Abstract
The article presents the results of the prospective study of 266 patients with dirofilariasis who received medical and diagnostic assistance in Rostov Scientific Research Institute of Microbiology and Parasitology in Rostov-on-Don, Russia from 2000 to 2016. We have assessed the features of the dynamics of epidemiology of this infection in several territories of the Russian Federation, depending on the social structure of patients. Immature females of dirofilaria were found most commonly in humans (82.9 ± 2.6%), the proportion of maturity females and adult males of worms respectively was 10.5 ± 2.1% and 0.9 ± 0.6%. All mature worms were localized inside a capsule. Peripheral blood eosinophilia was detected only in patients with the migration of helminths (19 of 116 persons – 16.4%). Blood samples of patients examined by the method of concentration in 3% acetic acid for detection of microfilariae, showed negative result in all patients.
Our data are consistent with the opinion of KI Skriabin about that human as «dual facultative host» for dirofiliaria. It is rare that parasite in human body is able to develop to the imago stage (according to our observations – 11.4%). The immune response to invasion by dirofiliaria in human is manifested as dense connective tissue which forms a capsule. According to our study the rare cases (22) of detection the sexual mature D. repens (10.4%) were localized inside the capsule. Observations of patients with D. repens infection allowed concluding that human for this helminth is «a biological deadend».
Keywords: Dirofilaria repens, Subcutaneous dirofilariasis, Epidemiology, Laboratory detection, Obligate relationship human-dirofilaria
Introduction
For the past several years problem of human infection caused by Dirofilaria spp. has attracted great attention of researchers. Several authors have discussed the obligate and facultative relationships between human and the dirofilariasis pathogen Dirofilaria (Nochtiella) repens infection [1], as well as the role of the human as a potential source of this roundworm infection in nature [1], [2]. This hypothesis is based on the cases of detection of larvae, adult, and gravid females of D. repens in subcutaneous nodes [3]. Russian literature described the case where D. repens larvae detected in human peripheral blood [2], [4]. There are a few reports about autochthonous pleuropulmonary dirofilariasis caused by D. repens in Russia [4].
In the 1920s, KI Skriabin systematized some cases, which were described by both foreign and Russian researchers, and came to the conclusion that the cause of infection was the nematode – Dirofilaria repens Railliet et Henry, 1911 [5]. In 1930, KI Skriabin, AJ Altgauzen and ES.Shulman described the case of detection of mature males of D. repens in the subcutaneous node on the right lower eyelid [6]. As suggested by KI Skriabin, humans are a «double facultative host» for dirofilariasis due to quantitative restrictions cases of invasion, and failure for parasite to reach the sexual maturity in human. Because of the immune response, human organism forms dense connective tissue capsule, which prevents the completion of ontogenesis of dirofilaria [7].
Until 2014 human dirofilariasis in the Russian Federation was considered as a rare helminthiasis. But now, due to the sudden increase of infectious cases, the human dirofiliriasis is recording separately from other Helminthiasis in the official statistical reports. According to information by the Center of Hygiene and Epidemiology of Rospotrebnadzor, at the end of 2012, 1093 cases infected by D. repens have been recorded in Russian Federation. The largest number of cases was revealed in the Rostov, Volgograd and Nizhny Novgorod regions. At the beginning of 2013, 242 patients with dirofilariasis were recorded in the Rostov region. This is 22.1% of all cases recorded in the Russian Federation.
The purpose of this work is to describe and analyse the clinical and laboratory features of human infection of D. repens in the Rostov, Volgograd, Novgorod, Krasnodar regions during the period from 2000 to 2016.
Materials and methods
We observed 266 patients with dirofilariasis aged from 2 to 79 years who received medical and diagnostic assistance in Rostov Scientific Research Institute of Microbiology and Parasitology in Rostov-on-Don from 2000 to 2016.
The diagnosis of dirofilariasis in humans was based on the microscopy of removed parasites or their fragments, morphological and histopathological features, histological analysis of tissue sections of patients as well as objective data (photo, video) or visualization of moving threadlike parasite beneath skin of patients.
To identify microfilariae in blood of the patients, the samples assayed by concentration method with 3% acetic acid [8]. Serum of blood examined by enzyme immunoassay (ELISA) to detect Dirofilaria-specific antibodies [9]. We studied morphological and histological examination of the biological material: 211 nematode D. repens were studied directly, 31 histologically and 24 were not available for visualization. Sexual maturity determined by the presence of helminthic eggs in the uterus and according to the maturation of male reproductive apparatus [10].
Results
Epidemiologically, all patients denied travel into areas of prevalence of anthroponotic filariasis, not leaving the territory of their residence for 2 years or more. Of the 266 patients with dirofilariosis the proportion of patients of active working age (20–49 years) was 59.0% (Fig. 1). The study included 24 children, among them there were 2 infants (under 3 years old). Women significantly dominated in gender structure (72.5%). The infection was more common among the urban population. However, since 2008, we observed an increase of incidence of dirofilariasis in rural areas. From 2000 to 2007 the proportion of rural residents with D. repens was 8.9 ± 1.75%, and since 2008 to 2016 this number has increased more than 3 times – to 31.2 ± 2.84% (p < 0,05).
Fig. 1.
The age structure of patients with dirofilariasis.
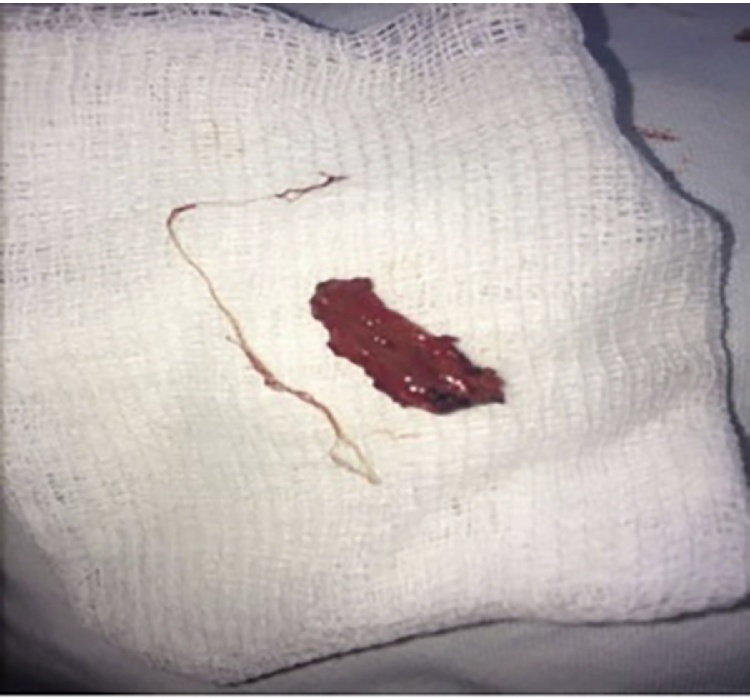
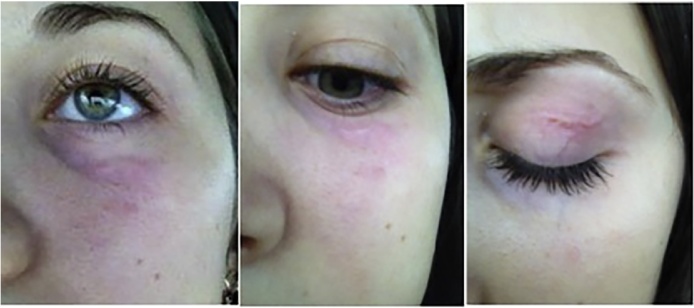
Patients sought medical assistance due to the detection of fixed cutaneous nodules up to 1.5 cm, or in cases of the visualization of thread like moving worms under the skin in various localization. The proportion of patients with encapsulated parasites was 56.4% (Fig. 2); active migration of the parasite was observed in 43.6% of patients (Fig. 3). In 17.7% cases worms moved considerable distances: from the knee to the groin area; lower back, upper eyelid or from periorbital region to shin and buttocks. Common localizations of Dirofilaria were head and neck (most often in the periorbital area, and conjunctivitis (face, scalp and forehead), or more rarely in the area of the trunk and extremities). In 6 patients (2.3%), worms were found in the external genitals. More than 1 nematodes was observed in three cases: in the first case – immature male and female, in the second – two immature females, in the third – two mature females.
Fig. 2.
Removed from tissue capsule Dirofilaria.
Fig. 3.
Migrating dirofilaria in the periorbital area.
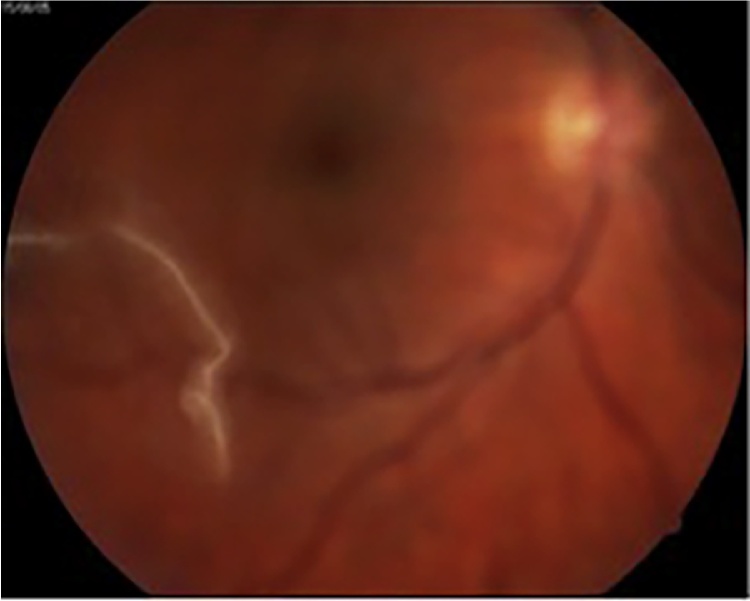
In 2005 only one patient was complaining on the floating object in the right eye view, which was changing the form permanently, sometimes becoming like a slowly squirming worm. At the ophthalmology examination in the vitreous body a threadlike worm was discovered (Fig. 4) [11]. Due to the high risk of total damage of eye, surgical treatment was not performed, therefore, determination the species of dirofilaria was not possible. According to our data it was the only case of detection of nematode inside the organ.
Fig. 4.
The threadlike worm was discovered in the vitreous body (Korkhov et al. [11]; photo taken with permission).
Peripheral blood eosinophilia was detected only in patients with the migration of helminthes (19 of 116 – 16.4%). Biochemical tests of liver and pancreas function in the most of patients were within the limits of age norm or were abnormal due to their premorbid conditions such as chronic cholecystitis, pancreatitis and diabetes, etc.
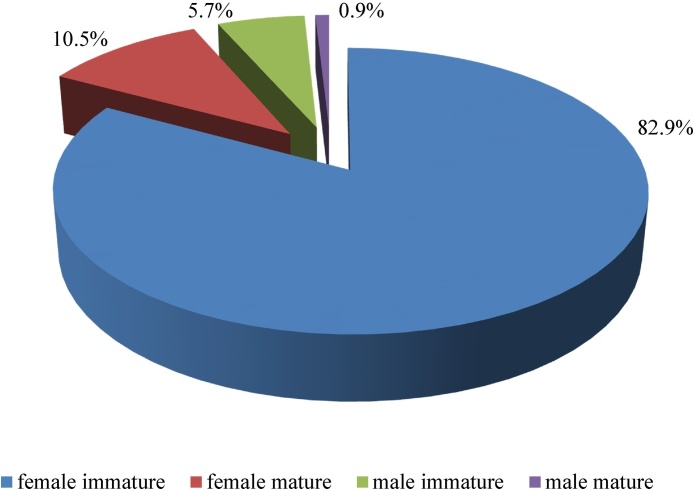
Blood samples of patients examined by the method of concentration in 3% acetic acid to detect microfilariae, showed negative results in all patients. Morphological examination of 211 removed parasites showed that length of the extracted roundworms varied from 75 to 170 mm, the most frequent nematode length was 110–120 mm (57.0%). The proportion of immature females was 82.9 ± 2.6% and significantly prevailed over the share of mature females (10.5 ± 2.1%), immature males (5.7 ± 1.6%), adult males (0.9 ± 0.6%) (Fig. 5).
Fig. 5.
Biological features of removed dirofilariasis.
Due to the active migration of parasites, and the impossibility of surgical treatment conservative treatment of 24 adult patients was conducted. Albendazole therapy was performed 800 mg/day and doxycycline 200 mg/day in 2 divided doses with intervals of 12 h for 5 days. Due to individual intolerance of doxycycline 2 patients were treated only with albendazole. In these patients, treatment failure was observed: after 10–12 days dirofilarias reappeared under the skin and were surgically removed.
Discussion
Dirofilariasis is the only vector-borne worm infection in countries with a temperate climate. The definitive hosts of D. repens are animals of the families Canidae, Felidae and Viverridae. The intermediate hosts are mosquitoes of the family Culicidae. People become infected when bitten by mosquitoes that contain third-stage filariform larvae of dirofilariae The territory of south Russia is an area of high risk of transmission of dirofiliariasis due to the suitable climatic conditions and high prevalence of worm in definitive and intermediate hosts [8]. The result is a significant number of cases of human invasion. The incidence of human infestation correlated with the extent of infestation of the definitive hosts [12], [13]. In recent decades, in the most part of the cities, diagnosis, prevention and treatment of dirofilariasis in definitive hosts (pet dogs) were optimized so that treatment could be given whereas in the rural areas their prevalence remains high [14]. This circumstance explains the significant increase in cases of human dirofilariasis in the rural area.
All cases of subcutaneous dirofilariasis were autochthonous.
The data of our observations are consistent with the opinion of KI Scriabin that the human is a «dual facultative host» for dirofiliaria [7]. It is extremely rare that parasite in human body is able to develop to the imago stage (according to our observations – 11.4%, according to the observations of KI Scriabin – 1 case). The immune response to invasion of the human organism by dirofiliaria manifested as dense connective tissue which forming a capsule where the parasite is unable to fully exercise its biological cycle [15]. According to our study the rare cases (22) of detection, the sexual mature of D. repens (10.4%) were localized inside the capsule, which is also consistent with the observations of KI Skriabin.
According to foreign researchers, pleuropulmonary and intraorganic damage in humans were reported only in cases of the infection with D. immitis [16], [17]. During our observation only 1 case with the parasite inside the vitreous humor was registered, but due to the threat of serious complications the surgery was not performed, so the species of the helminth was not estimated. Results of epidemiological monitoring in the Rostov region indicate that from 2011, the extent of infestation of definitive hosts with D. immitis is beginning to prevail over D. repens [12]. Perhaps in the future cases of human infestation by D. immitis will be registered in this area.
According to some scientists, human dirofilariasis is not a serious disease and has value mainly in the aspect of the differential diagnosis of tumors [16], [17].
Rare cases of complications after subcutaneous dirofilariasis were associated with surgery [9] and the formation of allergic reactions [18].
Our observations of patients with D. repens infection correspond to a row of European scientists from Slovakia [19], Czech Republic [20], Greece [21], Italy [13], Austria [22], and others who came to conclusion that the human is a biological deadend for this helminth.
Conclusion
For completion of biological cycle of Dirofilaria newborn larvae must circulate in human blood for two weeks to become infectious, then actively migrate to the lung capillaries. Under the favorable conditions for the start of the circulation phase, larvae must appear in the peripheral blood and become accessible for the vector of transmission. The only case of the detection of microfilariae in the peripheral human blood was observed in 2012 [2], [4], but this patient returned after a travel in South East Asia which is endemic for anthroponotic filariasis. In our study, the hypothesis about the obligate relationship between human and pleuropulmonary dirofilariasis, caused by D. repens [1], [4] in the Russian Federation today cannot be considered proven.
Authors’ contributions
Ermakova LA and Pshenichnaya NY performed short literature review and drafted the manuscript, Nagorny SA and Boltachiev KKh provided data collection, Ambalov YM performed the data analysis and design of the study.
Conflict of interest declaration
The authors declare that they have no any competing financial interests.
The content is solely the responsibility of the authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Supryaga V.G., Rakova V.M., Morozov E.N. Сurrent ideas on obligate and facultative relationships between man and the dirofilariasis pathogen Dirofilaria (N.) repens. Med Parazitol (Mosk) 2016;2:3–7. in Russian. [PubMed] [Google Scholar]
- 2.Fedianina L.V., Shatova S.M., Rakova V.M., Shaĭtanov V.M., Lebedeva M.N., Frolova A.A. Microfilaraemia in human dirofilariasis caused by Dirofilaria repens Raiet et Henry, 1911. A case report. Med Parazitol (Mosk) 2013;2:3–7. in Russian. [PubMed] [Google Scholar]
- 3.Fontanelli Sulekova L., Gabrielli S., De Angelis M., Milardi G.L., Magnati C., Di Marco B. Dirofilaria repens microfilariae from a human node fine-needle aspirate: a case report. BMC Infect Dis. 2016;16(1):248. doi: 10.1186/s12879-016-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronstein A.M., Malyshev N.A., Fedyanina L.V., Frolova A.A., Davydova I.V. Clinical masks of lung and pleurisy dirofilariasis: an analysis of own observations and a literature review. Epidemiol Infect Dis. 2015;20(1):43–49. in Russian. [Google Scholar]
- 5.Scriabin K.I., Petrow A.I. The systematic position of filaria nodulosum. Rud. 1819. Ann Trop Med Parazit. 1928;XXII(#2) in Russian. [Google Scholar]
- 6.Scriabin K.I., Altgauzen A.Z., Shulman E.S. The first case of detection of Dirofilaria repens in humans. Trop Med Vet. 1930;8(2):9–11. in Russian. [Google Scholar]
- 7.Scriabin K.I., Shikhobalova N.P. Ogiz-Selkhozgiz; Moscow: 1948. Filarias of ananimals and human. pp. 608 [in Russian] [Google Scholar]
- 8.Nagorny S.A., Ermakova L.A., Krivorotova E.Y. The specific features of the epidemiology and epizootology of dirofilariasis in Rostov-on-Don and the Rostov region. Med Parasitol (Mosk) 2012;4:46–48. in Russian. [PubMed] [Google Scholar]
- 9.Ermakova L.A., Nagorny S.A., Krivorotova E.Y., Pshenichnaya N.Y., Matina O.N. Dirofilaria repens in the Russian Federation: current epidemiology, diagnosis, and treatment from a federal reference center perspective. Int J Infect Dis. 2014;23:47–52. doi: 10.1016/j.ijid.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Lent H., Freitas J.F.F. Contribuciao ao estudo do genero Dirofilaria Raillet et Henry, 1911. Mems Inst Osw Cruz. 1937;32:37–54. [Google Scholar]
- 11.Korkhov A.P., Temirov N.E., Nagornyĭ S.A., Ermakova L.A., Dumbadze O.S., Beskrovnaya Y.G. A case of the rare intraocular site of Dirofilaria spp. in man. Med Parazitol (Mosk) 2009;1:59. in Russian. [PubMed] [Google Scholar]
- 12.Ermakova L.A., Nagorny S.A., Pshenichnaya N.Y., Krivirotova E.Y. Comments in response to the authors of «Human dirofilariasis due to Dirofilaria repens in the Russian Federation—remarks concerning epidemiology». Int J Infect Dis. 2014;28(225) doi: 10.1016/j.ijid.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Otranto Domenico, Dantas-Torres Filipe, Brianti Emanuele, Traversa D., Petrić D., Genchi C. Vector-borne helminths of dogs and humans in Europe. Parasit Vectors. 2013;6:16. doi: 10.1186/1756-3305-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medvedev A.Yu. Bios; Moscow: 2007. Distribution of dog dirofilariasis in the Krasnodar Territory and development of its diagnosis by an enzyme immunoassay. PhD thesis; pp. 3–24. [in Russian] [Google Scholar]
- 15.Shulyak B.F., Arkhipov I.A. 2010. Nematodoses of dogs (zoonoses and zooanthropoiesis) Moscow, pp. 495. [Google Scholar]
- 16.Haro Akira, Tamiya Sadafumi, Nagashima Akira. A rare case of human pulmonary dirofilariasis with a growing pulmonary nodule after migrating infiltration shadows, mimicking primary lung carcinoma. Int J Surg Case Rep. 2016;22:8–11. doi: 10.1016/j.ijscr.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob S., Parameswaran A., Santosham R., Santosham R. Human pulmonary dirofilariasis masquerading as a mass. Asian Cardiovasc Thorac Ann. 2016;24(7):722–725. doi: 10.1177/0218492316658569. [DOI] [PubMed] [Google Scholar]
- 18.Araya J., Kawabata Y., Tomichi N., Kaneko K., Hayashi K., Iwabuchi K. Allergic inflammatory reaction is involved in necrosis of human pulmonary dirofilariasis. Histopathology. 2007;51(4):484–490. doi: 10.1111/j.1365-2559.2007.02822.x. [DOI] [PubMed] [Google Scholar]
- 19.Antolová D., Miterpáková M., Paraličová Z. Case of human Dirofilaria repens infection manifested by cutaneous larva migrans syndrome. Parasitol Res. 2015;114(8):2969–2973. doi: 10.1007/s00436-015-4499-7. [DOI] [PubMed] [Google Scholar]
- 20.Matějů Jana, Chanová Marta, Modrý David, Mitková Barbora, Hrazdilová Kristýna, Žampachová Víta. Dirofilaria repens: emergence of autochthonous human infections in the Czech Republic (case reports) BMC Infect Dis. 2016;16:171. doi: 10.1186/s12879-016-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chris Kalogeropoulos D., Maria Stefaniotou I., Konstantina Gorgoli E., Chrissanthy Papadopoulou V., Chrysavgi Pappa N., Costas Paschidis A. Ocular dirofilariasis: a case series of 8 patients. Middle East Afr J Ophthalmol. 2014;21(4):312–316. doi: 10.4103/0974-9233.142267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuehrer H.P., Auer H’Leschnik M., Silbermayr K. Dirofilaria in humans, dogs, and vectors in Austria (1978–2014)—from imported pathogens to the endemicity of Dirofilaria repens. PLoS Negl Trop Dis. 2016;10(5):e004547. doi: 10.1371/journal.pntd.0004547. Published online 2016 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]