Abstract
Transpulmonary pressure (PL) is computed as the difference between airway pressure and pleural pressure and separates the pressure delivered to the lung from the one acting on chest wall and abdomen. Pleural pressure is measured as esophageal pressure (PES) through dedicated catheters provided with esophageal balloons. We discuss the role of PL in assessing the effects of mechanical ventilation in patients with acute respiratory distress syndrome (ARDS). In the supine position, directly measured PL represents the pressure acting on the alveoli and airways. Because there is a pressure gradient in the pleural space from the non-dependent to the dependent zones, the pressure in the esophagus probably represents the pressure at a mid-level between sternal and vertebral regions. For this reason, it has been proposed to set the end-expiratory pressure in order to get a positive value of PL. This improves oxygenation and compliance. PL can also be estimated from airway pressure plateau and the ratio of lung to respiratory elastance (elastance-derived method). Some data suggest that this latter calculation may better estimate PL in the nondependent lung zones, at risk for hyperinflation. Elastance-derived PL at end-inspiration (PLend-insp) may be a good surrogate of end-inspiratory lung stress for the “baby lung”, at least in non-obese patients. Limiting end-inspiratory PL to 20–25 cmH2O appears physiologically sound to mitigate ventilator-induced lung injury (VILI). Last, lung driving pressure (∆PL) reflects the tidal distending pressure. Changes in PL may also be assessed during assisted breathing to take into account the additive effects of spontaneous breathing and mechanical breaths on lung distension. In summary, despite limitations, assessment of PL allows a deeper understanding of the risk of VILI and may potentially help tailor ventilator settings.
Keywords: Acute respiratory distress syndrome (ARDS), ventilator-induced lung injury (VILI), respiratory mechanics, driving pressure, mechanical ventilation, esophageal pressure (PES)
Introduction
Mechanical ventilation to restore aeration in the collapsed lung and reverse hypoxemia is a life-saving treatment for patients with the acute respiratory distress syndrome (ARDS). However, despite recent advances in the diagnosis and management, ARDS mortality remain as high as 30–45%, with inappropriate ventilatory settings contributing to morbidity through the so-called ventilator-induced lung injury (VILI) (1,2). Ventilator induced lung injury is a dysregulated inflammatory response that occurs as a means of excessive volume/pressure (volu- and barotrauma) load in the aerated lung (i.e., the baby lung) along with the cyclic opening and closing of distal airways and/or flooded or collapsed alveoli during tidal ventilation (atelectrauma) (1,3,4). Since its introduction in 1950s, mechanical ventilation was aimed at treating the impairment in gas exchange; recent years have witnessed a radical shift towards a mechanistic ‘lung protective’ approach, so that nowadays limiting VILI has arisen as a priority in the management of patients with ARDS (5). Limiting tidal volume (VT) to 6 mL/kg of predicted body weight (PBW) with plateau pressure (PPLAT) within 30 cmH2O for most patients has been shown to improve survival as compared to higher VT (12 mL/kg of PBW) (6). Subsequent physiological studies have also suggested that further reduction of VT to 3–4 mL/kg may benefit patients who are at higher risk of overdistension (7-10).
Respiratory system compliance (CRS) is directly affected by the size of the aerated lung. Amato et al. suggested that the impact of tidal ventilation on lung injury could be better predicted if VT is normalized to CRS rather than to PBW. The ratio VT/CRS is the driving pressure of the respiratory system (∆P), and can be easily calculated at the bedside as PPLAT-PEEP. It was shown to be the final mediator of the effects of lowering VT and PPLAT on mortality (11). Essentially, ∆P estimates the mechanical distortion provided by VT to the baby lung (i.e., the dynamic strain), while PPLAT roughly measures the pressure delivered to the baby lung with VT and PEEP (the lung stress): both contribute to rate the risk of barotrauma. Introducing transpulmonary pressure (PL) into the bedside management has then been proposed for two main purposes: know the influence of the chest wall on airway pressure and determine the pressure needed to keep the lung open. In addition, esophageal pressure (PES) is essential to assess patient’s effort and the PL generated during partial ventilatory support.
In the present review, we will describe how PL helps assessing mechanical ventilation harms and benefits, its importance and limitations in respiratory mechanics monitoring and its possible usefulness in tailoring patient’s management. Because PL is the pressure distending the lungs, it is referred to as PL.
PL allows to differentiate lungs and chest wall
Airway pressure is the sum of the pressure delivered to move the lung, the chest wall and the pressure required to overcome the resistive forces (when flow is present). In the absence of flow and if airways are open, airway pressure is equilibrated with alveolar pressure. This pressure, being also needed to overcome chest wall, does not always reliably reflect the pressure load the lung is exposed to.
Consequently, PPLAT during a short end-inspiratory occlusion (0.3–0.5 s) and total PEEP (and consequently ∆P) displayed by the ventilator, being measured at airway opening, represent alveolar pressure but are only surrogates of the actual pressure acting on the lung, which is best assessed with PL.
PL is the pressure delivered to the lung independently from the effects of the chest wall and the abdomen and is computed as the difference between airway pressure and pleural pressure. While alveolar pressure is applied to overcome the elastic recoil of the respiratory system [(respiratory system elastance (ERS)], that is the sum of the elastance of the lung (EL) and the elastance of the chest wall (ECW), PL, if measured in the absence of flow, represents the actual pressure dissipated across the lung tissue.
Given that both pleural pressure absolute values and chest wall elastance (changes in the pleural pressure due to changes in lung volume) are often impaired in a variable proportion during ARDS (12-15), this concept has relevant implications in clinical practice.
First, PEEP applied on the alveoli needs to overcome pleural pressure to generate recruitment. The effective recruiting PEEP is the one acting on the lung independently from pleural pressure (16,17).
Second, during tidal ventilation, high alveolar pressure may not be injurious per se if it is required to overcome ECW rather than being dissipated across the alveoli: in a ‘proof of concept’ study, in 1988 Dreyfuss et al. showed that lesional pulmonary edema occurs in paralyzed healthy animals during pressure-control ventilation with high VT and airway pressures, but not in those ventilated with similar airway pressures and lower VT because of straps applied around their abdomens and chests. The straps were a simple way to raise ECW; therefore, for the same airway pressure or PPLAT, the lung distending pressure (i.e., PL) was lower and non-injurious. Their experiments showed that volume (i.e., lung stretching), not airway pressure, was the most important factor in determining injury, a finding that led them to coin the term ‘volutrauma’ (18). We now interpret these findings as the indirect demonstration of the importance of PL in determining ‘lung trauma’ and injury, which indeed do not occur if PL is maintained within safe limits, no matter how high airway and alveolar pressures are.
PES vs. pleural pressure
Direct pleural pressure measurement is complex in experimental conditions and even harder in the clinical setting. Since 1950s, PES measured through dedicated balloons has been proposed to estimate pleural pressure and compute PL (19). Esophageal manometry has drawbacks that indeed have limited its use in the clinical field until the last decade: a great effort has been made to standardize the technical issues concerning catheter placement and signal validation (20). In addition, the recent renewed interest in the topic has provided data that allow a deeper understanding of PES meaning and validity in estimating pleural pressure, computing PL and understanding respiratory mechanics (21-24).
PL vs. transalveolar pressure
PL is calculated as the difference between the airway pressure and PES. When flow is absent, the role of resistive forces is ruled out and airway pressure is equilibrated with alveolar pressure, with PL corresponding to transalveolar pressure, provided that airways are fully open (25). Essentially, during end-inspiratory and end-expiratory occlusions, the difference between airway pressure and PES is the actual pressure the alveoli are exposed to.
Technique
PES is measured through dedicated catheters endowed with esophageal balloon associated or not with gastric balloon for contemporaneous measurement of gastric pressure.
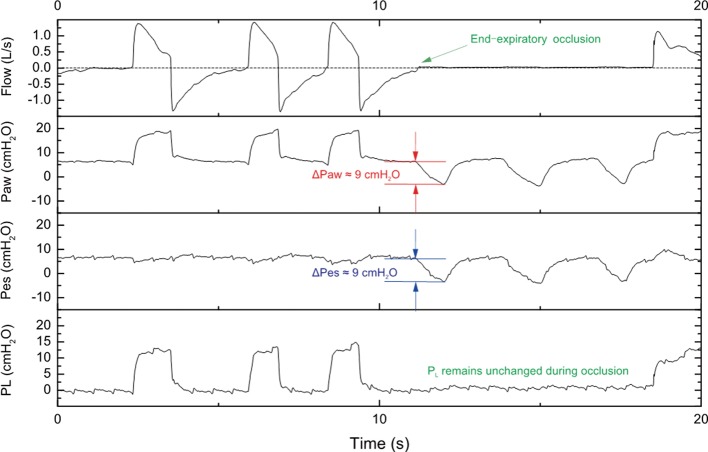
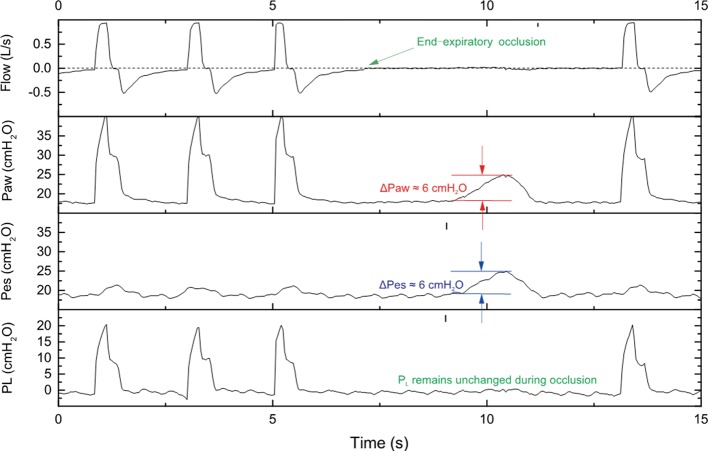
Briefly, the esophageal catheter is transorally/transnasally inserted in the esophagus, gently advanced to the stomach (usual depth 55–60 cm) and then the balloon is inflated with the minimum non-stress volume recommended by the manufacturer. An underfilled balloon does not properly transmit PES, whereas an overfilled balloon overestimates the value of the surrounding pressure. One technique is to start with inserting the catheter down in the stomach and, after confirming the intragastric placement of the balloon with a gentle epigastric compression, the catheter is progressively withdrawn into the esophagus, as suggested by the appearance of cardiac artifacts on the signal. The validity of the measured PES can be confirmed by either a negative pressure occlusion test (26) during spontaneous breathing (Figure 1) or a positive pressure occlusion test during passive ventilation (Figure 2). In spontaneous breathing patients, during an end-expiratory occlusion, a negative change in the intrathoracic pressure by patient’s inspiratory effort generates a consistent change in the airway pressure, as PL is necessarily unmodified given the constant lung volume. In patients without spontaneous breathing the change in the intrathoracic pressure is provided by a gentle compression on the chest and is positive, with similar principle and mechanism of action. Ratio of the change in PES to the change in airway pressure within 0.8–1.2 is an accepted value to confirm the validity of the measure.
Figure 1.
Negative pressure occlusion test during spontaneous breathing. During an end-expiratory occlusion, the patient generated three spontaneously inspiratory efforts (negative pleural pressure) against the occluded airway. The negative change in airway pressure (∆Paw) is identical with the negative change in esophageal pressure (∆Pes). Also, the transpulmonary pressure remains unchanged during end-expiratory occlusion. In this case, the change in esophageal pressure can be used to surrogate the change in pleural pressure. PL, transpulmonary pressure.
Figure 2.
Positive pressure occlusion test during passive ventilation. During an end-expiratory occlusion, the patient was not able to generate any inspiratory effort since the patient was ventilated passively (no spontaneous effort for inspiration). A clinician need to manually compress the chest wall or abdomen to generate a transit increase in pleural pressure (∆Pes). The positive change in airway pressure (∆Paw) is identical with the positive change in esophageal pressure. Also, the transpulmonary pressure remains unchanged during end-expiratory occlusion. In this case, the change in esophageal pressure can be used to surrogate the change in pleural pressure. PL, transpulmonary pressure.
A more detailed technical description of the procedure along with a precise instructional video (https://www.edge-cdn.net/video_1059118?playerskin=37016) have been recently published and made available online by the PLUG working group of the European Society of Intensive Care Medicine (21).
Pleural pressure gradient
Uncertainties exist concerning the reliability of PES in estimating pleural pressure. A vertical gradient in the pleural pressure in the supine patient has been documented in several experimental conditions, with higher values documented in dorsal (dependent) and lower in ventral (non-dependent) lung regions (27,28). This raised concerns about the lung regions in which PES allows to compute the actual distending pressure. Experimental data and translational results from our group showed that in the supine position pleural pressure increases from sternal to vertebral regions because of a vertical gradient generated by superimposed pressure. This gradient appears magnified by lung injury but is also present in the healthy lung. In such a context, PES reliably estimates pleural pressure in the area surrounding the esophagus, which is at a mid-value between ventral non-dependent and dorsal dependent lung regions (24,29).
Direct measurement of PL at end-expiration
Description
Directly measured PL at end expiration is computed as follows (14):
| PLend-exp = PEEPTOT − PESend-exp |
where PEEPTOT and PESend-exp are airway and esophageal pressure during an end-expiratory occlusion.
Some authors have proposed to subtract 5 cmH2O from the value of PES to account for the weight of the mediastinum, but uncertainties exist regarding the validity of this approximation in ICU patients (16,30).
Application
The optimal PEEP setting protocol during ARDS is hotly debated. Low-tidal volumes tend to reduce alveolar recruitment and further impair oxygenation: both effects can be reversed by PEEP (31,32). Moreover, PEEP-induced lung recruitment increases the size of the baby lung (i.e., the functional residual capacity) and, for a given VT, may reduce lung dynamic strain and mitigate lung injury (33-35).
Nevertheless, it is widely accepted that PEEP setting should aim to a balance between its capability to re-open the collapsed lung and the unavoidable damage generated in the already open alveoli that occurs as a means of static stress and strain in the baby lung. Hence, over the last decade, great effort has been made to identify the PEEP-setting strategy that best optimizes lung recruitment without producing excessive alveolar overdistension; PEEP titration methods based on CRS (36-38), oxygenation and shunt values (39,40) and pressure-volume curve (41) have been proposed. Three different randomized studies comparing higher versus lower levels of PEEP, in which higher PEEP values were set according to respiratory mechanics (37) or oxygenation impairment (39,40), failed to detect a significant effect on survival, although some benefits (less use of rescue therapy, reduced ventilation duration) were demonstrated in some studies. A meta-analysis showed a significant survival benefit in most severe patients treated with higher PEEP (42), but the most relevant drawback of such ‘universal’ approach stays in the fact that lung recruitability (increase in the size of the baby lung as a response to PEEP) may significantly vary among patients according to different degrees of lung inhomogeneity: high PEEP in patients with low recruitability may enhance lung injury in the aerated lung, while low PEEP in potentially recruiting patients cannot fully exert its beneficial effects (41,43,44).
Thus, it appears physiologically sound that PEEP setting should be rather mechanistically individualized on patient’s needs and requirements. In this sense, in 2008, Talmor et al. reported the results of a pilot mono centre randomized trial in patients with ARDS (PaO2/FiO2 ≤300 mmHg) assessing the effect on oxygenation of a PEEP-setting protocol measuring PES in all patients to achieve a positive PL, computed with the directly-measured method with no correction for the weight of the mediastinum (17). The authors showed a significant increase in oxygenation, compliance of the respiratory system and a trend to an improved clinical outcome in patients receiving higher PEEP based on PL. Despite the interest of these results, it is not possible to discriminate whether the positive PL or the higher absolute PEEP values irrespectively of PES drove the observed results. A larger multicentre study with similar design is currently ongoing and will allow to refine more concrete conclusions (45).
Given that the directly-measured method allows to measure PL in the lung surrounding the esophagus, setting PEEP according to the directly-measured PES may allow to overcome the superimposed pressure in that specific area, which likely is at the edge between dependent and non-dependent lung regions and could be worthy recruiting to minimize the risk of recurrent alveolar opening and closure. Results from clinical trials will shed some light on this important clinical question.
The safety of such approach may be also limited by the risk of hyperinflation of the baby lung, which indeed is the most relevant mechanism of lung injury (46).
In addition, some controversies have been raised around the concept of the absolute value of PES for this titration, highlighting that it did not represent the lung weight measured by CT scan nor correlates with ARDS severity; importantly, PEEP set to achieve a positive PL according to this protocol provides settings that seem unrelated to lung recruitability (44,47).
The elastance derived method at end-inspiration
Regional overdistension is probably the key mediator of VILI and the global effect of PEEP and VT needs to be addressed with criteria assessing tidal hyperinflation (35,46). PL and the PES may help rate the degree of overdistension, as explained below.
Definition
The most accepted clinical method for measuring regional overdistention in the baby lung is the total inflation pressure (PPLAT) due to VT and PEEP. PPLAT and the change in pressure (driving pressure, ∆P) during tidal ventilation have been proposed to better assess this risk, as they respectively surrogate the measure of lung stress and dynamic strain.
PPLAT and ∆P are measured in the airways: PL at end-inspiration (PLend-insp) and lung driving pressure (∆PL) are the parameters representing the corresponding pressure loads in the lung independently from the effects of the chest wall.
Three approaches are available for computing PLend-insp:
-
The directly-measured method already discussed, using absolute values (17):
PLend-insp = PPLAT − PESend-insp
where PPLAT and PESend-insp are airway and esophageal pressure during an end-expiratory occlusion.
-
The release-derived method (47):
PLend-insp = (PPLAT − PESend-insp) + PESzeep
The release-derived PLend-exp represents the total amount of PL increase from ZEEP to PEEP.
-
The elastance-derived method: this method does not require the measurement of PES atmospheric pressure but simply the change in PES (33):
PLend-insp = PPLAT × (EL/ERS)
with EL and ERS respectively representing the EL and of the respiratory system. EL can be measured from changes in PL. ECW can also be calculated from PES and subsequently EL calculated as ERS – ECW.
EL = [(PPLAT – PESend-insp) – (PEEPTOT – PESend-exp)]/VT
ERS = (PPLAT – PEEPTOT)/VT
Given that
∆P = (PPLAT – PEEPTOT)
PLend-insp according to the elastance derived method can be also expressed as
PLend-insp = PPLAT × (∆PL/∆P)
According to the elastance- and release-derived methods, PL and PES are interpreted to partition the change in the elastic pressure of the respiratory system between the lung and the chest wall. Both these methods rely on the assumption that PL is 0 at atmospheric pressure, are highly correlated and consistently represent the total increase in lung stress due to PEEP and VT (i.e., static and dynamic) from atmospheric to the inflation pressure.
Conversely, the directly measured method provides significantly lower values, potentially underestimating the risk of overdistension (47).
Assessment of PLend-insp seems important to fully understand the effect of different ventilator settings and to stratify patients’ severity, in order to optimize interventions and define the need for rescue therapies (48).
A debate has arisen from the evidence that the direct measurement and the release-derived methods provide values that are not interchangeable (47,49). Recent preliminary data from our group suggest that both approaches may give interesting results for clinical application but with different meanings: in particular, the directly measured PLend-insp describes the actual PLend-insp in the area situated at the level of the esophagus. This is a region that is often collapsed, at risk for repeated closing and opening, and less exposed to the risk of overdistension than non-dependent regions. On the contrary, in non-obese patients, the elastance derived PLend-insp could surrogate the PL mostly in the non-dependent lung regions, which are most exposed to lung injury due to hyperinflation (29).
Application
The elastance-derived PLend-insp is the lung stress and is mathematically coupled to the ∆PL, being itself a surrogate of lung strain (50,51). Large datasets describing the epidemiology of PLend-insp are not available, but it could represent a novel tool to better target and determine the effects of mechanical ventilation during ARDS. It may potentially be also used during assisted ventilation to assess the risk of patient self-inflicted lung injury, since PPLAT and PLend-insp measurement seems feasible in such context (52,53).
Limiting elastance-derived PLend-insp lower than 20-25 cmH2O is probably a reasonable approach (21,33,48): unfortunately, setting PEEP to achieve a positive directly measured PLend-exp while keeping PLend-insp below 20–25 cmH2O is not always possible (49) when VT is set at 6 mL/kg IBW (45).
ΔPL
Definition
ΔPL, defined as the difference between PLend-insp and PLend-exp, stands for the VT-induced lung stress and reflects the distending pressure taken by the lungs when VT delivered. This parameter provides two potential advantages: first and similar to ∆P, ∆PL removes the stress caused by PEEP from transpulmonary PPLAT, which does not necessarily contribute to lung injury and sometimes can mitigate it (35). Second, ∆PL has removed the distending pressure taken by the chest wall from ∆P, which is barely relevant to the risk of VILI. Hence, it sounds reasonable to suspect that ∆PL might be better associated with the risk of VILI and even clinical outcomes than ∆P.
It is computed as:
| ∆P = (PPLAT − PEEPTOT) |
| ∆PL = (PPLAT – PESend-insp) – (PEEPTOT − PESend-exp) |
Application
A retrospective analysis on 56 patients by Baedorf Kassis et al. (54) suggested that ∆PL, after 24 h receiving two different PEEP strategies is associated with 28-day mortality. The ∆P demonstrated similar association with mortality in this interventional study. In an ongoing prospective, observational study for investigating epidemiology of respiratory mechanics in ARDS (NCT02623192), ∆P and ∆PL had similar statistical power and did not differ, suggested by receiver-operating-characteristic curve analysis (55).
From a physiological view, ∆PL represents lung stress and should be a better surrogate of lung strain comparing to ∆P. However, both the cardiac and pulmonary circulation effects of pleural pressure may also play a role in the outcome and are not represented by ∆PL. In addition, some studies suggested that the chest wall compliance is not so widely affected in patients with ARDS; the change in pleural pressure induced by VT is relatively similar among the majority of patients (23), so that the ∆P may be sufficient to represent the ∆PL in many circumstances (50).
Further physiological and epidemiological studies are required to thoroughly elucidate the potential association and/or causation between transpulmonary driving pressure, VILI and clinical outcomes.
Changes in PL may also be assessed during assisted breathing to take into account the combined effects of spontaneous breathing and of mechanical breaths on lung distension. Indeed, the pressure generated by the patient is added to the ventilator pressure. It was shown that under similar conditions of flow and volume, PL change is similar between controlled mechanical ventilation and pressure support ventilation. Spontaneous breathing during mechanical ventilation can cause remarkably negative swings in alveolar pressure, a mechanism by which spontaneous breathing might potentially induce lung injury on top of high changes in PL (52).
Limitations
Although PL provides useful information concerning respiratory physiology that may potentially help clinical decision making, a large observational study dealing with patients’ management in the clinical field recently showed that PES is monitored only in approximately 1% of ARDS patients (1). Even if esophageal manometry is an old technique, different aspects have hampered the widespread diffusion in the clinical setting.
Technical aspects
Recently, newer equipment facilitates the use in the clinical setting. Naso- or oro-gastric feeding tubes equipped with one or two balloons are now available to foster the clinical feasibility of esophageal manometry; similarly, some modern ventilators have been equipped with an auxiliary port connected to a pressure transducer that allows plugging an external pressure whose signal is displayed on the ventilator screen in phase with airway pressure and flow.
Signal validation
Assessment of PES requires accuracy in esophageal balloon positioning and precision in signal validation. In order to enhance the usefulness of PES measurement, techniques for in vivo calibration of the esophageal balloon taking into account intra-thoracic pressure and esophageal elastance have been recently reported and appear of interest (56,57). In particular, the optimal filling volume of the balloon may significantly differ from the one reported by the manufacturer (often reported for standing spontaneously breathing patients) and is dependent on the intrathoracic pressure, i.e., needing higher volumes in the supine patient. Balloon overinflation warrants complete transmission of PES swings, but is associated with significant elevation of absolute values; conversely, balloon underfilling generates incomplete transmission of PES swings (with consequent overestimation of lung elastance) and lower absolute values. To identify the optimal individual filling volume and obtain reliable measurements, an in vivo calibration is necessary. The full technique suggests to record static PES at end-expiration as the balloon is inflated with increasing volumes from 0 to 8 mL: afterwards, a pressure-volume curve of the balloon is generated and its intermediate linear section graphically identified. The limits of this intermediate section are the minimum and maximum filling volumes of the balloon, while the filling volume that generates the maximum change in PES during the occlusion test represents the best filling volume (57). More simply, finding the inflation volume which gives the largest tidal swing in PES usually allows to find the best filling volume (57).
Pleural pressure gradient and interpretation
PES reliably measures pleural pressure in the lung surrounding the esophagus. It may therefore underestimate pleural pressure of the dependent regions and overestimate pleural pressure of the nondependent zones (24). Accordingly, PL computed as the absolute difference between airway pressure and PES represents the PL at mid-chest. As already discussed, the elastance-derived method may give an estimate of the PL in the non-dependent baby lung, although these data are still preliminary and may not be applicable to wide categories of patients (obese, patients in the prone position) (29).
Clinical outcome
Respiratory mechanics measurements allow better stratify severity of illness and optimize ventilator settings (58). Data fostering the clinical usefulness of PES in supporting decision making during ARDS are limited to few studies, although the results appear encouraging (17,48). We recently reported that a bundle for the assessment of respiratory mechanics including esophageal manometry leads to significant adjustments in the ventilator settings in two thirds of ARDS patients, with the final effect of improving oxygenation and reducing the risk of overdistension at the same time (58).
Conclusions
In patients with ARDS, assessment of PL is a minimally invasive technique that allows accurate respiratory monitoring and better assessment of the physiological effects of mechanical ventilation.
Ongoing research will clarify whether and to what extent PL is more effective than airway pressure in stratifying patients’ severity, assessing the risk of VILI and predicting outcome. Preliminary data regarding its use to tailor ventilator settings appear encouraging but further adequately powered studies are warranted.
Acknowledgements
L Brochard is supported by the Keenan Chair in Critical Care Medicine and Acute Respiratory Failure.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 2.Laffey JG, Bellani G, Pham T, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 2016;42:1865-76. 10.1007/s00134-016-4571-5 [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Marini JJ, Pesenti A, et al. The "baby lung" became an adult. Intensive Care Med 2016;42:663-73. 10.1007/s00134-015-4200-8 [DOI] [PubMed] [Google Scholar]
- 4.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. New England Journal of Medicine 2013;369:2126-36. 10.1056/NEJMra1208707 [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS. History of Mechanical Ventilation. From Vesalius to Ventilator-induced Lung Injury. Am J Respir Crit Care Med 2015;191:1106-15. 10.1164/rccm.201503-0421PP [DOI] [PubMed] [Google Scholar]
- 6.ARDSnet Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 7.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2007;175:160-6. 10.1164/rccm.200607-915OC [DOI] [PubMed] [Google Scholar]
- 8.Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013;39:847-56. 10.1007/s00134-012-2787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. American Journal of Respiratory and Critical Care Medicine 2005;172:1241-5. 10.1164/rccm.200501-048CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanelli V, Ranieri MV, Mancebo J, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care 2016;20:36. 10.1186/s13054-016-1211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- 12.Ranieri VM, Brienza N, Santostasi S, et al. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med 1997;156:1082-91. 10.1164/ajrccm.156.4.97-01052 [DOI] [PubMed] [Google Scholar]
- 13.Pelosi P, Cereda M, Foti G, et al. Alterations of lung and chest wall mechanics in patients with acute lung injury: effects of positive end-expiratory pressure. Am J Respir Crit Care Med 1995;152:531-7. 10.1164/ajrccm.152.2.7633703 [DOI] [PubMed] [Google Scholar]
- 14.Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985) 2010;108:515-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinoni L, Chiumello D, Carlesso E, et al. Bench-to-bedside review: Chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Critical Care 2004;8:350-5. 10.1186/cc2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talmor D, Sarge T, O'Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 2006;34:1389-94. 10.1097/01.CCM.0000215515.49001.A2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. 10.1056/NEJMoa0708638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 1988;137:1159-64. 10.1164/ajrccm/137.5.1159 [DOI] [PubMed] [Google Scholar]
- 19.Cherniack RM, Farhi LE, Armstrong BW, et al. A comparison of esophageal and intrapleural pressure in man. J Appl Physiol 1955;8:203-11. [DOI] [PubMed] [Google Scholar]
- 20.Milic-Emili J, Mead J, Turner JM, et al. Improved Technique for Estimating Pleural Pressure from Esophageal Balloons. J Appl Physiol 1964;19:207-11. [DOI] [PubMed] [Google Scholar]
- 21.Mauri T, Yoshida T, Bellani G, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016;42:1360-73. 10.1007/s00134-016-4400-x [DOI] [PubMed] [Google Scholar]
- 22.Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189:520-31. 10.1164/rccm.201312-2193CI [DOI] [PubMed] [Google Scholar]
- 23.Henderson WR, Chen L, Amato MB, et al. Fifty Years of Research in ARDS. Respiratory Mechanics in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017. [Epub ahead of print]. 10.1164/rccm.201612-2495CI [DOI] [PubMed] [Google Scholar]
- 24.Pelosi P, Goldner M, McKibben A, et al. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001;164:122-30. 10.1164/ajrccm.164.1.2007010 [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Del Sorbo L, Grieco DL, et al. Airway Closure in Acute Respiratory Distress Syndrome: An Underestimated and Misinterpreted Phenomenon. Am J Respir Crit Care Med 2017. [Epub ahead of print]. 10.1164/rccm.201702-0388LE [DOI] [PubMed] [Google Scholar]
- 26.Baydur A, Behrakis PK, Zin WA, et al. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 1982;126:788-91. [DOI] [PubMed] [Google Scholar]
- 27.Agostoni E. Mechanics of the pleural space. Physiol Rev 1972;52:57-128. [DOI] [PubMed] [Google Scholar]
- 28.Agostoni E, D'Angelo E. Pleural liquid pressure. J Appl Physiol (1985) 1991;71:393-403. [DOI] [PubMed] [Google Scholar]
- 29.Grieco DL, Richard JC, Delisle S, et al. Oesophageal And Directly Measured Pleural Pressure: A Validation Study On Thiel Cadavers. Am J Respir Crit Care Med 2017;195:A3701. [Google Scholar]
- 30.Guérin C, Richard JC. Comparison of 2 correction methods for absolute values of esophageal pressure in subjects with acute hypoxemic respiratory failure, mechanically ventilated in the ICU. Respir Care 2012;57:2045-51. [DOI] [PubMed] [Google Scholar]
- 31.Richard JC, Maggiore SM, Jonson B, et al. Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med 2001;163:1609-13. 10.1164/ajrccm.163.7.2004215 [DOI] [PubMed] [Google Scholar]
- 32.Maggiore SM, Jonson B, Richard JC, et al. Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med 2001;164:795-801. 10.1164/ajrccm.164.5.2006071 [DOI] [PubMed] [Google Scholar]
- 33.Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2008;178:346-55. 10.1164/rccm.200710-1589OC [DOI] [PubMed] [Google Scholar]
- 34.Protti A, Cressoni M, Santini A, et al. Lung stress and strain during mechanical ventilation any safe threshold? American Journal of Respiratory and Critical Care Medicine 2011;183:1354-62. 10.1164/rccm.201010-1757OC [DOI] [PubMed] [Google Scholar]
- 35.Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: Any difference between statics and dynamics? Critical Care Medicine 2013;41:1046-55. 10.1097/CCM.0b013e31827417a6 [DOI] [PubMed] [Google Scholar]
- 36.Grasso S, Terragni P, Mascia L, et al. Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med 2004;32:1018-27. 10.1097/01.CCM.0000120059.94009.AD [DOI] [PubMed] [Google Scholar]
- 37.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. 10.1001/jama.299.6.646 [DOI] [PubMed] [Google Scholar]
- 38.Kacmarek RM, Villar J, Sulemanji D, et al. Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial. Crit Care Med 2016;44:32-42. 10.1097/CCM.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 39.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. 10.1056/NEJMoa032193 [DOI] [PubMed] [Google Scholar]
- 40.Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. 10.1001/jama.299.6.637 [DOI] [PubMed] [Google Scholar]
- 41.Grasso S, Stripoli T, Sacchi M, et al. Inhomogeneity of lung parenchyma during the open lung strategy a computed tomography scan study. American Journal of Respiratory and Critical Care Medicine 2009;180:415-23. 10.1164/rccm.200901-0156OC [DOI] [PubMed] [Google Scholar]
- 42.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 43.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. 10.1056/NEJMoa052052 [DOI] [PubMed] [Google Scholar]
- 44.Chiumello D, Cressoni M, Carlesso E, et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med 2014;42:252-64. 10.1097/CCM.0b013e3182a6384f [DOI] [PubMed] [Google Scholar]
- 45.Fish E, Novack V, Banner-Goodspeed VM, et al. The Esophageal Pressure-Guided Ventilation 2 (EPVent2) trial protocol: a multicentre, randomised clinical trial of mechanical ventilation guided by transpulmonary pressure. BMJ Open 2014;4:e006356. 10.1136/bmjopen-2014-006356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Güldner A, Braune A, Ball L, et al. Comparative Effects of Volutrauma and Atelectrauma on Lung Inflammation in Experimental Acute Respiratory Distress Syndrome. Crit Care Med 2016;44:e854-65. 10.1097/CCM.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiumello D, Cressoni M, Colombo A, et al. The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014;40:1670-8. 10.1007/s00134-014-3415-4 [DOI] [PubMed] [Google Scholar]
- 48.Grasso S, Terragni P, Birocco A, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012;38:395-403. 10.1007/s00134-012-2490-7 [DOI] [PubMed] [Google Scholar]
- 49.Gulati G, Novero A, Loring SH, et al. Pleural Pressure and Optimal Positive End-Expiratory Pressure Based on Esophageal Pressure Versus Chest Wall Elastance: Incompatible Results. Crit Care Med 2013;41:1951-7. 10.1097/CCM.0b013e31828a3de5 [DOI] [PubMed] [Google Scholar]
- 50.Chiumello D, Carlesso E, Brioni M, et al. Airway driving pressure and lung stress in ARDS patients. Crit Care 2016;20:276. 10.1186/s13054-016-1446-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grieco DL, Chen L, Dres M, et al. Should we use driving pressure to set tidal volume? Curr Opin Crit Care 2017;23:38-44. 10.1097/MCC.0000000000000377 [DOI] [PubMed] [Google Scholar]
- 52.Bellani G, Grasselli G, Teggia-Droghi M, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care 2016;20:142. 10.1186/s13054-016-1290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195:438-42. 10.1164/rccm.201605-1081CP [DOI] [PubMed] [Google Scholar]
- 54.Baedorf Kassis E, Loring SH, Talmor D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 2016;42:1206-13. 10.1007/s00134-016-4403-7 [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Xu M, Chen GQ, et al. Respiratory Mechanics in Acute Respiratory Distress Syndrome: Variables and Indexes Associated with Clinical Outcome. Am J Respir Crit Care Med 2016;193:A1839. [Google Scholar]
- 56.Mojoli F, Chiumello D, Pozzi M, et al. Esophageal pressure measurements under different conditions of intrathoracic pressure. An in vitro study of second generation balloon catheters. Minerva Anestesiol 2015;81:855-64. [PubMed] [Google Scholar]
- 57.Mojoli F, Iotti GA, Torriglia F, et al. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care 2016;20:98. 10.1186/s13054-016-1278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Chen GQ, Shore K, et al. Implementing a bedside assessment of respiratory mechanics in patients with acute respiratory distress syndrome. Crit Care 2017;21:84. 10.1186/s13054-017-1671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]