Abstract
AIM
To investigate the possible predictive role of routinely used glycemic parameters for a first venous thromboembolism (VTE) episode in gastrointestinal (GI) cancer ambulatory patients - with or without clinically diagnosed type 2 diabetes (T2D) or obesity - treated with chemotherapy.
METHODS
Pre-treatment fasting blood glucose, insulin, glycated hemoglobin (HbA1c) and homeostasis model of risk assessment (HOMA) were retrospectively evaluated in a cohort study of 342 GI cancer patients. Surgery was performed in 142 (42%) patients with primary cancer, 30 (21%) and 112 (79%) of whom received neoadjuvant and adjuvant therapies, respectively. First-line chemotherapy was administered in 200 (58%) patients with metastatic disease. The study outcome was defined as the occurrence of a first symptomatic or asymptomatic VTE episode during active treatment.
RESULTS
Impaired glucose tolerance (IGT) or T2D were diagnosed in 30% of GI cancer patients, while overweight/obesity had an incidence of 41%. VTE occurred in 9.4% of patients (7% of non-diabetic non-obese), especially in those with a high ECOG score (P = 0.025). No significant association was found between VTE incidence and T2D, obesity, different tumor types, metastatic disease, Khorana class of risk, or different anti-cancer drugs, although VTE rates were substantially higher in patients receiving bevacizumab (17% vs 8%, P = 0.044). Conversely, all glucose metabolic indexes were associated with increased VTE risk at ROC analysis. Multivariate Cox proportional analyses confirmed that HOMA index (HR = 4.13, 95%CI: 1.63-10.5) or fasting blood glucose (HR = 3.56, 95%CI: 1.51-8.39) were independent predictors of VTE occurrence during chemotherapy.
CONCLUSION
The results here reported demonstrate that evaluating glucose metabolic asset may allow for VTE risk stratification in GI cancer, helping to identify chemotherapy-treated patients who might benefit from thromboprophylaxis. Further multicenter prospective studies involving a larger number of patients are presently needed.
Keywords: Gastrointestinal cancer, Type 2 diabetes, Venous thromboembolism, Chemotherapy, Insulin resistance
Core tip: The predictive value of pre-treatment fasting blood glucose, insulin, HbA1c and homeostasis model of risk assessment (HOMA) was investigated in a cohort of gastrointestinal (GI) cancer patients. Despite all investigated metabolic markers were associated with an increased VTE risk during chemotherapy at ROC analysis, only elevated HOMA index (HR = 4.13) or fasting blood glucose (HR = 3.56) had an independent predictive value in survival analyses after adjustment for major confounders. These results suggest that glycemic metabolic markers, mainly HOMA index, should be carefully monitored in chemotherapy-treated GI cancer patients, as they could help to identify patients who might benefit from thromboprophylaxis.
INTRODUCTION
Cancer patients are at increased risk for venous thromboembolism (VTE)[1]. Among the different subtypes of tumors, pancreas, stomach, or other gastrointestinal (GI) (i.e., esophagus, liver, biliary) cancers have been reported to have the highest risk for incident VTE, whereas colorectal cancer is generally considered at low risk[2,3]. Furthermore, the individual risk of VTE in GI cancers could be boosted by active treatment, and the use of new biological drugs has led to a clinically relevant increase in thromboembolic complications, as in the case of bevacizumab in colorectal carcinoma[1,4].
Beside cancer- and treatment-related factors, VTE risk might be influenced by patient’s individual factors and co-morbidities[1]. The possibility that co-morbidities, such as type 2 diabetes (T2D)[5-8] or obesity[9-11] may be linked to an increased risk of VTE has been raised in the general population, and VTE has been proposed as a marker of underlying cancer - especially of the GI tract - in patients with T2D[12]. However, data are often confusing and no consensus exists on the impact of these two co-morbid conditions on VTE incidence[13], especially in the oncology setting.
Independently of an association between clinically diagnosed T2D, or obesity, and VTE, elevated levels of fasting blood glucose, glycated hemoglobin (HbA1c) or insulin resistance [IR, evaluated by the homeostasis model of risk assessment (HOMA)] have all been associated with an increased risk of VTE, either unprovoked[14-19] or cancer-associated[20]. The possibility of a causal link between hyperglycemia (and, thus, HbA1c or the composite HOMA index) and VTE occurrence is biologically plausible and supported by the experimental finding that, in healthy non-diabetic subjects, increased blood glucose levels enhances blood coagulation[21]. However, the association between hyperglycemia (assessed according to HbA1c) and VTE risk has been recently disproved[22].
It is worth noticing that all these evidences derived from studies performed in non-cancer patients. To the best of our knowledge, there are no data, so far, that specifically addressed this issue in oncologic patients, with the only exception of a study by our group demonstrating that breast cancer women with IR had an increased risk of chemotherapy-associated VTE - independently of T2D, or other related risk factors[20]. IR, hyperglycemia and T2D are associated with several cancer types, other than breast, and accumulating evidence indicates that they could represent shared pathophysiological mechanisms in GI cancer and its co-morbidities. Accordingly, we hypothesized that, as in the case of breast cancer, a de-regulated glucose metabolism could be involved in GI cancer-associated VTE, as well. Therefore, the present study was designed to investigate the possible predictive role of routinely used glycemic parameters for a first VTE episode in GI cancer out-patients - with or without clinically diagnosed T2D - in whom chemotherapy might act as a thrombotic trigger.
MATERIALS AND METHODS
Patients and sample collection
Starting from January 2007, the PTV Bio.Ca.Re. (Policlinico Tor Vergata Biospecimen Cancer Repository) and the Interinstitutional Multidisciplinary Biobank of the IRCCS San Raffaele Pisana (SR-BioBIM, Rome, Italy) are actively involved in the recruitment of ambulatory patients with primary or relapsing/metastatic cancer, who are prospectively followed under the appropriate Institutional ethics approvals, as part of a Clinical Database and Biobank project. Among them, 342 patients with GI cancer completed the clinical assessment for VTE. Inclusion criteria for patients whose serum samples were stored in our Biobanks were: age above 18 years, to be at the start of a first chemotherapy regimen, an Eastern Cooperative Oncology Group performance status (ECOG-PS) ≤ 2 and adequate hematological, hepatic and renal functions. Exclusion criteria were: therapeutic doses of any heparin before enrolment or treatment with anticoagulant or anti-platelet drugs. No patient underwent surgery during follow-up, nor was admitted to clinic for an acute medical illness requiring thromboprophylaxis.
GI cancer was staged according to the TNM classification. Surgery was performed in 142 patients with primary cancer. The remaining 200 patients had metastatic disease and entered the study prior to the start of chemotherapy. Among the non-metastatic population, 30/142 (21%) and 112/142 (79%) patients received neoadjuvant and adjuvant therapies, respectively. First-line chemotherapy was instituted in all patients with metastatic disease. Details on anti-cancer drugs are summarized in Supplementary Table 1. Erythropoiesis stimulating agents (2.6%), granulocyte colony stimulating factor (3.5%) and steroids (17.6%) were used as supportive drugs. Patients’ characteristics are summarized in Table 1.
Table 1.
Patients’ characteristics n (%)
| Characteristics | P value |
| Age (yr), mean ± SD (range) | 65 ± 10 (30-85) |
| Gender | |
| Male | 197 (58) |
| Female | 145 (42) |
| Length of follow-up (mo), median (IQR) | 11 (6-24) |
| Venous thromboembolism | |
| Pulmonary embolism | 9 (2.6) |
| Deep venous thrombosis | 22 (6.4) |
| Portal vein thrombosis | 2 (0.6) |
| Port-a-Cath | 1 (0.3) |
| Cumulative frequency | 34 (9.9) |
| Khorana Class of risk | |
| Low | 199 (58) |
| Intermediate | 118 (35) |
| High | 25 (7) |
| Site of primary | |
| Colon-rectum | 237 (69) |
| Stomach | 38 (11) |
| Pancreas | 36 (11) |
| Biliary tract | 16 (5) |
| Oesophagus | 15 (4) |
| Stage of disease | |
| Primary | 142 (42) |
| Metastatic | 200 (58) |
| Performance status (ECOG) | |
| 0 | 274 (80) |
| 1 | 60 (18) |
| 2 | 8 (2) |
| Body mass index, mean ± SD (range) | 24.9 ± 4.0 (14.8-39.5) |
| Normoweight | 200 (58) |
| Overweight | 106 (31) |
| Obese | 36 (11) |
| Type 2 diabetes | 79 (23) |
| Impaired glucose tolerance | 23 (7) |
IQR: Interquartile range; ECOG: Eastern Cooperative Oncology Group.
Patients were regularly seen at scheduled visits; additional visits were arranged at the occurrence of clinically suspected VTE. Initial VTE risk stratification was performed by the Khorana Score (KS) at a ≥ 3-point cutoff, as per current recommendation[23]. All patients were followed up for a median period of 11 mo, during which outcomes were prospectively recorded. The study outcome was defined as the occurrence of a first symptomatic or asymptomatic VTE episode during active treatment. Deep vein thrombosis (DVT) was confirmed by venography or color-coded duplex sonography (in proximal DVT only). Pulmonary embolism (PE) was diagnosed by spiral computed tomography.
The study was performed in accordance with the principles embodied in the Declaration of Helsinki. All patients gave written informed consent, previously approved by our Institutional Review Boards.
Blood sampling and assessment of glycemic indexes
Fasting serum samples were obtained prior to chemotherapy from each recruited subject, aliquoted and stored at -80 °C in the facilities of the PTV Bio.Ca.Re. or of the SR-BioBIM. Routine chemistry studies, including fasting blood glucose (Hexokinase/Glucose-6-phosphate dehydrogenase-based methodology; Abbott Laboratories, Abbott Park, IL, United States), were performed on fresh samples within one hour from blood withdrawal on an ARCHITECT c8000 System (Abbott Laboratories). Fasting insulin levels were analyzed on serum samples using a fully automated Lumipulse G 600 II chemiluminescent enzyme immunoassay analyzer (Fujirebio Inc. Tokyo, Japan) according to the manufacturer’s instructions. The HOMA index (a marker of insulin resistance) was retrospectively calculated for each participating subject from fasting blood glucose and insulin according to the formula: glucose (mg/dL) × insulin (μIU/mL)/405.
HbA1C levels were immediately measured on EDTA anticoagulated whole blood by a Tosoh G7 Automated HPLC Analyzer - HbA1c Variant Analysis Mode (Tosoh Bioscience, Rivoli, TO, Italy), certified by the NGSP (National Glycohemoglobin Standardization Program) and traceable to the Diabetes Control and Complications Trial.
All measurements were ascertained while blinded to the sample origin and to the study endpoint.
Statistical analysis
Sample size of the study was based on the agreement to inclusion criteria and willingness to provide informed consent rather than on sample size calculations. However, estimation was later performed and showed that the recruited population was capable of yielding a power > 90%, at a two-sided 5% significance level. This was based on the assumption of a true HR of at least 2 (based on previous data on breast cancer)[20], an accrual period of no less than 2 years, an elapsed time between cycles within 30 d, and a median time-to-event of 2.5 mo.
Data are presented as percentages, mean ± SD, or median and interquartile range (IQR). Differences between percentages were assessed by χ2 test. Appropriate parametric or nonparametric tests were employed for group comparison. The cut-off values were generated from continuous data by receiver operating characteristic (ROC) curve analyses performed by MedCalc Statistical Software version 13.1.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). Bayesian analysis was performed, and positive (+LR) and negative (-LR) likelihood ratios were used to estimate the probability of having or not VTE. Cox proportional hazards analysis was performed with a free web-based application (http://statpages.org/). Survival curves were calculated by the Kaplan-Meier and log-rank methods using a computer software package (Statistica 8.0, StatSoft Inc., Tulsa, OK, United States). VTE-free survival time was calculated from the date of enrolment until the date of any VTE occurrence, or of the last follow-up. For administrative censoring, follow-up was ended on September 30th, 2016. For patients receiving neoadjuvant chemotherapy follow-up was terminated at completion of an entire antiblastic treatment and before surgery. All tests were two-tailed and only P values lower than 0.05 were regarded as statistically significant.
RESULTS
A condition of impaired glucose tolerance (IGT)/T2D was diagnosed in 30% of patients, while overweight (n = 105) or obesity (n = 35) was observed in 41% of cases. It is well recognized that overweight/obesity increases the chances of developing T2D; accordingly, the rate of T2D rose from 19% in normoweight to 27% and 36% in overweight and obese patients, respectively (P = 0.122). The distribution of glucose metabolic indexes is reported in Table 2. As shown, median pre-chemotherapy HOMA index was 3.0 in the overall GI cancer cohort (IQR: 2.0-5.6). As expected, median HOMA index was associated with BMI and increased steadily from 2.5 in normoweight to 3.8 and 3.9 in overweight and obese patients, respectively (Kruskal-Wallis H = 18.8, P = 0.0001). Similarly, IR was associated with the presence of IGT (median HOMA index 4.3) or T2D (median HOMA index 6.7).
Table 2.
Glycemic indexes in gastrointestinal cancer patients - Comparison between patients who developed or not venous thromboembolism during chemotherapy
| Whole cohort |
Venous thromboembolism |
P value1 | ||
| Yes (n = 34) | No (n = 308) | |||
| Glycemia (mg/dL), mean ± SD (range) | 107 ± 38 (51-415) | 122 ± 53 (60-339) | 105 ± 36 (51-415) | 0.019 |
| Insulinemia (μIU/mL), median (IQR) | 12.5 (8.8-20.1) | 17.2 (11.9-24.5) | 11.6 (8.7-18.6) | 0.016 |
| HOMA index, median (IQR) | 3.0 (2.0-5.6) | 4.8 (3.0-8.1) | 2.8 (2.0-4.9) | 0.006 |
| HbA1c (%), mean ± SD (range) | 6.1 ± 0.8 (4.3-13.0) | 6.2 ± 0.6 (5.2-8.0) | 6.1 ± 0.8 (4.3-13.0) | 0.436 |
Student t-test or Mann-Whitney U test were used for normally distributed or non parametric variables, respectively. IQR: Interquartile range.
VTE occurred in 9.9% (Table 1) of GI cancer patients (median TTE: 3.2 mo) (7.5% of non-diabetic non-obese patients), in agreement with previous reports[1]. In particular, 15 (3 non fatal sub-segmental PE, 2 portal vein thrombosis and 10 DVT) patients were incidentally diagnosed with asymptomatic VTE at time of CT-scan for restaging. Symptomatic VTE was diagnosed in the remaining 19 patients. Clinical characteristics of all 34 patients with VTE are reported in Supplementary Table 2.
Of interest, only 2 (8%) VTE events were recorded among the 25 classified as high-risk for VTE, as per current guidelines[23]. Conversely, VTE occurred in 10% and 11% of patients classified as low- or intermediate-risk according to Khorana[23], respectively. VTE incidence increased with high ECOG-PS (P = 0.025), but no significant difference was observed for VTE rates among patients with colorectal (n = 25, 11%), gastro-esophageal (n = 5, 9.5%) or pancreatobiliary (n = 4, 7.7%) carcinomas. Similarly, no association was found with other clinical variables, such as metastatic disease, T2D, obesity, or different chemotherapy regimens, although VTE rates were higher in patients receiving bevacizumab (17% vs 8%, P = 0.044). On the other hand, pre-treatment fasting blood glucose, insulin, HOMA index, but not HbA1c, were higher in patients who developed VTE during treatment compared with those who did not (Table 2).
ROC curves and bayesian analysis was, then, carried out to analyze the predictive performances of glycemic parameters. Details are reported in Table 3, showing that pre-treatment fasting blood glucose, insulin and the composite HOMA index, but to a lesser extent HbA1c levels, were all associated with a significant probability of having or not VTE during chemotherapy. Hence, cut-off levels corresponding to the criteria associated with the highest Youden indexes were employed for patients’ categorization and subsequent analyses. Multivariate Cox proportional analysis showed that, among all variables listed in Table 4, only ECOG-PS (HR = 2.47, 95%CI: 1.22-4.99) and HOMA index (HR = 4.13, 95%CI: 1.63-10.5) acted as independent VTE predictors (Overall Model Fit: Chi Square = 30.5, P = 0.0004). Of interest, when HOMA index was replaced by the individual parameters in an otherwise similar model of Cox analysis, only fasting blood glucose retained significance for VTE risk prediction (HR = 3.56, 95%CI: 1.51-8.39), along with ECOG-PS (HR = 2.34, 95%CI: 1.17-4.69) (Overall Model Fit: χ2 = 36.9, P = 0.0001). Inclusion of anti-cancer drugs other than bevacizumab into multivariate models did not substantially modify the results obtained (data not shown).
Table 3.
Receiver operating characteristics and Bayesian analysis of venous thromboembolism predictive value of glycemic parameters
| Fasting blood glucose | Fasting insulin | HOMA | HbA1c | |
| AUC (SE) | 0.636 (0.06) | 0.630 (0.05) | 0.647 (0.05) | 0.574 (0.06) |
| 95%CI | 0.582-0.687 | 0.576-0.681 | 0.593-0.697 | 0.519-0.627 |
| Criterion1 | 103 mg/dL | 12 μIU/mL | 2.6 | 6.0% |
| Sensitivity | 75% | 75% | 81% | 59% |
| Specificity | 65% | 51% | 48% | 63% |
| PPV | 18% | 14% | 14% | 14% |
| NPV | 96% | 95% | 96% | 94% |
| +LR (CI) | 2.12 (1.54-2.57) | 1.54 (1.13-1.85) | 1.56 (1.19-1.81) | 1.60 (1.07-2.13) |
| -LR (CI) | 0.39 (0.19-0.68) | 0.49 (0.23-0.87) | 0. 39 (0.16-0.78) | 0.65 (0.38-0.96) |
| P value2 | 0.0135 | 0.0128 | 0.0045 | 0.1989 |
Corresponding with highest Youden index;
Significance level P (Area = 0.5). AUC: Area under the curve; PPV: Positive predictive value; NPV: Negative predictive value; +LR: Positive likelihood ratio; -LR: Negative likelihood ratio.
Table 4.
Cox proportional hazards survival regression analysis of the predictive value of clinical-pathological variables and glycemic indexes on venous thromboembolism-free survival of gastrointestinal cancer patients n (%)
| Variable | n |
VTE |
HR (CI) | P value | |
| Yes | No | ||||
| Sex | |||||
| Male | 197 | 17 (9) | 180 (91) | ||
| Female | 145 | 17 (12) | 128 (88) | 0.48 (0.24-0.97) | 0.040 |
| Age | |||||
| ≤ 65 yr | 157 | 9 (6) | 148 (94) | ||
| > 65 yr | 185 | 25 (14) | 160 (86) | 2.15 (0.96-4.79) | 0.062 |
| Diabetes | |||||
| No | 240 | 20 (8) | 220 (92) | ||
| IGT | 23 | 2 (9) | 21 (91) | ||
| T2D | 79 | 12 (15) | 67 (85) | 0.94 (0.62-1.41) | 0.748 |
| Tumor site | |||||
| Colorectal | 237 | 25 (11) | 212 (89) | ||
| Stomach | 38 | 3 (8) | 35 (92) | ||
| Esophagus | 15 | 2 (13) | 13 (87) | ||
| Pancreas | 36 | 3 (8) | 33 (92) | ||
| Biliary tract | 16 | 1 (6) | 15 (94) | 0.78 (0.53-1.14) | 0.201 |
| Khorana class of risk | |||||
| Low | 199 | 19 (10) | 180 (90) | ||
| Intermediate | 118 | 13 (11) | 105 (89) | ||
| High | 25 | 2 (8) | 23 (92) | 1.20 (0.64-2.26) | 0.562 |
| Stage of disease | |||||
| Primary | 142 | 9 (6) | 133 (94) | ||
| Metastatic | 200 | 25 (12) | 175 (88) | 1.81 (0.75-4.33) | 0.186 |
| ECOG-PS | |||||
| 0 | 274 | 24 (9) | 250 (91) | ||
| 1 | 60 | 7 (12) | 53 (88) | ||
| 2 | 8 | 3 (37) | 5 (62) | 2.47 (1.22-4.99) | 0.012 |
| Homa index | |||||
| ≤ 2.6% | 155 | 7 (5) | 148 (95) | ||
| > 2.6% | 187 | 27 (14) | 160 (86) | 4.13 (1.63-10.5) | 0.003 |
| Bevacizumab | |||||
| No | 270 | 22 (8) | 248 (92) | ||
| Yes | 72 | 12 (17) | 60 (83) | 1.77 (0.74-4.25) | 0.199 |
ECOG-PS: Eastern Cooperative Oncology Group performance status; T2D: Type 2 diabetes; IGT: Impaired glucose tolerance; VTE: Venous thromboembolism.
Figure 1 demonstrates the Kaplan-Meier curves for VTE-free survival of GI cancer patients stratified on the basis of pre-treatment HOMA index (Figure 1A) or blood glucose levels (Figure 1B). As shown, patients with a HOMA index > 2.6 had a worse 1-year VTE-free survival rate compared to patients with a HOMA index below this cutoff (83% vs 94%, log-rank = 3.0, P = 0.003) (Figure 1A). Similarly, patients with glucose levels >103 mg/dL had a worse 1-year VTE-free survival rate compared to patients with glucose levels below this cutoff (78% vs 94%, log-rank = 4.5, P < 0.0001) (Figure 1B). These results were substantially confirmed in a sub-set of 261 non-obese non-diabetic GI cancer patients (Figure 2).
Figure 1.
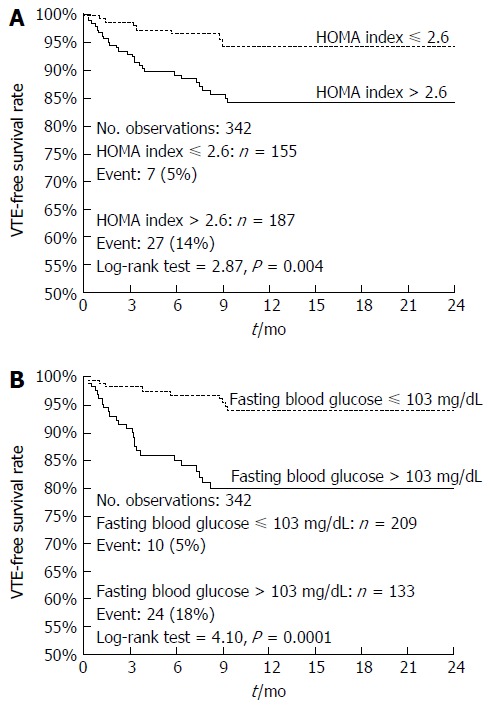
Kaplan-Meier curves of venous thromboembolism-free survival of the whole cohort of gastrointestinal cancer patients. Comparison between patients stratified on HOMA index (Panel A; HR = 3.17, 95%CI: 1.61-6.23) or fasting blood glucose (Panel B; HR = 4.21, 95%CI: 2.09-8.46).
Figure 2.
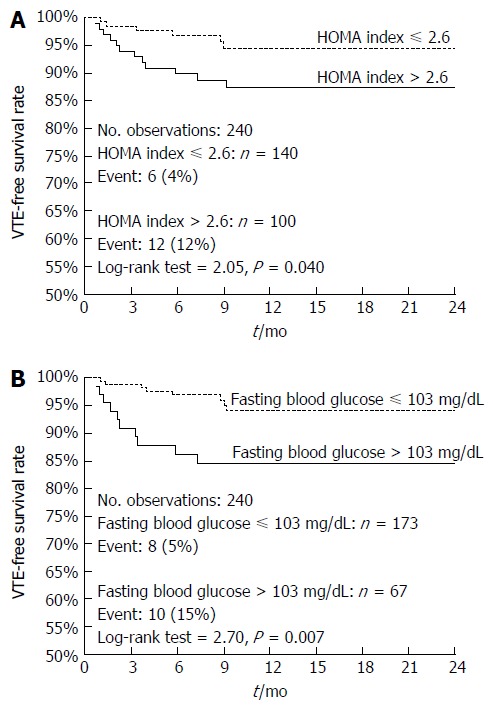
Kaplan-Meier curves of venous thromboembolism-free survival of non-diabetic non-obese gastrointestinal cancer patients. Comparison between patients stratified on HOMA index (Panel A; HR = 2.67, 95%CI: 1.05-6.80) or fasting blood glucose (Panel B; HR = 3.38, 95%CI: 1.19-9.57).
DISCUSSION
Diabetes and obesity have often been related with increased risk of VTE in the general population, but the evidences on their association in the oncology setting are sparse. Here we report, for the first time to our knowledge, that a condition of IR (assessed by the glucose/insulin composite HOMA index) associates to an increased risk of VTE in GI cancer out-patients on active chemotherapy. In particular, we demonstrate that a HOMA index > 2.6 at time of chemotherapy start is predictive for a first VTE event, independently of T2D, obesity or other well known risk factors, such as tumor site and stage or ECOG-PS. This correlation appears to be strongly dependent on blood glucose levels, rather than on insulin, as demonstrated by multivariate analyses in which glycemia, but not insulinemia, confirmed its independent association with VTE. Of note, HbA1c - a marker of sustained hyperglycemia over the previous 2-3 mo - associated with VTE only in non diabetic GI cancer patients, with a HR of 3.12 (95%CI: 1.21-8.07, P = 0.02) independently of sex, age, BMI, IGT, ECOG-PS, and tumor site and stage, possibly as a result of the confounding effect of glycemic pharmacologic control in T2D (data not shown).
These results are scarcely comparable with literature data, as the majority of the studies investigated the predictive role of these metabolic features in the general population. Nonetheless, the finding of an independent predictive role of elevated fasting blood glucose levels in the GI cancer setting is in agreement with the results by Tala et al[18], demonstrating a 4.1 odds ratio of VTE in critically ill children with hyperglycemia. Similarly, Di Minno et al[14] indicated that impaired fasting glucose independently predicted idiopathic VTE in adults. Furthermore, the data here reported are partially in agreement with previously published results demonstrating that high HOMA scores were associated with an increased risk of VTE in the general population, which, however, was dependent on BMI[19]. This finding was further confirmed by Gariani et al[24], who suggested that the increased risk of diabetes-associated VTE might result from confounders (i.e., obesity) rather than an intrinsic effect of diabetes. On the other hand, the predictive value of HOMA index in our analysis was independent of a condition of overweight/obesity, validating our previous results in a breast cancer cohort, in which a HOMA index greater than 2.5 (the cutoff for normality) predicted chemotherapy-associated VTE, independently of other components of the metabolic syndrome (BMI, T2D and HDL cholesterol)[20].
As stated above, the possibility of a causal link between hyperglycemia and VTE occurrence is biologically plausible and supported by the finding that increased blood glucose levels enhances blood coagulation in healthy non-diabetic individuals[21]. Additional evidence on the role of hyperglycemia on coagulation activation derives from experimental data suggesting that hyperglycemia may cause vessel damage through at least three apparently unrelated pathways: advanced glycation end product formation, activation of protein kinase C, and sorbitol accumulation by way of the polyol pathway (for a review see[25]). Furthermore, hyperglycemia via increased oxidative stress, and receptor for advanced glycation end products activation, increases the activation of transcription factor-κB in endothelial cells, thus causing a switch of the endothelial functions toward a pro-thrombotic, pro-inflammatory condition. This, together with an altered platelet metabolism and changes in intraplatelet signaling pathways, contributes to the pathogenesis of the thrombotic complications of T2D[25]. Of interest, in this study, elevated HbA1c levels were significantly associated with increased mean platelet volumes (a marker of platelet hyperactivity available for 214 patients) either in the overall cohort (regression coefficient = 0.235, P = 0.0006) or in non-diabetic non-obese (regression coefficient = 0.239, P = 0.004) GI cancer patients, independently of age, sex or BMI (data not shown), thus suggesting a role for sustained hyperglycemia in the procoagulant status of these patients. However, this hypothesis is purely speculative, since the present study was not specifically designed to address this issue and, as such, it deserves further investigation.
Whichever the mechanism involved, cancer-associated VTE poses serious concerns both in terms of patient care and health costs, while thromboprophylaxis could provide an opportunity to substantially improve clinical management. Nonetheless, international consensus guidelines do not recommend routine prophylaxis for the primary prevention of VTE in cancer outpatients receiving chemotherapy, except for pancreatic cancer or selected high-risk patients[23,25,26]. To aid in VTE risk assessment, the use of Khorana score is currently recommended[23]. In the present study, the Khorana score was used to provide an initial estimate of VTE risk in GI cancer patients. However, it correctly identified VTE only in two gastric cancer patients (one normoweight diabetic and one overweight non diabetic) out of 25 patients classified as high-risk, both with IR. Conversely, 8.6% and 11% of patients classified as low- or intermediate-risk according to Khorana, had VTE. This is in agreement with recent findings suggesting that the major weakness of this scoring system is represented by the high proportion of the patients (> 50%) falling into the intermediate risk category[27], which also encompasses the majority of events[27-29]. At this point, it should be emphasized that, in the low- or intermediate-risk classes, 13 of 17 (77%) and 11 of 13 (85%) patients with VTE, respectively, had a HOMA index above the 2.6 cut-off. Thus, HOMA index, or other glucose metabolism parameters, could be employed in expanded risk scoring models, or newly developed clinical decision support systems[30] without causing an excessive increase in patients’ management costs.
There are some limitations to acknowledge for this study. First of all, glucose metabolic parameters were retrospectively evaluated. However, all eligible consecutive patients within the designated timeframe were included and prospectively followed up, and all measurements were performed while blinded to the patient outcome. Secondly, recruitment was mono-institutional, and therefore might have limited external validity. Finally, analyses were conducted on a relatively small sample size, ultimately leading to a small number of events. Nonetheless, the results here reported suggest that a deregulation of glucose metabolism might contribute to VTE pathogenesis in chemotherapy-treated ambulatory cancer patients. To the best of our knowledge, this is the first evidence reporting an association between HOMA index and VTE in GI cancer patients. At present, we may hypothesize that chemotherapy triggers a pro-thrombotic state, subdued in patients with hyperglycemia, independently of clinically overt T2D. Additional studies are required to validate this theory, as VTE can be influenced by many environmental or inherited factors that increase the likelihood of detecting spurious associations.
In conclusion, the results here reported demonstrate that the evaluation of glucose metabolic asset may allow for VTE risk stratification in GI cancer, helping to identify chemotherapy-treated patients who might benefit from thromboprophylaxis. Further multicenter prospective studies involving a larger number of patients are presently needed.
ACKNOWLEDGMENTS
The team expresses deep gratitude to all patients and their families for providing the opportunity to conduct the present research project. Authors also wish to thank the nursery staff of the Day Hospital of the Medical Oncology Unit, Tor Vergata Clinical Center who have contributed and supported the researchers to the overall success of the PTV Bio.Ca.Re. project.
COMMENTS
Background
The possibility of a causal link between hyperglycemia and venous thromboembolism (VTE) occurrence is supported by the experimental finding that, in healthy non-diabetic subjects, increasing blood glucose levels enhances blood coagulation. Accordingly, metabolic markers of impaired glucose metabolism have been associated with an increased risk of VTE in the general population, but little is known about their predictive significance in chemotherapy-treated cancer patients. The few available data come from a recent study demonstrating that breast cancer women with insulin resistance had an increased risk of chemotherapy-associated VTE, independently of type 2 diabetes (T2D), obesity, or other related risk factors.
Research frontiers
Future investigations specifically designed to address the predictive role of insulin resistance or hyperglycemia for VTE risk assessment may provide the rationale for their inclusion in expanded risk scoring models, or newly developed clinical decision support systems.
Innovations and breakthroughs
This study provides evidences that pre-treatment blood glucose levels or the composite homeostasis model of risk assessment (HOMA) index may allow for VTE risk stratification in GI cancer patients. Glycemic metabolic markers should be carefully monitored, independently of T2D or obesity, as they could provide important information for VTE risk prediction.
Applications
Clinicians should be alert to the pro-coagulant risk of impaired glycemic control and advise patients about lifestyle intervention, weight loss, and exercise as a part of their therapeutic plan. In the context of a precision medicine approach, incorporation of pre-treatment fasting glycemia - or HOMA index - in expanded risk scoring models, or newly developed clinical decision support systems might improve risk prediction without causing an excessive increase in patients’ management costs.
Terminology
HbA1c provides an estimate of average blood glucose levels over the previous three months and is used as a marker of glycemic control in the management of T2D. HOMA index, derived from blood glucose and insulin levels, is a non invasive parameter of insulin resistance in pathologic states, such as diabetes and obesity.
Peer-review
The article is interesting, methods of the study are presented concisely and there are no objections about it. The study deals with an important problem of treatment strategy in the large group of cancer patients, though the small number of subjects tends to show preliminary character of the study and does not allow to draw population conclusions.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Scientific Institute for Research, Hospitalization and Health Care San Raffaele Pisana and by the Tor Vergata University Institutional Review Boards.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: All the Authors have no conflict of interest related to the manuscript.
Data sharing statement: No additional data are available.
Peer-review started: February 9, 2017
First decision: April 21, 2017
Article in press: July 13, 2017
P- Reviewer: Wiewiora M S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
References
- 1.Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 2.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Petterson TM, Marks RS, Ashrani AA, Bailey KR, Heit JA. Risk of site-specific cancer in incident venous thromboembolism: a population-based study. Thromb Res. 2015;135:472–478. doi: 10.1016/j.thromres.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferroni P, Formica V, Roselli M, Guadagni F. Thromboembolic events in patients treated with anti-angiogenic drugs. Curr Vasc Pharmacol. 2010;8:102–113. doi: 10.2174/157016110790226660. [DOI] [PubMed] [Google Scholar]
- 5.Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;48:1017–1021. doi: 10.1007/s00125-005-1715-5. [DOI] [PubMed] [Google Scholar]
- 6.Stein PD, Goldman J, Matta F, Yaekoub AY. Diabetes mellitus and risk of venous thromboembolism. Am J Med Sci. 2009;337:259–264. doi: 10.1097/MAJ.0b013e31818bbb8b. [DOI] [PubMed] [Google Scholar]
- 7.Eyadiel C, Hamm RM, Scheid DC. In adults with type 2 diabetes mellitus, are patients with poor control more likely to develop venous thromboembolism compared to patients with good control? J Okla State Med Assoc. 2014;107:65–66. [PubMed] [Google Scholar]
- 8.Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: A PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore) 2016;95:e4495. doi: 10.1097/MD.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffen LM, Cushman M, Peacock JM, Heckbert SR, Jacobs DR Jr, Rosamond WD, Folsom AR. Metabolic syndrome and risk of venous thromboembolism: Longitudinal Investigation of Thromboembolism Etiology. J Thromb Haemost. 2009;7:746–751. doi: 10.1111/j.1538-7836.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delluc A, De Moreuil C, Kerspern H, Le Moigne E, Mottier D, Tromeur C, Carre JL, Le Gal G, Lacut K. Body mass index, a major confounder to insulin resistance association with unprovoked venous thromboembolism. Results from the EDITH case-control study. Thromb Haemost. 2013;110:593–597. doi: 10.1160/TH13-01-0048. [DOI] [PubMed] [Google Scholar]
- 11.Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res. 2016;145:27–33. doi: 10.1016/j.thromres.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen DH, Horváth-Puhó E, Thomsen RW, Knudsen ST, Dekkers O, Prandoni P, Sørensen HT. Venous thromboembolism and risk of cancer in patients with diabetes mellitus. J Diabetes Complications. 2016;30:603–607. doi: 10.1016/j.jdiacomp.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Minno MN, Tufano A, Guida A, Di Capua M, De Gregorio AM, Cerbone AM, Tarantino G, Di Minno G. Abnormally high prevalence of major components of the metabolic syndrome in subjects with early-onset idiopathic venous thromboembolism. Thromb Res. 2011;127:193–197. doi: 10.1016/j.thromres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Dentali F, Squizzato A, Caprioli M, Fiore V, Bernasconi M, Paganini E, Ageno W, Venco A, Grandi AM. Prevalence of arterial and venous thromboembolic events in diabetic patients with and without the metabolic syndrome: a cross sectional study. Thromb Res. 2011;127:299–302. doi: 10.1016/j.thromres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Bell EJ, Selvin E, Lutsey PL, Nambi V, Cushman M, Folsom AR. Glycemia (hemoglobin A1c) and incident venous thromboembolism in the Atherosclerosis Risk in Communities cohort study. Vasc Med. 2013;18:245–250. doi: 10.1177/1358863X13506764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermanides J, Cohn DM, Devries JH, Kamphuisen PW, Huijgen R, Meijers JC, Hoekstra JB, Büller HR. Venous thrombosis is associated with hyperglycemia at diagnosis: a case-control study. J Thromb Haemost. 2009;7:945–949. doi: 10.1111/j.1538-7836.2009.03442.x. [DOI] [PubMed] [Google Scholar]
- 18.Tala JA, Silva CT, Pemira S, Vidal E, Faustino EV. Blood glucose as a marker of venous thromboembolism in critically ill children. J Thromb Haemost. 2014;12:891–896. doi: 10.1111/jth.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Schouwenburg IM, Mahmoodi BK, Veeger NJ, Bakker SJ, Kluin-Nelemans HC, Meijer K, Gansevoort RT. Insulin resistance and risk of venous thromboembolism: results of a population-based cohort study. J Thromb Haemost. 2012;10:1012–1018. doi: 10.1111/j.1538-7836.2012.04707.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferroni P, Roselli M, Riondino S, Cavaliere F, Guadagni F. Insulin resistance as a predictor of venous thromboembolism in breast cancer. Endocr Relat Cancer. 2016;23:L25–L28. doi: 10.1530/ERC-16-0187. [DOI] [PubMed] [Google Scholar]
- 21.Stegenga ME, van der Crabben SN, Blümer RM, Levi M, Meijers JC, Serlie MJ, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerstad G, Brodin EE, Enga KF, Jorde R, Schirmer H, Njølstad I, Svartberg J, Braekkan SK, Hansen JB. Hyperglycemia, assessed according to HbA1c , and future risk of venous thromboembolism: the Tromsø study. J Thromb Haemost. 2014;12:313–319. doi: 10.1111/jth.12498. [DOI] [PubMed] [Google Scholar]
- 23.Khorana AA, Otten HM, Zwicker JI, Connolly GC, Bancel DF, Pabinger I; Subcommittee on Haemostasis and Malignancy of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1928–1931. doi: 10.1111/jth.12725. [DOI] [PubMed] [Google Scholar]
- 24.Gariani K, Mavrakanas T, Combescure C, Perrier A, Marti C. Is diabetes mellitus a risk factor for venous thromboembolism? A systematic review and meta-analysis of case-control and cohort studies. Eur J Intern Med. 2016;28:52–58. doi: 10.1016/j.ejim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Ferroni P, Basili S, Falco A, Davì G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–656. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14:1773–1778. doi: 10.1111/jth.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srikanthan A, Tran B, Beausoleil M, Jewett MA, Hamilton RJ, Sturgeon JF, O’Malley M, Anson-Cartwright L, Chung PW, Warde PR, et al. Large retroperitoneal lymphadenopathy as a predictor of venous thromboembolism in patients with disseminated germ cell tumors treated with chemotherapy. J Clin Oncol. 2015;33:582–587. doi: 10.1200/JCO.2014.58.6537. [DOI] [PubMed] [Google Scholar]
- 29.van Es N, Franke VF, Middeldorp S, Wilmink JW, Büller HR. The Khorana score for the prediction of venous thromboembolism in patients with pancreatic cancer. Thromb Res. 2017;150:30–32. doi: 10.1016/j.thromres.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Ferroni P, Zanzotto FM, Scarpato N, Riondino S, Nanni U, Roselli M, Guadagni F. Risk Assessment for Venous Thromboembolism in Chemotherapy-Treated Ambulatory Cancer Patients. Med Decis Making. 2017;37:234–242. doi: 10.1177/0272989X16662654. [DOI] [PubMed] [Google Scholar]


