in varying behavioral context. While the ventral object vision pathway is directed at integrating such object information with memory systems for recognition, the human dorsal object vision pathway may have emerged to integrate such information for flexible tool use. Importantly, these object representations emerge in similar regions as in the macaque suggesting that an expansion of functional properties in human IPS1/2, an area similar to monkey LIP, may be one of the prerequisites for developing a specific tool network. In our account, these areas provide direct input to IPS5 and the tool area (see Fig. 2). Importantly, this input system would need to represent high-level object information in flexible ways. However, it should not be specialized for representing tool information, so that other object- and cognitive-based information can be integrated. As reviewed above, the response properties of human posterior PPC indeed reflect these predictions.
2. Integration of object information with cognitive information, particularly related to working memory
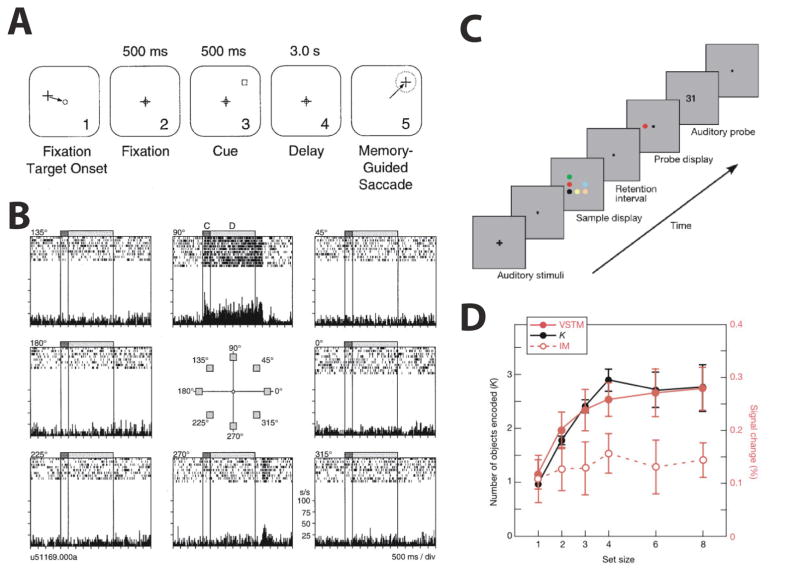
The ability to interact with tools and particularly to build them requires that information about action sequences is not only integrated with information about object properties, but also that it can be maintained over some period of time. Accordingly, one would predict that the same areas that advance functionality to represent object information would also advance cognitive function, particularly with respect to working memory. Indeed, much evidence has demonstrated parietal mechanisms important for visual working memory 1. In monkeys, spatial working memory, in particular, has been readily demonstrated in LIP neurons. For example, spatially-specific delay activity has been found in an oculomotor delayed response task in which monkeys were trained to make a saccade to a remembered location after a delay (Fig. 5A–B; Chafee & Goldman-Rakic, 1998; Gnadt & Andersen, 1988). While spatial working memory function has also been demonstrated in human IPS (Courtney, Ungerleider, Keil, & Haxby, 1996; Jonides, et al., 1993; Killebrew, et al., 2015; Schluppeck, et al., 2006; Sheremata, et al., 2010), the posterior IPS regions, possibly including IPS1/2, appear to be one, if not the, major hub for working memory more generally in the human brain. This is best exemplified by the seminal work of Todd and Marois (2004). Using fMRI they found a strong correlation between fMRI activity in posterior IPS and the behavioral working memory capacity to encode visual items of individual participants (Fig. 5C–D). Such correlation was not found in an iconic memory task that did not require maintenance of visual information and not in other parts of the brain (notably not in PFC) suggesting that posterior IPS activity reflects working memory storage and is a major hub for this cognitive function in the human brain.
Figure 5. Working memory in primate parietal cortex.
(A) Oculomotor delayed response (ODR) task: Monkeys were required to maintain fixation and encode the location of a cue presented in the periphery. After fixation offset, monkeys made a saccade to the remembered location. (B) Delay period activity of an example LIP neuron in the ODR task. The center panel indicates eight locations at which a cue could appear and each raster plot indicates the resulting neural activity in response to the cue (C) and during the delay period (D). The LIP neuron showed elevated activity during the cue and delay period at the preferred receptive field location (90 degree). Panels A and B reproduced with permission from Chafee and Goldman-Rakic (1998). (C) Visual working memory task: Participants encoded colors in a sample display while rehearsing two digits. After a delay, participants responded whether the color of the probe item matched the remembered colors. (D) Correlation between behavioral working memory capacity and fMRI signals in IPS. The black and red solid lines indicate behavioral working memory capacity (Cohen’s K) and fMRI response amplitudes during a visual short-term memory task (VSTM) at each set size, respectively. FMRI signals and behavioral working memory capacity were strongly correlated. However, an iconic memory task (IM) that used the same sample display without working memory requirement did not show such correlation (indicated by the red dotted line). Panels C and D reproduced with permission from Todd and Marois (2004).
Berryhill and Olson (2008) extended these results by demonstrating causal evidence that PPC is involved in working memory. They found that patients with right PPC damage showed impaired performance in both spatial and object visual working memory tasks. Taken together, these findings support strong evidence for advanced cognitive functionality with respect to visual working memory in the human posterior PPC. Interestingly, this functionality is observed in the regions that also carry advanced object representations, thereby allowing for integration of perceptual and cognitive function in posterior PPC. This may be another prerequisite for the formation of an efficient tool network. According to our model, information relevant for tool use and manipulation will be input from the posterior IPS to IPS5 and the human tool area. Together with hPGR (and the more extended fronto-parietal action system), these regions form an integrated large-scale circuit supporting tool use and manipulation (see Fig. 2).
3. Tool-specific responses in IPS5
Despite the tool area, the most anterior region in the IPS, IPS5, also exhibits strong and selective responses to viewing tools (Mruczek, et al., 2013). As mentioned earlier, IPS5 is located just posterior to hPGR (Konen, et al., 2013) and posterior, medial and superior to the tool-specific region (Mruczek, et al., 2013). However there is some partial overlap between all of these regions and they may act as a coordinated network to guide sophisticated object interactions.
We alluded earlier to the functional correspondence between human IPS5 and area VIP in monkeys (Konen & Kastner, 2008b; Silver & Kastner, 2009). The tool specificity in IPS5 is consistent with the idea that neural processes in macaque VIP provided the evolutionary foundation for the tool-specific circuit in humans. Interestingly, evidence from monkey physiology (Iriki, Tanaka, & Iwamura, 1996), human psychophysics (Maravita, Spence, Kennett, & Driver, 2002) and human neuropsychology (Berti & Frassinetti, 2000) shows that tool usage dynamically modifies the brain’s internal “body schema”, a representation of posture and positioning in space that is hypothesized to be located in the PPC. Tools extend the internal body schema to include the spatial regions around or reachable by the tool, which suggests an integration of the tool as an effector for actions (Graziano & Botvinick, 2002; Maravita & Iriki, 2004).
4. The formation of category-specific tool regions
We have reviewed the evidence of tool-specific regions in left parietal cortex in a previous section. In our account, the tool-specific regions of the anterior IPS integrate object and cognitive information from the posterior IPS and information from IPS5 with respect to the body schema with experience-dependent knowledge of action associations, affordances, and goals, which are uniquely linked to tools.
The anterior parietal tool-specific region is an integral part of two other cortical networks. First, the human tool network includes regions of inferior temporal and medial temporal cortex. These regions have been implicated in conceptual and semantic knowledge related to tools and actions (Lewis, 2006). Regions of the ventral tool network likely interact with the parietal tool region (Lewis, 2006) and may provide one way in which object information from the dorsal and ventral streams interact. Second is the fronto-parietal action-to-perception system, of which hPGR is a part as well. Importantly, parts of this integrated network will also serve action recognition. The formation of an action recognition system may be another principal stepping stone towards the formation of a tool network. However, reviewing the extensive literature on this topic is beyond the scope of this review.
Conclusion
The primate parietal cortex underwent significant transformations during evolution, anatomically as well as functionally. While we are only at a beginning understanding of the functional transformations in the primate lineage, it appears that the significance of spatial processing that involves the dorsal visual pathway including PPC is preserved in human and non-human primates. In contrast, non-spatial information appears to be represented with greater complexity in humans. For example, extrastriate dorsal regions exhibit viewpoint- and size-dependent shape and object selectivity, whereas higher-order areas in PPC represent object information regardless of changes in viewpoint and size in humans, but not in monkeys. Thus, the human dorsal vision system shows a response profile indicative of a flexible object recognition system with the potential to contribute to a variety of sophisticated, visually-guided, object-directed behaviors. This expansion in functional repertoire of human posterior PPC may be paralleled by an expansion of cognitive function as well. An example for such expanded functionality is working memory. Working memory may be instrumental when deriving motor and action sequences directed at complex interactions with objects in the world such as tools. Remarkably, the evolutionarily novel and uniquely human tool network is located to a large extent in parietal cortex. Based on a comparative analysis of monkey and human PPC literature, we propose that the functional expansion of PPC, in its contribution to the representation of non-spatial information and enhanced cognitive function, reflect prerequisites for developing a highly refined tool network.
Highlights.
The dorsal object system in humans and monkeys supports action guidance.
The human dorsal object system mirrors the complexity of the ventral stream.
The human dorsal object system is integrated with working memory systems.
The human dorsal object system contains unique tool-specific representations.
These may support the human toolmaker’s sophisticated object-directed behavior.
Acknowledgments
We thank former members of the lab for their contributions to some of the work presented in this review, particularly Michael Arcaro, Christina Konen and Tanja Kassuba. Our research is supported by the National Science Foundation (BCS-1025149 to S.K.), the National Institutes of Health (2R01 MH64043 and 1R01 EY017699 to S.K.; F32 NS063619 to R.E.B.M.), the Henry Luce Foundation, a Deutscher Akademischer Austauschdienst grant, Foundation for Ophthalmology Research and Education - International, and the James S. McDonnell Foundation.
Footnotes
Of course, contributions to object working memory have also been observed in both frontal and ventral-temporal cortex. For example, and relevant to the current discussion, convergent evidence from neuroimaging (Singhal, Monaco, Kaufman, & Culham, 2013), neuropsychology (Goodale, Jakobson, & Keillor, 1994), and neuromodulation (Cohen, Cross, Tunik, Grafton, & Culham, 2009) suggests that delayed-action movements are guided in part by the reactivation of ventral stream object representations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Bracewell RM, Barash S, Gnadt JW, Fogassi L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a of macaque. J Neurosci. 1990;10:1176–1196. doi: 10.1523/JNEUROSCI.10-04-01176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Arcaro MJ, McMains SA, Singer BD, Kastner S. Retinotopic organization of human ventral visual cortex. J Neurosci. 2009;29:10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, Pinsk MA, Li X, Kastner S. Visuotopic organization of macaque posterior parietal cortex: a functional magnetic resonance imaging study. J Neurosci. 2011;31:2064–2078. doi: 10.1523/JNEUROSCI.3334-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol. 1991;66:1095–1108. doi: 10.1152/jn.1991.66.3.1095. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res. 2001;140:127–144. doi: 10.1007/s002210100785. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008;46:1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A, Frassinetti F. When far becomes near: remapping of space by tool use. J Cogn Neurosci. 2000;12:415–420. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain Lang. 2013;127:222–229. doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–1259. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Booth MC, Rolls ET. View-invariant representations of familiar objects by neurons in the inferior temporal visual cortex. Cereb Cortex. 1998;8:510–523. doi: 10.1093/cercor/8.6.510. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Distler C, Hoffmann KP. Eye position effects in monkey cortex. II. Pursuit- and fixation-related activity in posterior parietal areas LIP and 7A. J Neurophysiol. 1997;77:962–977. doi: 10.1152/jn.1997.77.2.962. [DOI] [PubMed] [Google Scholar]
- Brewer AA, Liu J, Wade AR, Wandell BA. Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat Neurosci. 2005;8:1102–1109. doi: 10.1038/nn1507. [DOI] [PubMed] [Google Scholar]
- Brewer AA, Press WA, Logothetis NK, Wandell BA. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J Neurosci. 2002;22:10416–10426. doi: 10.1523/JNEUROSCI.22-23-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt aufgrund des Zellensbaues. Leipzig, Germany: Barth; 1909. [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–636. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43:917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Res Cogn Brain Res. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41:1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA. FMRI-adaptation to highly-rendered color photographs of animals and manipulable artifacts during a classification task. Neuroimage. 2012;59:2941–2951. doi: 10.1016/j.neuroimage.2011.09.073. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Cross ES, Tunik E, Grafton ST, Culham JC. Ventral and dorsal stream contributions to the online control of immediate and delayed grasping: a TMS approach. Neuropsychologia. 2009;47:1553–1562. doi: 10.1016/j.neuropsychologia.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. The analysis of visual space by the lateral intraparietal area of the monkey: the role of extraretinal signals. Prog Brain Res. 1993a;95:307–316. doi: 10.1016/s0079-6123(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol. 1993b;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CS, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial Neglect and Attention Networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Craighero L, Fadiga L, Umilta CA, Rizzolatti G. Evidence for visuomotor priming effect. Neuroreport. 1996;8:347–349. doi: 10.1097/00001756-199612200-00068. [DOI] [PubMed] [Google Scholar]
- Daprati E, Sirigu A. How we interact with objects: learning from brain lesions. Trends Cogn Sci. 2006;10:265–270. doi: 10.1016/j.tics.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Dastjerdi M, Ozker M, Foster BL, Rangarajan V, Parvizi J. Numerical processing in the human parietal cortex during experimental and natural conditions. Nat Commun. 2013;4:2528. doi: 10.1038/ncomms3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Dehaene-Lambertz G, Cohen L. Abstract representations of numbers in the animal and human brain. Trends Neurosci. 1998;21:355–361. doi: 10.1016/s0166-2236(98)01263-6. [DOI] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Durand JB, Nelissen K, Joly O, Wardak C, Todd JT, Norman JF, Janssen P, Vanduffel W, Orban GA. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron. 2007;55:493–505. doi: 10.1016/j.neuron.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fize D, Vanduffel W, Nelissen K, Denys K, d’Hotel CC, Faugeras O, Orban GA. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J Neurosci. 2003;23:7395–7406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443:85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Georgieva S, Peeters R, Kolster H, Todd JT, Orban GA. The processing of three-dimensional shape from disparity in the human brain. J Neurosci. 2009;29:727–742. doi: 10.1523/JNEUROSCI.4753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S. Tool use and mechanical problem solving in apraxia. Neuropsychologia. 1998;36:581–589. doi: 10.1016/s0028-3932(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J. The neural basis of tool use. Brain. 2009;132:1645–1655. doi: 10.1093/brain/awp080. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Jakobson LS, Keillor JM. Differences in the visual control of pantomimed and natural grasping movements. Neuropsychologia. 1994;32:1159–1178. doi: 10.1016/0028-3932(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature. 1991;349:154–156. doi: 10.1038/349154a0. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Cooke DF. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:845–859. doi: 10.1016/j.neuropsychologia.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Botvinick MM. How the brain represents the body: insights from neurophysiology and psychology. In: Prinz W, Hommel B, editors. Common Mechanisms in Perception and Action: Attention and Performance XIX. XIX. Oxford: Oxford University Press; 2002. pp. 136–157. [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/s0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Gross CG, Rocha-Miranda CEd, Bender DB. Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–577. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Huang RS, Chen CF, Tran AT, Holstein KL, Sereno MI. Mapping multisensory parietal face and body areas in humans. Proc Natl Acad Sci U S A. 2012;109:18114–18119. doi: 10.1073/pnas.1207946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Hihara S, Iriki A. Acquisition and development of monkey tool-use: behavioral and kinematic analyses. Can J Physiol Pharmacol. 2000;78:958–966. [PubMed] [Google Scholar]
- Ishibashi H, Hihara S, Takahashi M, Heike T, Yokota T, Iriki A. Tool-use learning induces BDNF expression in a selective portion of monkey anterior parietal cortex. Mol Brain Res. 2002;102:110–112. doi: 10.1016/s0169-328x(02)00201-2. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. Differential effects of viewpoint on object-driven activation in dorsal and ventral streams. Neuron. 2002;35:793–801. doi: 10.1016/s0896-6273(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Janssen P, Srivastava S, Ombelet S, Orban GA. Coding of shape and position in macaque lateral intraparietal area. J Neurosci. 2008;28:6679–6690. doi: 10.1523/JNEUROSCI.0499-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Decety J, Michel F. Impairment of grasping movements following a bilateral posterior parietal lesion. Neuropsychologia. 1994;32:369–380. doi: 10.1016/0028-3932(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Jeong SK, Xu Y. Behaviorally relevant abstract object identity representation in the human parietal cortex. J Neurosci. 2016;36:1607–1619. doi: 10.1523/JNEUROSCI.1016-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Joly O, Vanduffel W, Orban GA. The monkey ventral premotor cortex processes 3D shape from disparity. Neuroimage. 2009;47:262–272. doi: 10.1016/j.neuroimage.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. J Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, Arcaro M, Li X, Konen CS. Investigations of visual object representations along the dorsal and ventral pathways in macaque monkeys using fMRI. Society for Neuroscience Abstracts 35 : Program Number 802.4 2009 [Google Scholar]
- Katsuyama N, Yamashita A, Sawada K, Naganuma T, Sakata H, Taira M. Functional and histological properties of caudal intraparietal area of macaque monkey. Neuroscience. 2010;167:1–10. doi: 10.1016/j.neuroscience.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Killebrew K, Mruczek R, Berryhill ME. Intraparietal regions play a material general role in working memory: Evidence supporting an internal attentional role. Neuropsychologia. 2015;73:12–24. doi: 10.1016/j.neuropsychologia.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H, Mandeville JB, Arsenault JT, Ekstrom LB, Wald LL, Vanduffel W. Visual field map clusters in macaque extrastriate visual cortex. J Neurosci. 2009;29:7031–7039. doi: 10.1523/JNEUROSCI.0518-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nat Neurosci. 2008a;11:224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci. 2008b;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Mruczek RE, Montoya JL, Kastner S. Functional organization of human posterior parietal cortex: grasping-and reaching-related activations relative to topographically organized cortex. J Neurophysiol. 2013;109:2897–2908. doi: 10.1152/jn.00657.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Erb M, Grodd W, Bulthoff HH. Representation of the perceived 3-D object shape in the human lateral occipital complex. Cereb Cortex. 2003;13:911–920. doi: 10.1093/cercor/13.9.911. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Gottlieb J, Goldberg ME. The lateral intraparietal area as a salience map: the representation of abrupt onset, stimulus motion, and task relevance. Vision Res. 2000;40:1459–1468. doi: 10.1016/s0042-6989(99)00212-6. [DOI] [PubMed] [Google Scholar]
- Larsson J, Heeger DJ. Two retinotopic visual areas in human lateral occipital cortex. J Neurosci. 2006;26:13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen TZ, D’Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis. 2009;9:18, 11–14. doi: 10.1167/9.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Sheinberg DL. Visual object recognition. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas J, Benson R, Kwong K, Jiang H, Kennedy W, Ledden P, Brady T, Rosen B, Tootell R. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Trends Cogn Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Maravita A, Spence C, Kennett S, Driver J. Tool-use changes multimodal spatial interactions between vision and touch in normal humans. Cognition. 2002;83:B25–34. doi: 10.1016/s0010-0277(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Michalka SW, Rosen ML, Kong L, Shinn-Cunningham BG, Somers DC. Auditory spatial coding flexibly recruits anterior, but not posterior, visuotopic parietal cortex. Cereb Cortex. 2016;26:1302–1308. doi: 10.1093/cercor/bhv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- Mruczek REB, von Loga IS, Kastner S. The representation of tool and non-tool object information in the human intraparietal sulcus. J Neurophysiol. 2013;109:2883–2896. doi: 10.1152/jn.00658.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Cohen YE, Groh JM. Eye-centered, head-centered, and complex coding of visual and auditory targets in the intraparietal sulcus. J Neurophysiol. 2005;94:2331–2352. doi: 10.1152/jn.00021.2005. [DOI] [PubMed] [Google Scholar]
- Mullette-Gillman OA, Cohen YE, Groh JM. Motor-related signals in the intraparietal cortex encode locations in a hybrid, rather than eye-centered reference frame. Cereb Cortex. 2009;19:1761–1775. doi: 10.1093/cercor/bhn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kuroda T, Wakita M, Kusunoki M, Kato A, Mikami A, Sakata H, Itoh K. From three-dimensional space vision to prehensile hand movements: the lateral intraparietal area links the area V3A and the anterior intraparietal area in macaques. J Neurosci. 2001;21:8174–8187. doi: 10.1523/JNEUROSCI.21-20-08174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Dehaene S. Representation of Number in the Brain. Annu Rev Neurosci. 2009;32:185–208. doi: 10.1146/annurev.neuro.051508.135550. [DOI] [PubMed] [Google Scholar]
- Obayashi S, Suhara T, Kawabe K, Okauchi T, Maeda J, Akine Y, Onoe H, Iriki A. Functional brain mapping of monkey tool use. Neuroimage. 2001;14:853–861. doi: 10.1006/nimg.2001.0878. [DOI] [PubMed] [Google Scholar]
- Orban GA. The extraction of 3D shape in the visual system of human and nonhuman primates. Annu Rev Neurosci. 2011;34:361–388. doi: 10.1146/annurev-neuro-061010-113819. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Ro T, Beauchamp MS. TMS of posterior parietal cortex disrupts visual tactile multisensory integration. Eur J Neurosci. 2010;31:1783–1790. doi: 10.1111/j.1460-9568.2010.07193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel GH, Shulman GL, Baker JT, Akbudak E, Snyder AZ, Snyder LH, Corbetta M. Topographic organization of macaque area LIP. Proc Natl Acad Sci U S A. 2010;107:4728–4733. doi: 10.1073/pnas.0908092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic ataxia: a specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain. 1988;111(Pt 3):643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Grafton ST. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. J Neurosci. 2006;26:8176–8182. doi: 10.1523/JNEUROSCI.1641-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenhuber M, Poggio T. Neural mechanisms of object recognition. Curr Opin Neurobiol. 2002;12:162–168. doi: 10.1016/s0959-4388(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Romero MC, Pani P, Janssen P. Coding of shape features in the macaque anterior intraparietal area. J Neurosci. 2014;34:4006–4021. doi: 10.1523/JNEUROSCI.4095-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MC, Van Dromme I, Janssen P. Responses to two-dimensional shapes in the macaque anterior intraparietal area. Eur J Neurosci. 2012;36:2324–2334. doi: 10.1111/j.1460-9568.2012.08135.x. [DOI] [PubMed] [Google Scholar]
- Romero MC, Van Dromme IC, Janssen P. The role of binocular disparity in stereoscopic images of objects in the macaque anterior intraparietal area. PLoS One. 2013;8:e55340. doi: 10.1371/journal.pone.0055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Cowan NJ, Angelaki DE. The visual representation of 3D object orientation in parietal cortex. J Neurosci. 2013;33:19352–19361. doi: 10.1523/JNEUROSCI.3174-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y. The TINS Lecture. The parietal association cortex in depth perception and visual control of hand action. Trends Neurosci. 1997;20:350–357. doi: 10.1016/s0166-2236(97)01067-9. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Mine S, Murata A. Control of arm movement in space: neurophysical and computational approaches. Berlin: Springer; 1992. The hand-movement-related neurons of the posterior parietal cortex of the monkey: their role in the visual guidance of hand movement; pp. 185–198. [Google Scholar]
- Sakata H, Tsutsui K, Taira M. Toward an understanding of the neural processing for 3D shape perception. Neuropsychologia. 2005;43:151–161. doi: 10.1016/j.neuropsychologia.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. London: Academic Press; 2007. [Google Scholar]
- Sawamura H, Georgieva S, Vogels R, Vanduffel W, Orban GA. Using functional magnetic resonance imaging to assess adaptation and size invariance of shape processing by humans and monkeys. J Neurosci. 2005;25:4294–4306. doi: 10.1523/JNEUROSCI.0377-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma SJ, Duysens J. Neurons in the ventral intraparietal area of awake macaque monkey closely resemble neurons in the dorsal part of the medial superior temporal area in their responses to optic flow patterns. J Neurophysiol. 1996;76:4056–4068. doi: 10.1152/jn.1996.76.6.4056. [DOI] [PubMed] [Google Scholar]
- Schaafsma SJ, Duysens J, Gielen CC. Responses in ventral intraparietal area of awake macaque monkey to optic flow patterns corresponding to rotation of planes in depth can be explained by translation and expansion effects. Vis Neurosci. 1997;14:633–646. doi: 10.1017/s0952523800012608. [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. J Physiol. 2003;551:551–561. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlack A, Sterbing-D’Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KA, Richter MC, Kastner S. Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: a high-resolution functional magnetic resonance imaging study. J Neurosci. 2004;24:8975–8985. doi: 10.1523/JNEUROSCI.2413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale A, Reppas J, Kwong K. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. Multisensory maps in parietal cortex. Curr Opin Neurobiol. 2014;24:39–46. doi: 10.1016/j.conb.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Serino A, Canzoneri E, Avenanti A. Fronto-parietal areas necessary for a multisensory representation of peripersonal space in humans: an rTMS study. J Cogn Neurosci. 2011;23:2956–2967. doi: 10.1162/jocn_a_00006. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Motion perception: seeing and deciding. Proc Natl Acad Sci U S A. 1996;93:628–633. doi: 10.1073/pnas.93.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sheremata SL, Bettencourt KC, Somers DC. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. J Neurosci. 2010;30:12581–12588. doi: 10.1523/JNEUROSCI.2689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker RW, Walkup KR, Beck BB. Animal Tool Behavior: The Use and Manufacture of Tools by Animals. Johns Hopkins University Press; Baltimore: 2011. [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Singhal A, Monaco S, Kaufman LD, Culham JC. Human fMRI reveals that delayed action re-recruits visual perception. PLoS One. 2013;8:e73629. doi: 10.1371/journal.pone.0073629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Cohen L, Duhamel JR, Pillon B, Dubois B, Agid Y. A selective impairment of hand posture for object utilization in apraxia. Cortex. 1995;31:41–55. doi: 10.1016/s0010-9452(13)80104-9. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Orban GA, De Maziere PA, Janssen P. A distinct representation of three-dimensional shape in macaque anterior intraparietal area: fast, metric, and coarse. J Neurosci. 2009;29:10613–10626. doi: 10.1523/JNEUROSCI.6016-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of Spatial Attention Control in Frontal and Parietal Cortex. J Neurosci. 2010;30:148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H. Parietal cortex neurons of the monkey related to the visual guidance of hand movement. Exp Brain Res. 1990;83:29–36. doi: 10.1007/BF00232190. [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Vanduffel W, Sasaki Y, Fize D, Knutsen TA, Mandeville JB, Wald LL, Dale AM, Rosen BR, Van Essen DC. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003;39:555–568. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Sakata H, Naganuma T, Taira M. Neural correlates for perception of 3D surface orientation from texture gradient. Science. 2002;298:409–412. doi: 10.1126/science.1074128. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Taira M, Sakata H. Neural mechanisms of three-dimensional vision. Neurosci Res. 2005;51:221–229. doi: 10.1016/j.neures.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Tsutsui KI, Jiang M, Sakata H, Taira M. Short-term memory and perceptual decision for three-dimensional visual features in the caudal intraparietal sulcus (area CIP) J Neurosci. 2003;23:5486–5495. doi: 10.1523/JNEUROSCI.23-13-05486.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 2005;8:505–511. doi: 10.1038/nn1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyear KF, Cavina-Pratesi C, Stiglick AJ, Culham JC. Does tool-related fMRI activity within the intraparietal sulcus reflect the plan to grasp? Neuroimage. 2007;36(Suppl 2):T94–T108. doi: 10.1016/j.neuroimage.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Surface-based approaches to spatial localization and registration in primate cerebral cortex. Neuroimage. 2004;23:S97–S107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Lewis JW, Drury HA, Hadjikhani N, Tootell R, Bakircioglu M, Miller MI. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson R, Driver J, Dolan R. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nat Neurosci. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jeong SK. In: The contribution of human superior intra-parietal sulcus to visual short-term memory and perception. Jolicoeur P, Martinez-Trujillo J, editors. 2015. [Google Scholar]