Abstract
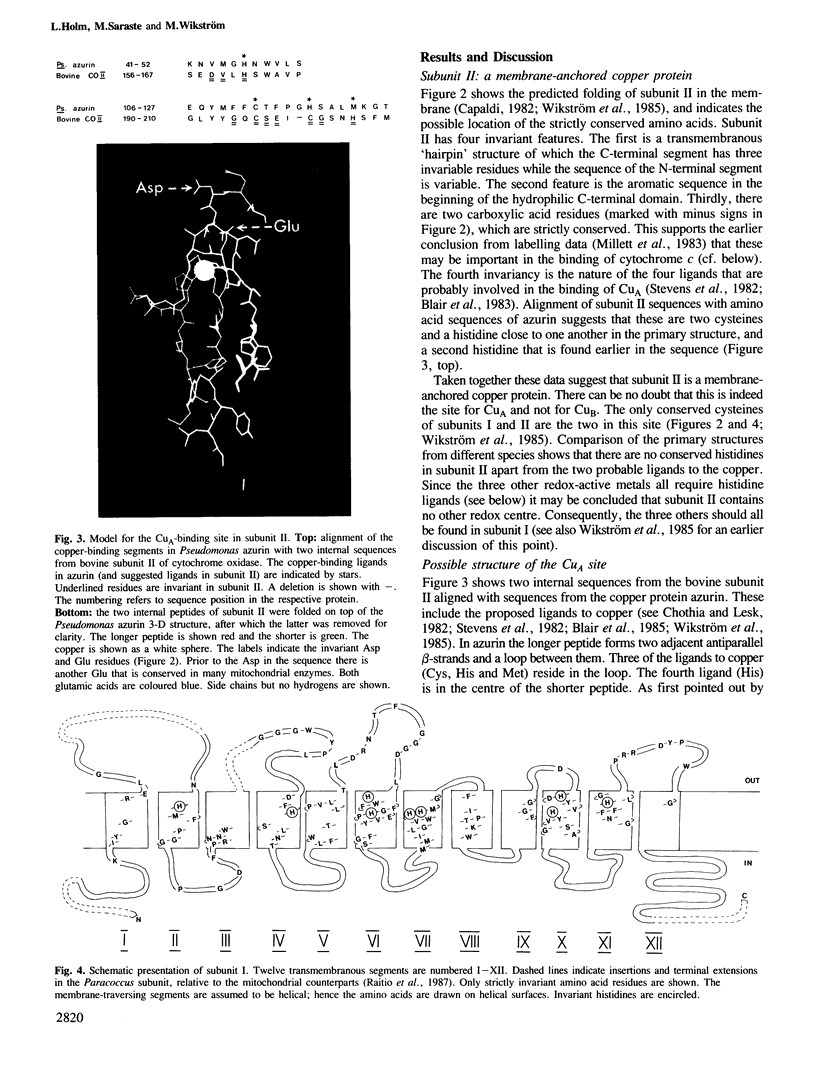
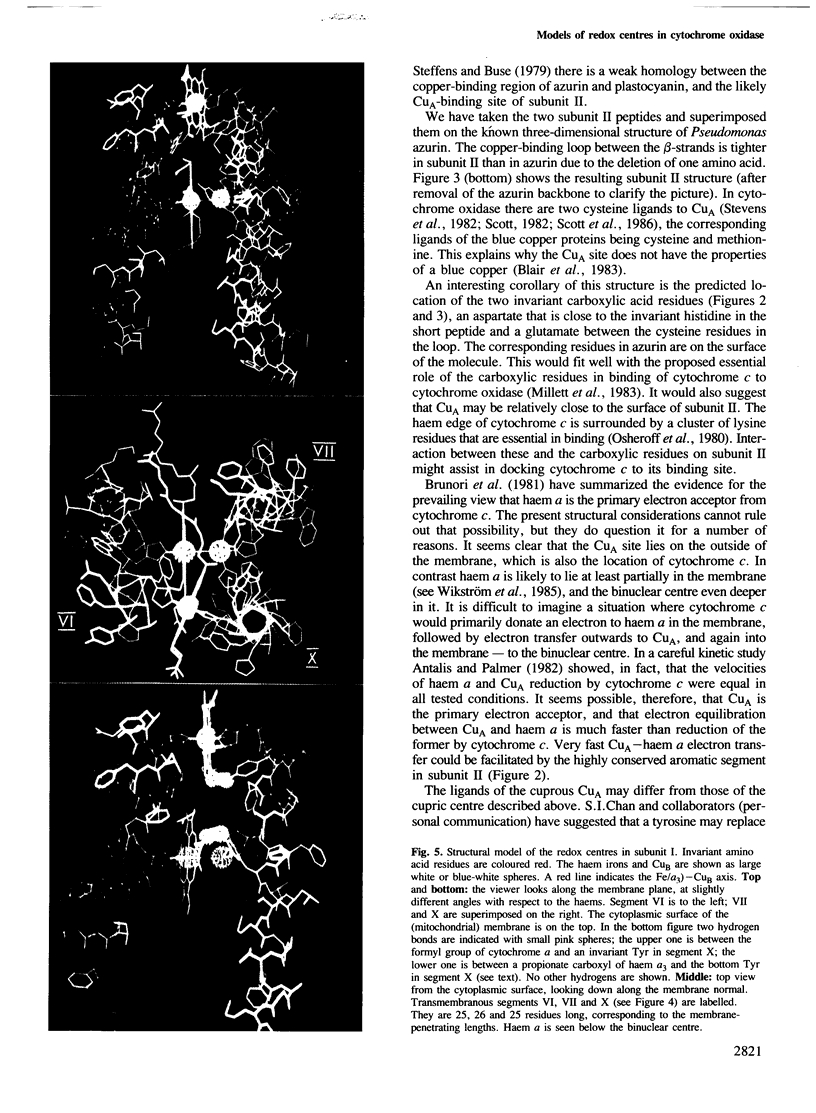
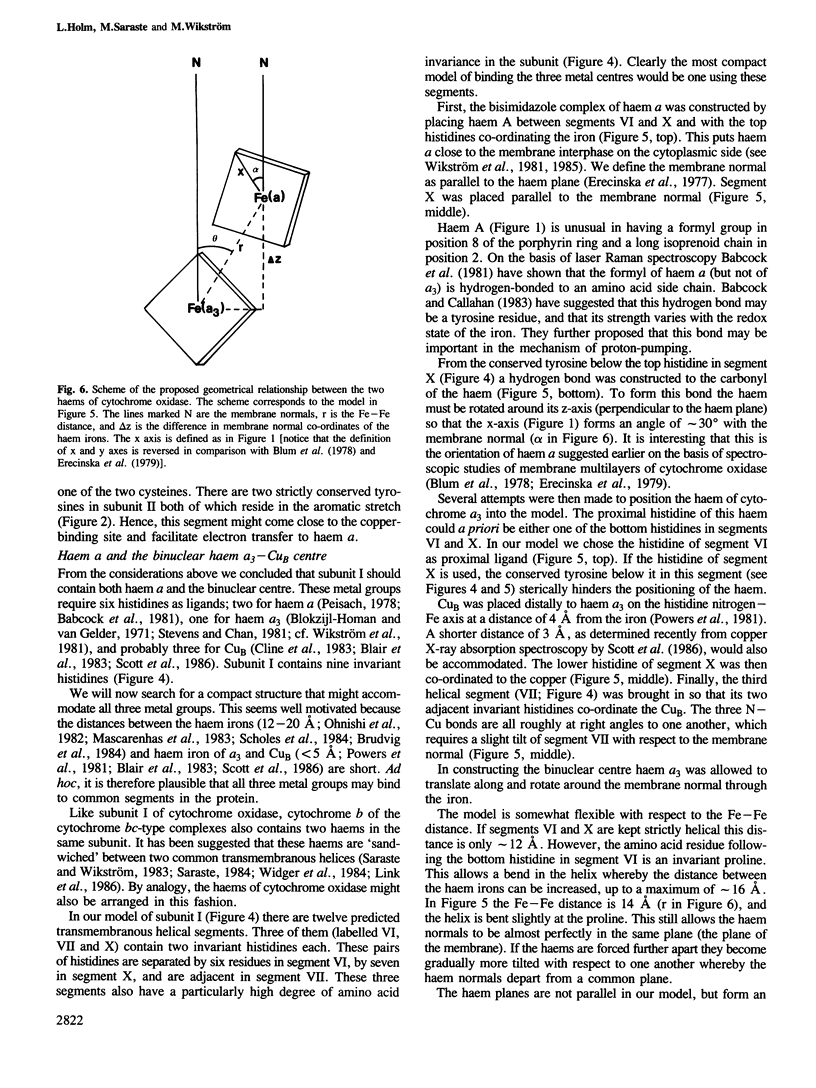
Evolutionary conservation, predicted membrane topography of the subunits, and known chemical and physical properties of the catalytic metals in cytochrome oxidase provided the basis for plausible structural models of the enzyme's redox centres. Subunit II probably binds one of the copper ions (CuA) whilst subunit I is likely to bind the two haems (a and a3) and the other redox-active copper (CuB). Two cysteine and two histidine residues of subunit II are the likely ligands of CuA, forming a centre that may be structurally similar to that in azurin. The two haems may be sandwiched between two transmembranous segments of subunit I, one of which also provides a histidine ligand to CuB. A third segment may provide two more histidine ligands to the latter. The model was constructed with a 4 A Fe-Cu distance in the binuclear haem a3-CuB centre, and a 14 A distance between the haem irons. The subunit I model involves only three transmembranous helices which bind three catalytic metal groups. The fit of this model to several known physicochemical properties of the redox centres is analysed.
Full text
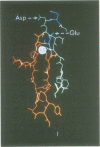
PDF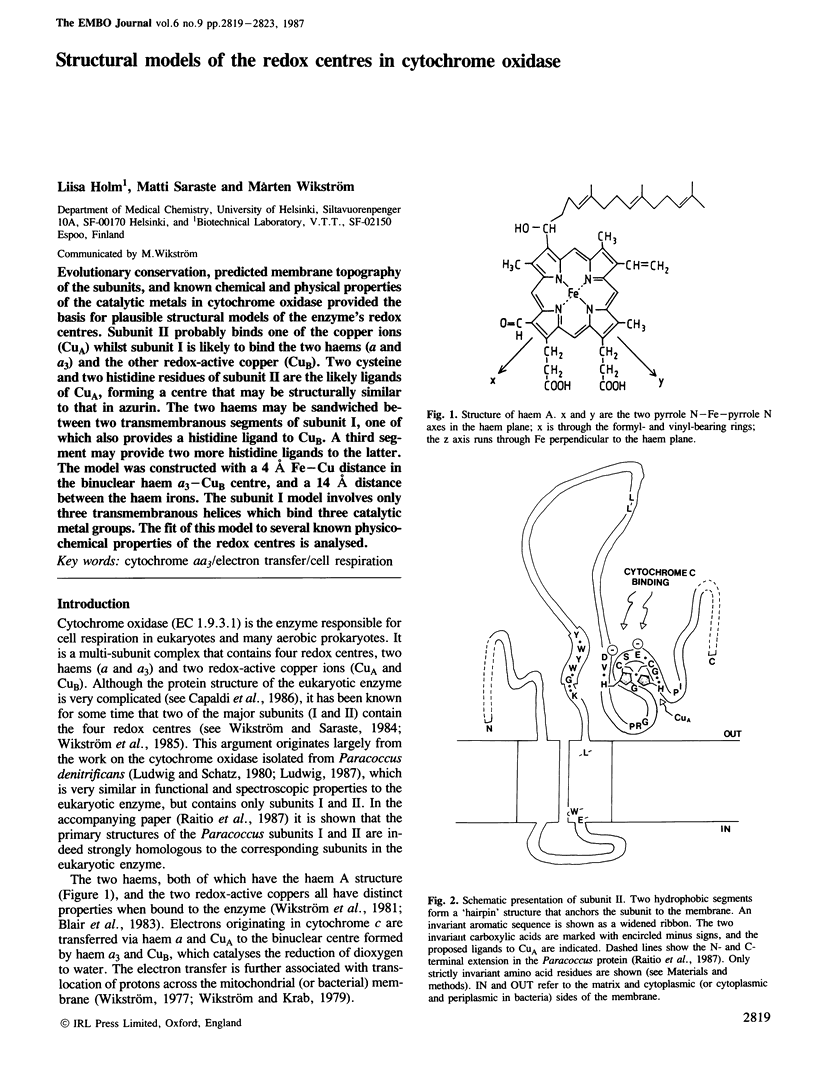

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antalis T. M., Palmer G. Kinetic characterization of the interaction between cytochrome oxidase and cytochrome c. J Biol Chem. 1982 Jun 10;257(11):6194–6206. [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M., Ondrias M. R., Salmeen I. Coordination geometries and vibrational properties of cytochromes alpha and alpha 3 in cytochrome oxidase from Soret excitation Raman spectroscopy. Biochemistry. 1981 Feb 17;20(4):959–966. doi: 10.1021/bi00507a049. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Blasie J. K., Pachence J. M., Tavormina A., Erecinska M., Dutton P. L., Stamatoff J., Eisenberger P., Brown G. The location of redox centers in biological membranes determined by resonance x-ray diffraction. II. Analysis of the resonance diffraction data. Biochim Biophys Acta. 1982 Feb 17;679(2):188–197. doi: 10.1016/0005-2728(82)90290-0. [DOI] [PubMed] [Google Scholar]
- Blokzijl-Homan M. F., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . 3. The EPR spectrum of NO-ferrocytochrome a 3 . Biochim Biophys Acta. 1971 Jun 15;234(3):493–498. doi: 10.1016/0005-2728(71)90215-5. [DOI] [PubMed] [Google Scholar]
- Blum H., Harmon H. J., Leigh J. S., Salerno J. C., Chance B. The orientation of a heme of cytochrome c oxidase in submitochondrial particles. Biochim Biophys Acta. 1978 Apr 11;502(1):1–10. doi: 10.1016/0005-2728(78)90125-1. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Blair D. F., Chan S. I. Electron spin relaxation of CuA and cytochrome a in cytochrome c oxidase. Comparison to heme, copper, and sulfur radical complexes. J Biol Chem. 1984 Sep 10;259(17):11001–11009. [PubMed] [Google Scholar]
- Capaldi R. A. Arrangement of proteins in the mitochondrial inner membrane. Biochim Biophys Acta. 1982 Nov 30;694(3):291–306. doi: 10.1016/0304-4157(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Gonzalez-Halphen D., Takamiya S. Sequence homologies and structural similarities between the polypeptides of yeast and beef heart cytochrome c oxidase. FEBS Lett. 1986 Oct 20;207(1):11–17. doi: 10.1016/0014-5793(86)80004-7. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Evolution of proteins formed by beta-sheets. I. Plastocyanin and azurin. J Mol Biol. 1982 Sep 15;160(2):309–323. doi: 10.1016/0022-2836(82)90178-4. [DOI] [PubMed] [Google Scholar]
- Cline J., Reinhammar B., Jensen P., Venters R., Hoffman B. M. Coordination environment for the type 3 copper center of tree laccase and CuB of cytochrome c oxidase as determined by electron nuclear double resonance. J Biol Chem. 1983 Apr 25;258(8):5124–5128. [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Blasie J. K., Wilson D. F. Orientation of the hemes of cytochrome c oxidase and cytochrome c in mitochondria. FEBS Lett. 1977 Apr 15;76(2):235–239. doi: 10.1016/0014-5793(77)80159-2. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Blasie J. K. Studies of the orientation of the mitochondrial redox carriers. III. Orientation of the gx and gy axes of the hemes of cytochrome oxidase with respect to the plane of the membrane in oriented membrane multilayers. Biochim Biophys Acta. 1979 Feb 8;545(2):352–364. doi: 10.1016/0005-2728(79)90212-3. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Brakenhoff J., De Vries B. F., Sloof P., Tromp M. C., Van Boom J. H., Benne R. The sequence of the gene for cytochrome c oxidase subunit I, a frameshift containing gene for cytochrome c oxidase subunit II and seven unassigned reading frames in Trypanosoma brucei mitochondrial maxi-circle DNA. Nucleic Acids Res. 1984 Oct 11;12(19):7327–7344. doi: 10.1093/nar/12.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Link T. A., Schägger H., von Jagow G. Analysis of the structures of the subunits of the cytochrome bc1 complex from beef heart mitochondria. FEBS Lett. 1986 Aug 11;204(1):9–15. doi: 10.1016/0014-5793(86)81378-3. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas R., Wei Y. H., Scholes C. P., King T. E. Interaction in cytochrome c oxidase between cytochrome a3 ligated with nitric oxide and cytochrome a. J Biol Chem. 1983 May 10;258(9):5348–5351. [PubMed] [Google Scholar]
- Millett F., de Jong C., Paulson L., Capaldi R. A. Identification of specific carboxylate groups on cytochrome c oxidase that are involved in binding cytochrome c. Biochemistry. 1983 Feb 1;22(3):546–552. doi: 10.1021/bi00272a004. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., LoBrutto R., Salerno J. C., Bruckner R. C., Frey T. G. Spatial relationship between cytochrome a and a3. J Biol Chem. 1982 Dec 25;257(24):14821–14825. [PubMed] [Google Scholar]
- Osheroff N., Brautigan D. L., Margoliash E. Definition of enzymic interaction domains on cytochrome c. Purification and activity of singly substituted carboxydinitrophenyl-lysine 7, 25, 73, 86, and 99 cytochromes c. J Biol Chem. 1980 Sep 10;255(17):8245–8251. [PubMed] [Google Scholar]
- Powers L., Chance B., Ching Y., Angiolillo P. Structural features and the reaction mechanism of cytochrome oxidase: iron and copper X-ray absorption fine structure. Biophys J. 1981 Jun;34(3):465–498. doi: 10.1016/S0006-3495(81)84863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Scholes C. P., Janakiraman R., Taylor H., King T. E. Temperature dependence of the electron spin-lattice relaxation rate from pulsed EPR of CUA and heme a in cytochrome c oxidase. Biophys J. 1984 May;45(5):1027–1030. doi: 10.1016/S0006-3495(84)84248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. A., Schwartz J. R., Cramer S. P. Structural aspects of the copper sites in cytochrome c oxidase. An X-ray absorption spectroscopic investigation of the resting-state enzyme. Biochemistry. 1986 Sep 23;25(19):5546–5555. doi: 10.1021/bi00367a030. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Buse G. Studies on cytochrome c oxidase, IV[1--3]. Primary structure and function of subunit II. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):613–619. [PubMed] [Google Scholar]
- Stevens T. H., Chan S. I. Histidine is the axial ligand to cytochrome alpha 3 in cytochrome c oxidase. J Biol Chem. 1981 Feb 10;256(3):1069–1071. [PubMed] [Google Scholar]
- Stevens T. H., Martin C. T., Wang H., Brudvig G. W., Scholes C. P., Chan S. I. The nature of CuA in cytochrome c oxidase. J Biol Chem. 1982 Oct 25;257(20):12106–12113. [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]