Abstract
Hypercholesterolemia and hypertension are among the most important risk factors for cardiovascular (CV) disease. They are also important contributors to metabolic diseases including diabetes that further increase CV risk. Updated guidelines emphasize targeted reduction of overall CV risks but do not explicitly incorporate potential adverse metabolic outcomes that also influence CV health. Hypercholesterolemia and hypertension have synergistic deleterious effects on interrelated insulin resistance and endothelial dysfunction. Dysregulation of the renin-angiotensin system is an important pathophysiological mechanism linking insulin resistance and endothelial dysfunction to atherogenesis. Statins are the reference standard treatment to prevent CV disease in patients with hypercholesterolemia. Statins work best for secondary CV prevention. Unfortunately, most statin therapies dose-dependently cause insulin resistance, increase new onset diabetes risk and exacerbate existing type 2 diabetes mellitus. Pravastatin is often too weak to achieve target low-density lipoprotein cholesterol levels despite having beneficial metabolic actions. Renin-angiotensin system inhibitors improve both endothelial dysfunction and insulin resistance in addition to controlling blood pressure. In this regard, combined statin-based and renin-angiotensin system (RAS) inhibitor therapies demonstrate additive/synergistic beneficial effects on endothelial dysfunction, insulin resistance, and other metabolic parameters in addition to lowering both cholesterol levels and blood pressure. This combined therapy simultaneously reduces CV events when compared to either drug type used as monotherapy. This is mediated by both separate and interrelated mechanisms. Therefore, statin-based therapy combined with RAS inhibitors is important for developing optimal management strategies in patients with hypertension, hypercholesterolemia, diabetes, metabolic syndrome, or obesity. This combined therapy can help prevent or treat CV disease while minimizing adverse metabolic consequences.
Keywords: Hypercholesterolemia, Hypertension, Statins, Renin-angiotensin system inhibitors, Cardiovascular disease
Introduction
Hypertension and/or hypercholesterolemia are among the most important risk factors for cardiovascular (CV) disease, the leading cause of death in developed nations. The new USA guidelines target reducing overall cardiovascular risks but do not explicitly consider adverse metabolic actions of statins that may promote additional CV risk.1),2) Atherosclerosis plays a pivotal role in the pathogenesis of CV disease. Endothelial dysfunction and insulin resistance are mechanistically interrelated through insulin signaling and contribute to the pathogenesis of atherosclerosis. Hypercholesterolemia and hypertension are both associated with endothelial dysfunction and insulin resistance and their coexistence is a vicious cycle that increases CV disease incidence.
Statins prevent CV disease by lowering low-density lipoprotein (LDL) cholesterol, improving endothelial dysfunction, and have other anti-atherosclerotic effects.3),4),5) Recently published hypertension guidelines state that diuretics, beta-blockers, calcium antagonists, angiotensin converting enzyme (ACE) inhibitors and angiotensin II type I (AT1) receptor blockers (ARBs) are equally recommended for the initiation and maintenance of anti-hypertensive treatment. However, various classes of anti-hypertensive drugs have differential impacts on insulin sensitivity despite similar blood pressure reduction. Only some classes of these drugs, including ACE inhibitors and ARBs, ameliorate insulin resistance.6) The renin-angiotensin system (RAS) is involved in many atherosclerosis steps and also modulates insulin action. Angiotensin II promotes superoxide anion generation and endothelial dysfunction. Angiotensin II activates nuclear transcription factor induced by oxidative stress, mediated by AT1 receptors.7),8),9) We reported that candesartan significantly improved flow-mediated vasodilation and reduced biomarkers of oxidant stress, inflammation, and hemostasis in patients with hypertension, independent of blood pressure reduction.10) ACE inhibitors and ARBs also significantly reduced insulin resistance, thus improved metabolic outcomes in diabetes with a further secondary benefit for CV risk.
Whether statin benefits to cardiovascular status outweigh non-cardiovascular harm in patients above a certain threshold of cardiovascular risk remains untested, especially when comparing similar levels of CV risk and lipid lowering in the absence or presence of adverse metabolic outcomes that secondarily increase CV risk. Indeed, ideal therapy would simultaneously lower LDL cholesterol to target levels while reducing instead of increasing the risk for new onset diabetes and progression of existing diabetes. Statins attenuate increases in cardiorespiratory fitness and skeletal muscle mitochondrial content when combined with exercise training in overweight or obese patients at risk for metabolic syndrome.11) Statin use is associated with modestly lower physical activity among community-living men, even after accounting for medical history and other potential confounding factors.12) Muscle pain, fatigue, and weakness are common adverse side effects of statin medications. Importantly, we have demonstrated that statin therapy dose-dependently caused insulin resistance and increased the risk for type 2 diabetes mellitus.13),14) Interestingly, we observed that statin-based combination treatment with ACE inhibitors or ARBs improved metabolic outcomes and had additive and/or synergistic effects in changing blood pressure, lipid profiles, endothelial dysfunction, inflammation, and hemostasis by both separate and interrelated mechanisms15),16),17) that may help explain outcomes in recent clinical trials.18),19),20),21) The HOPE-3 study examined 12705 subjects with at least one known CV risk factor, but who had not been diagnosed with CV disease (at intermediate risk). Participants were randomly assigned to one of four groups: rosuvastatin 10 mg plus a combination pill of candesartan 16 mg and hydrocholothiazide 12.5 mg daily, rosuvastatin 10 mg plus a placebo daily, a placebo plus the combination pill daily, or two placebo pills daily. Over 5.6 years of follow-up, CV death, myocardial infarction or stroke occurred in 3.5 percent of patients receiving both drugs and in 5 percent of patients receiving only placebo. The relative risk reduction in those taking both drugs was 30 percent overall, 40 percent in those with hypertension and 20 percent in those without hypertension.21) Here, we discuss the promising treatment strategies of statins-based combination treatment with ACE inhibitors or ARBs in patients to ameliorate risk of both CV disease and diabetes mellitus simultaneously.
Effects of Statin Therapy on Insulin Resistance
Statin therapy is very important for decreasing CV morbidity and mortality especially in secondary prevention.2),18) Unfortunately high dose potent statins cause adverse metabolic effects including worse insulin sensitivity and glucose intolerance that contribute to increased risk of new onset diabetes and progression of existing diabetes.13),14) Pravastatin is the only statin which has beneficial metabolic actions at equal lipid lowering doses with rosuvastatin or simvastatin.22) It is possible that lipophilic statins are taken up by the brain and fat tissue where they may cause unfavorable pleiotropic effects including secondary actions on the regulation of insulin secretion and exacerbation of insulin resistance. By contrast, the hydrophilic statin, pravastatin improved insulin sensitivity and increased circulating adiponectin levels in humans that might have beneficial metabolic effects as well as atherogenic reduction.22),23),24) Pravastatin is the only statin that requires a transporter to get across the cell membrane. Even rosuvastatin which is relatively hydrophilic (but still lipophilic relative to pravastatin) does not need a transporter to cross the cell membrane.
Rosuvastatin is less hydrophilic than pravastatin. We have demonstrated rosuvastatin treatment significantly increased fasting insulin levels, decreased plasma adiponectin levels and worsened insulin sensitivity and glucose control, while pravastatin treatment had opposite beneficial effects in hypercholesterolemic patients.22) Diabetes onset and progression related to statin use has not been shown to increase CV events because appropriately controlled outcome studies have not been performed. Nonetheless, long-term adverse effects of new-onset diabetes mellitus and accelerated diabetes progression may generate a relative increase in CV and all cause morbidity or mortality when compared with equal lipid lowering in the absence of adverse metabolic actions.25) Indeed, a recent large-scale randomized clinical trial strongly supports this concept. When compared to placebo, rosuvastatin treatment increased the incidence of diabetes mellitus by 28% in individuals with one or more risk factors for diabetes, but reduced CV events by 39%. By contrast, in individuals with no major diabetes risk factors, rosuvastatin treatment did not cause diabetes mellitus and reduced CV events by 52%: 13% more than the former group.26) This may just be due to the fact that it is easier to detect adverse metabolic outcomes in patients with more risk factors. Indeed, in the first large outcome study where the primary outcome for statin therapy was prospectively designated as increased risk of diabetes, there was both a significant time and dose-dependent increase in metabolic risk for all statins except pravastatin. Moreover, the average increased risk of statin therapy for diabetes was huge (46%) in a cohort of 8749 patients with the metabolic syndrome.25) Further, the physiological mechanisms evaluated in this study supported our previous studies suggesting the major cause of statin-induced diabetes was increased insulin resistance with a minor component of reduced insulin secretion.13),14),22) Two clinical genomic studies clearly demonstrated that exposure to LDL cholesterol-lowering genetic variants was associated with a higher risk of type 2 diabetes despite a significant reduction in coronary artery disease risk.27),28) Furthermore, the effects of these variants were independent and additive.28) These data provide insights into potential adverse effects of LDL cholesterol-lowering therapy. Although, the increased risk of type 2 diabetes appeared confined to persons with impaired fasting glucose levels, it might simply have been more difficult to detect increased diabetes risk in the absence of initial metabolic abnormalities.28) It is likely that previous large outcome studies of statin therapy underestimated the magnitude of metabolic risk because they did not have diabetes onset as a primary study outcome, and the patient population was different with fewer initial metabolic risks.
Insulin Resistance Associated with ACE Inhibitors or ARBs
Insulin resistance plays a pivotal role in hypertension, hypercholesterolemia, and atherosclerosis. About half of hypertensive individuals are hyperinsulinemic with insulin resistance, and up to three fourths of people with type 2 diabetes have hypertension. The prevalence of dyslipidemia is more than double in hypertensive patients when compared with a normotensive population.8)
When compared with other anti-hypertensive drugs, ACE inhibitors and ARBs improved insulin sensitivity in hypertensive patients.7) RAS also has multiple effects in the central nervous system, skeletal muscle, liver, and adipose tissue that may interfere with insulin action. Thus, RAS dysregulation may contribute to the evolution of insulin resistance. Conversely, RAS blockade may potentially help prevent new-onset diabetes. Indeed, RAS blockade directly augmented insulin-stimulated glucose uptake, promoted adipogenesis, and induced peroxisome proliferator-activated receptor-γ activity that promoted adipocyte differentiation.10) ACE inhibitors and ARBs are associated with reductions in the incidence of new-onset diabetes independent of blood pressure reduction.29)
Another important benefit of RAS inhibitors is that they may attenuate vascular complications associated with insulin resistance.30) In the milieu of insulin resistance, the CV system is sensitized to adverse RAS tropic effects, evidenced by the frequent occurrence of diffuse vascular disease and left ventricular hypertrophy in diabetic patients, even when lipid and blood pressure levels are normal. High insulin levels stimulate AT1 receptors, which activate RAS and also activate the cardiac sympathetic nervous system.10) Accordingly, ACE inhibitors and ARBs reverse endothelial dysfunction and reduce oxidant stress and inflammatory cytokines, suggesting that ACE inhibitors and ARBs have anti-atherogenic effects in hypertensive patients that contribute to reduction of insulin resistance.
Statin-Based Combined Treatment with ACE Inhibitors or ARBs to Maximize Cardiovascular Protection
Endothelial dysfunction and insulin resistance play crucial roles in the pathogenesis of atherosclerosis. Importantly, elevated levels of free fatty acids associated with insulin resistance, obesity, diabetes mellitus, and the metabolic syndrome cause endothelial dysfunction by activating innate immune inflammatory pathways upstream of NF-κB. Thus, inflammation and oxidative stress contribute to endothelial dysfunction and insulin resistance while endothelial dysfunction and insulin resistance promote oxidative stress and inflammation.8),9),10)
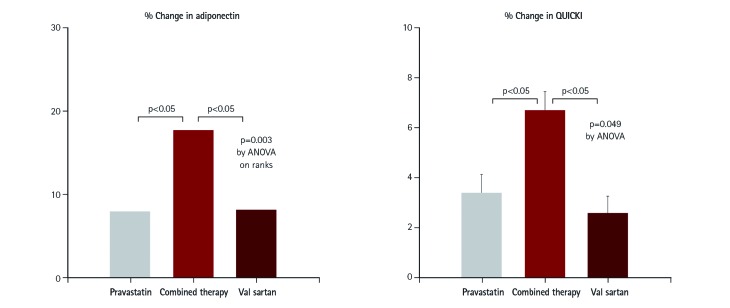
Of note, experimental and clinical studies demonstrate crosstalk between hypercholesterolemia and RAS at multiple levels. Hypercholesterolemic rabbits display enhanced vascular expression of AT1 receptors that mediate increased angiotensin II activity, thus increasing blood pressure.31) Statins reverse the blood pressure elevating response to angiotensin II infusion by decreasing AT1 receptor density.32) Therefore, statins and ACE inhibitors or ARBs may have the potential to exert additive/synergistic beneficial effects on both endothelial function and insulin sensitivity when compared to monotherapy in patients with cardiovascular risk factors affected by both separate and interrelated mechanisms (Fig. 1).8),9),10) We reported additive beneficial vascular effects of statin and ACE inhibitors or ARB combined therapy when compared with monotherapy alone.15),16) Combination therapy also showed a metabolic benefit due to ACE inhibitors or ARBs therapy. Recently, we observed that pravastatin combined with valsartan therapy had additive beneficial effects on endothelial dysfunction and simultaneously had additive beneficial effects that increased plasma adiponectin, lowered fasting insulin levels, and improved insulin sensitivity when compared with monotherapy alone in a hypertensive population (Fig. 2).17)
Fig. 1. Synergistic effect of statins and angiotensin-receptor blocker on insulin sensitivity. In 48 hypercholesterolemic patients, both pravastatin 40 mg and valsartan 160 mg increased plasma adiponectin levels, reduced fasting insulin levels, and increased insulin sensitivity (QUICKI) relative to baseline measurements. When pravastatin was combined with valsartan, their response increased in an additive manner when compared with monotherapy alone. Median values (adiponectin) or mean with SEM (QUICKI) are provided. Modified from Koh et al.17) QUICKI: quantitative insulin sensitivity check index, SEM: standard error of the mean. ANOVA: analysis of variance.
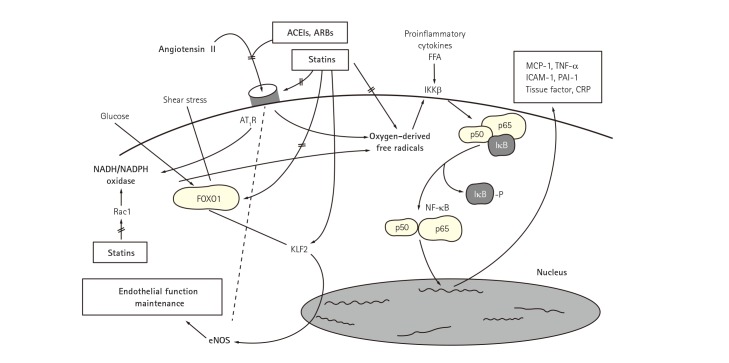
Fig. 2. Synergistic effect of statins and ACEIs or ARBs for insulin resistance and endothelial dysfunction. Dysregulation of the RAS contributes to the pathogenesis of atherosclerosis. Angiotensin II binds to AT1R resulting in enzymatic production of oxygen-derived free radicals. FFA also promote oxygenderived free radical generation in vascular endothelial cells and smooth muscle cells. This leads to dissociation of inhibitory factor with subsequent activation of NF-κB which stimulates expression of proinflammatory genes, chemokines, and cytokines. Importantly, elevated levels of FFA associated with insulin resistance, obesity, diabetes mellitus, and the metabolic syndrome cause endothelial dysfunction by activating innate immune inflammatory pathways upstream of NF-κB. Thus, inflammation and oxidative stress contribute to endothelial dysfunction and insulin resistance while endothelial dysfunction and insulin resistance promote oxidative stress and inflammation. These have shown the reciprocal relationships between insulin resistance and endothelial dysfunction. Statins down-regulate the expression of AT1R via reducing lowering low-density lipoprotein-cholesterol levels. KLF2 is implicated as a key molecule maintaining endothelial function. High glucose-induced, FOXO1-mediated KLF2 suppression was reversed by statin treatment. Further, experimental studies have shown a cross-talk between hypercholesterolemia and RAS at multiple steps. Accordingly, combined therapy with statins and RAS inhibitors show additive/synergistic beneficial effects on endothelial dysfunction and insulin resistance when compared with monotherapy in patients with cardiovascular risk factors by both distinct and interrelated mechanisms. Reproduced with permission from Koh et al.8),9),35),36),37) ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin-receptor blocker, FFA: free fatty acids, MCP: monocyte chemotactic protein, TNF: tumor necrosis factor, ICAM: intercellular adhesion molecule, PAI: plasminogen activator inhibitor, CRP: C-reactive protein, AT1R: angiotensin II type I receptor, IKKB: inhibitor of nuclear factor kappa B kinase subunit, NADH/NADPH: nicotinamide dehydrogenase/nicotinamide diphosphate dehydrogenase, FOXO1: forkhead box protein O1, Rac1: Ras-related C3 botulinum toxin substrate 1, KLF2: Kruppel-like factor 2, RAS: renin-angiotensin system, NF-κB: nuclear transcription factor, eNOS: endothelial nitric oxide synthase.
Indeed, large-scale clinical studies assessed the ‘synergy’ of statins and ACE inhibitors in reducing vascular events in patients with coronary heart disease during a 3-year follow-up. The statin and ACE inhibitor combination reduced CV events more than statin monotherapy and considerably more than ACE inhibitor monotherapy.19) This benefit may be related to combined beneficial effects on endothelial function, vascular inflammation and the initiation, progression and rupture of atheromatous plaques.20) The HOPE-3 trial, designed to focus on preventing CV disease before it starts, was the first to assess outcomes of preventative combination treatment with statin and ARB drugs in a large, globally diverse population at intermediate risk for developing CV disease.21)
Clinical Implication
From 1988-1994 to 2005-2010, the control rate of concomitant hypertension and LDL cholesterol rose from 5.0 to 30.7%. Multivariate logistic regression showed the most important contributing factors to concomitant hypertension, LDL cholesterol, and non-high-density lipoprotein cholesterol control were statin (10.7) and anti-hypertensive (3.32) medications. Of note, 69.3% of hypertensive hypercholesterolemic patients failed to be concomitantly controlled in 2005–2010.33) Further, various strategies to reduce residual CV disease risk in hypertensive patients using different classes of anti-hypertensive medications reduced CV disease risk by only 20 to 25%.1) However, controlling hypercholesterolemia in hypertensive patients with statins reduced residual CV disease risk by 35 to 40%.2) These data suggest that effective combined treatment of both hypertension and hypercholesterolemia reduced CV disease risk by ≥50%. Thus, statins are paramount of importance for reducing CV disease risk.2) Unfortunately, most hypertension guidelines currently focus on which single agents to use and what blood pressure to aim for without emphasizing the potential benefits of combination treatment in patients with hypertension.
There is strong scientific evidence obtained from experimental and clinical studies that supports combined statin and ACE inhibitor or ARB therapy to optimize CV protection in high risk patients while minimizing metabolic risk. Hypercholesterolemia and hypertension share common pathophysiologies such as endothelial dysfunction and insulin resistance, and both are common risk factors for CV disease. Indeed, more than 60% of hypertensives are hypercholesterolemic. Alternate classes of antihypertensive medications in hypertensive patients do not substantially reduce residual CV disease risk. However, treating moderate cholesterol elevations with low-dose statins reduces CV disease by 35% to 40%. Therefore, statins are very important.34) However, statin therapy alone dose-dependently causes insulin resistance and increases the risk of type 2 diabetes mellitus. On the other hand, ACE inhibitors or ARBs improve both endothelial dysfunction and insulin resistance in addition to lowering blood pressure by mechanisms distinct from those of statins. Of interest, cross-talk between hypercholesterolemia and RAS exists at multiple levels of insulin resistance and endothelial dysfunction. Combined therapy with statins and ACE inhibitors or ARBs demonstrate additive/synergistic beneficial effects on endothelial function and insulin sensitivity in addition to lowering both cholesterol levels and blood pressure when compared with either monotherapy in patients with CV risk factors. This is mediated by both distinct and interrelated mechanisms. Therefore, there is a strong scientific rationale for recommending statins combined with ACE inhibitors or ARBs therapy to treat or prevent CV disease in patients with hypertension, hypercholesterolemia, diabetes, metabolic syndrome, or obesity to optimize reduction in CV risk while avoiding further metabolic dysregulation which itself raises CV risk.8),35),36),37) Combination therapy with statins and other classes of drugs such as ACE inhibitors or ARBs is a promising approach to maximize therapeutic benefit while reducing inherent metabolic risks of potent statins.
Now we propose a simple but practical recommendation regarding combining potent statin therapy with other drugs to optimize simultaneous CV and metabolic benefits while minimizing adverse events. For patients with acute coronary syndrome, high doses of potent statins such as atorvastatin or rosuvastatin +/− ezetimibe are recommended because the impact of CV events is likely to be greater than additional diabetogenic or other risks. If patients stabilize after 3 months or in patients with stable angina, optimal doses of statins +/− ezetimibe combined with RAS blockades or peroxisome proliferator-activated receptor α agonists are recommended. For the primary prevention of heart disease, if patients do not have risk factors for diabetes such as impaired glucose tolerance, obesity, or metabolic syndrome, we recommend low (for Asian) or optimal (for Caucasian) dose statins alone; if patients do not have risk factors for diabetes we recommend statins with beneficial metabolic actions such as pravastatin. However, if patients have risk factors for diabetes, we recommend low or optimal dose statins +/− ezetimibe combined with RAS blockades or peroxisome proliferator-activated receptor α agonists to reduce the diabetogenic effect of statins. These treatment guidelines might provide the beneficial effects of lowering LDL cholesterol while minimizing adverse outcomes from high-dose statin administration for CV disease amelioration (Table 1).36),37),38),39),40),41),42),43),44),45),46),47),48)
Table 1. Statins guideline to maximize cardio metabolic benefits.
| Primary prevention | Secondary prevention |
|---|---|
| Without risk factors* for diabetes: low (for Asian) or optimal (for Caucasian) dose statins alone; statins with beneficial metabolic actions such as pravastatin | In acute coronary syndrome state; potent, high dose statins +/− ezetimibe because cardiovascular benefits of statins exveed diabetogenic or other risks |
| With risk factors for diabetes: low or optimal dose statins +/− ezetimibe combined with RASS blockades or PPARα agonists to reduce diabetogenic effect of statins | In stable coronary artery diseaseddiseases: optimal dose statins +/- ezetimibe combined with RAAS blockades or PPARα agonists |
*Impaired glucose tolerance, obesity, metabolic syndrome individuals should lose weight and take regular physical exercise. RAAS: renin-angiotensin-aldosterone system, PPAR: peroxisome proliferator-activated receptor
Acknowledgments
Dr. Koh holds a certificate of patent, 10-1579656 (pravastatin+valsartan).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Go AS, Bauman MA, Coleman King SM, et al. An effective approach to high blood pressure control a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. J Am Coll Cardiol. 2014;63:1230–1238. doi: 10.1016/j.jacc.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 3.Koh KK. Effects of statins on vascular wall: Vasomotor function, inflammation, and plaque stability. Cardiovasc Res. 2000;47:648–657. doi: 10.1016/s0008-6363(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim JW, Yun KH, Kim EK, et al. Effect of high dose rosuvastatin loading before primary percutaneous coronary intervention on infarct size in patients with ST-segment elevation myocardial infarction. Korean Circ J. 2014;44:76–81. doi: 10.4070/kcj.2014.44.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang JY, Lee SH, Kim BS, et al. Additive beneficial effects of valsartan combined with rosuvastatin in the treatment of hypercholesterolemic hypertensive patients. Korean Circ J. 2015;45:225–233. doi: 10.4070/kcj.2015.45.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh KK, Quon MJ, Han SH, et al. Distinct vascular and metabolic effects of different classes of anti-hypertensive drugs. Int J Cardiol. 2010;140:73–81. doi: 10.1016/j.ijcard.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh KK, Han SH, Oh PC, Shin EK, Quon MJ. Combination therapy for treatment or prevention of atherosclerosis: focus on the lipid-RAAS interaction. Atherosclerosis. 2010;209:307–313. doi: 10.1016/j.atherosclerosis.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, Sakuma I, Ihm SH, Goh CW, Koh KK. Statins and renin-angiotensin system inhibitor combination treatment to prevent cardiovascular disease. Circ J. 2014;78:281–287. doi: 10.1253/circj.cj-13-1494. [DOI] [PubMed] [Google Scholar]
- 9.Koh KK, Sakuma I, Hayashi T, Kim SH, Chung WJ. Renin-angiotensin system inhibitor and statins combination therapeutics - what have we learnt? Expert Opin Pharmacother. 2015;16:949–953. doi: 10.1517/14656566.2015.1019464. [DOI] [PubMed] [Google Scholar]
- 10.Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–910. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- 11.Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62:709–714. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DS, Markwardt S, Goeres L, et al. Statins and physical activity in older men: the osteoporotic fractures in men study. JAMA Intern Med. 2014;174:1263–1270. doi: 10.1001/jamainternmed.2014.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh KK, Quon MJ, Han SH, et al. Simvastatin improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Diabetes Care. 2008;31:776–782. doi: 10.2337/dc07-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55:1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 16.Koh KK, Quon MJ, Han SH, et al. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005;45:1088–1093. doi: 10.1161/01.HYP.0000166722.91714.ba. [DOI] [PubMed] [Google Scholar]
- 17.Koh KK, Lim S, Choi H, et al. Combination pravastatin and valsartan treatment has additive beneficial effects to simultaneously improve both metabolic and cardiovascular phenotypes beyond that of monotherapy with either drug in patients with primary hypercholesterolemia. Diabetes. 2013;62:3547–3552. doi: 10.2337/db13-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim S, Sakuma I, Quon MJ, Koh KK. Potentially important considerations in choosing specific statin treatments to reduce overall morbidity and mortality. Int J Cardiol. 2013;167:1696–1702. doi: 10.1016/j.ijcard.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. Effect of statins and ACE inhibitors alone and in combination on clinical outcome in patients with coronary heart disease. J Hum Hypertens. 2004;18:781–788. doi: 10.1038/sj.jhh.1001748. [DOI] [PubMed] [Google Scholar]
- 20.Athyros VG, Katsiki N, Karagiannis A, Mikhailidis DP. Combination of statin plus renin angiotensin system inhibition for the prevention or the treatment of atherosclerotic cardiovascular disease. Curr Pharm Des. 2014;20:6299–6305. doi: 10.2174/1381612820666140620115756. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–2043. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 22.Koh KK, Quon MJ, Sakuma I, et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int J Cardiol. 2013;166:509–515. doi: 10.1016/j.ijcard.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215:1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Sakuma I, Quon MJ, Koh KK. Differential metabolic actions of specific statins: clinical and therapeutic considerations. Antioxid Redox Signal. 2014;20:1286–1299. doi: 10.1089/ars.2013.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cederberg H, Stanč áková A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia. 2015;58:1109–1117. doi: 10.1007/s00125-015-3528-5. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotta LA, Sharp SJ, Burgess S, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA. 2016;316:1383–1391. doi: 10.1001/jama.2016.14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–2153. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 29.Bavishi C, Bangalore S, Messerli FH. Renin angiotensin aldosterone system inhibitors in hypertension: is there evidence for benefit independent of blood pressure reduction? Prog Cardiovasc Dis. 2016;59:253–261. doi: 10.1016/j.pcad.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Oh PC, Sakuma I, Hayashi T, Koh KK. Angiotensin converting enzyme inhibitors remain the first treatment of choice. Korean J Intern Med. 2016;31:237–241. doi: 10.3904/kjim.2016.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickenig G, Sachinidis A, Michaelsen F, Böhm M, Seewald S, Vetter H. Upregulation of vascular angiotensin II receptor gene expression by low-density lipoprotein in vascular smooth muscle cells. Circulation. 1997;95:473–478. doi: 10.1161/01.cir.95.2.473. [DOI] [PubMed] [Google Scholar]
- 32.Nickenig G, Bäumer AT, Temur Y, Kebben D, Jockenhövel F, Böhm M. Statin-sensitive dysregulated AT1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 33.Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients: national health and nutrition examination surveys 1988-2010. Circulation. 2013;128:29–41. doi: 10.1161/CIRCULATIONAHA.112.000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 35.Koh KK, Lim S, Sakuma I, Quon MJ. Caveats to aggressive lowering of lipids by specific statins. Int J Cardiol. 2012;154:97–101. doi: 10.1016/j.ijcard.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Lim S, Oh PC, Sakuma I, Koh KK. How to balance cardiorenometabolic benefits and risks of statins. Atherosclerosis. 2014;235:644–648. doi: 10.1016/j.atherosclerosis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Koh KK. Is it not timely to consider how to balance cardiorenometabolic benefits and risks of statins? J Am Coll Cardiol. 2014;63(25 Pt A):2880–2881. doi: 10.1016/j.jacc.2014.01.080. [DOI] [PubMed] [Google Scholar]
- 38.Koh KK, Han SH, Quon MJ, Yeal Ahn J, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28:1419–1424. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 39.Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of patients with combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649–1653. doi: 10.1016/j.jacc.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 40.Lee BS, Choi JY, Kim JY, Han SH, Park JE. Simvastatin and losartan differentially and synergistically inhibit atherosclerosis in apolipoprotein e(-/-) mice. Korean Circ J. 2012;42:543–550. doi: 10.4070/kcj.2012.42.8.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh KK. How to control residual risk during statin era? J Am Coll Cardiol. 2015;66:1848. doi: 10.1016/j.jacc.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 42.Koh KK, Oh PC, Sakuma I, et al. Vascular and metabolic effects of ezetimibe combined with simvastatin in patients with hypercholesterolemia. Int J Cardiol. 2015;199:126–131. doi: 10.1016/j.ijcard.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Koh KK. Intriguing off-target effects of ezetimibe. J Am Coll Cardiol. 2015;66:2808. doi: 10.1016/j.jacc.2015.08.1130. [DOI] [PubMed] [Google Scholar]
- 44.Koh KK, Oh PC, Sakuma I, Lee Y, Han SH, Shin EK. Rosuvastatin dose-dependently improves flow-mediated dilation, but reduces adiponectin levels and insulin sensitivity in hypercholesterolemic patients. Int J Cardiol. 2016;223:488–493. doi: 10.1016/j.ijcard.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 45.Han SH, Nicholls SJ, Sakuma I, Zhao D, Koh KK. Hypertriglyceridemia and cardiovascular diseases: revisited. Korean Circ J. 2016;46:135–144. doi: 10.4070/kcj.2016.46.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh KK, Han SH, Sakuma I, Zhao D. Calming down chaos regarding redefining blood pressure targets- the importance of statin-based therapy. Int J Cardiol. 2016;221:572–574. doi: 10.1016/j.ijcard.2016.06.121. [DOI] [PubMed] [Google Scholar]
- 47.Koh KK. Letter by Koh regarding article, “long-term effectiveness and safety of pravastatin in patients with coronary heart disease: 16 years of follow-up of the LIPID study”. Circulation. 2016;134:e294–e295. doi: 10.1161/CIRCULATIONAHA.116.023680. [DOI] [PubMed] [Google Scholar]
- 48.Koh KK. What is the best disease-guided approach to statin? J Am Coll Cardiol. 2017;69:600. doi: 10.1016/j.jacc.2016.11.035. [DOI] [PubMed] [Google Scholar]