Abstract.
Aggrecan, encoded by ACAN, is a major proteoglycan component of the extracellular matrix in the growth plate and articular cartilage. Aggrecan provides the hydrated gel structure important for the load-bearing properties of joints and plays a key role in cartilage and bone morphogenesis. At least 25 pathological ACAN mutations have been identified in patients with highly variable phenotypes of syndromic or non-syndromic short stature. This review provides an overview of the current understanding of ACAN and the clinical and genetic findings concerning aggrecan-associated diseases.
Keywords: aggrecan, ACAN, short stature, bone age, disc herniation
Introduction
Linear bone growth is determined by the division and growth of matrix-producing chondrocytes at the growth plate. The rate of growth plate chondrogenesis is regulated by many systems including multiple hormones (GH, thyroid hormone, and sex steroids), paracrine factors, extracellular matrix molecules, and intracellular proteins (1). Short stature can be caused by decreased chondrogenesis due to mutations in any gene that directly or indirectly affects growth plate chondrocytes and the process of growth plate chondrogenesis (1).
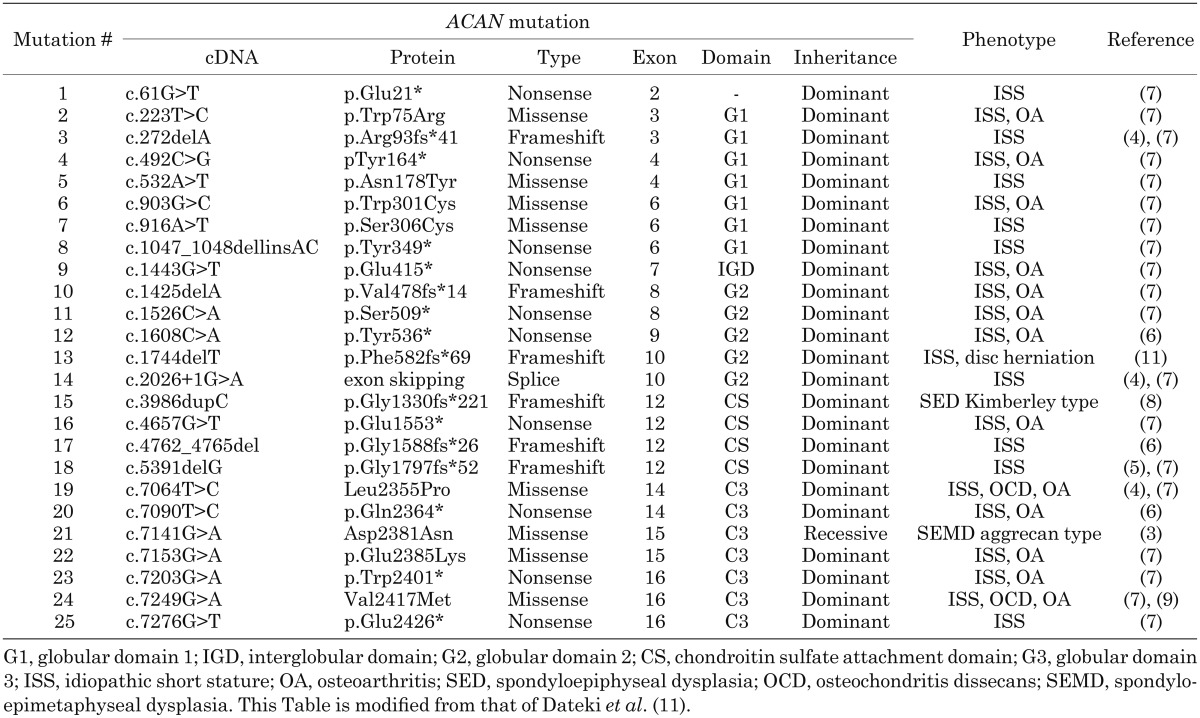
Aggrecan, encoded by ACAN, is a major proteoglycan component in the extracellular matrix of the growth plate and articular cartilage. Aggrecan provides the hydrated gel structure important for the load-bearing properties of joints (2). At least 25 pathological ACAN mutations have been identified in patients with highly variable phenotypes of syndromic or non-syndromic short stature (Table 1) (3,4,5,6,7,8,9,10,11). This review article provides an overview of the current understanding of ACAN and the clinical and genetic findings concerning aggrecan-associated diseases.
Table 1. Summary of molecular and clinical findings in patients with ACAN mutations.
Structure and Function of Aggrecan
Aggrecan consists of a 250-kDa protein core with approximately 100 chondroitin sulfate and 30 keratan sulfate glycosaminoglycan chains that attach to a large domain located between three globular domains (N-terminal G1 and G2 domains and a C-terminal G3 domain) (Fig. 1). The globular domains mediate interactions with other components of the extracellular matrix. The G1 domain is responsible for interaction with hyaluronan, and the G3 domain binds tenascin and fibulin through its C-type lectin repeat domain. The C-type lectin domain may also be involved in the secretion of aggrecan from chondrocytes (2).
Fig. 1.
The structure of ACAN and the position of mutations. The black and white boxes on genomic DNA (gDNA) denote the coding regions on exons 1–18 and the untranslated regions, respectively. The mutations identified in previous studies are shown (NM_013227.3, NP_037359.3). G1, globular domain 1; IGD, interglobular domain; G2, globular domain 2; KS, keratin sulfate attachment domain; CS, chondroitin sulfate attachment domain; G3, globular domain 3. The figure is modified from that of Dateki et al. (11).
Extensive sulfation of keratan sulfate/chondroitin sulfate chains and aggregation with hyaluronan generate a substantial fixed negative charge that renders the aggregates highly hydrated, and the resulting swelling pressure confers load-bearing properties on cartilage. In addition, aggrecan regulates the expression of key growth factors and signaling molecules during the development of cartilaginous tissue and plays a key role in cartilage and bone morphogenesis (12, 13).
Animal Model of Aggrecan-associated Diseases
The cartilage matrix deficiency (cmd) mouse is the first known animal model of aggrecan-associated diseases (14). The mice harbor a 7-bp deletion in exon 5 of the aggrecan gene that leads to a premature termination codon in exon 6. The phenotype of homozygous cmd mice is characterized by dwarfism and a cleft palate, and the animals die shortly after birth because of respiratory failure. In contrast, the heterozygous mutant mice are apparently normal at birth; however, they develop proportional dwarfism with age-associated misalignment of the cervical and thoracic spine and usually die early from starvation followed by spastic paralysis. A histological examination revealed a high incidence of vertebral disc herniation and degeneration in the mutant mice. Electron microscopy of the disc cartilage in the mutant mice showed abnormal rough collagen fibrils with increased diameter, appearance of periodic banding patterns and banding formation. These findings indicate that a reduced level of aggrecan and an abnormal collagen network in the disc cartilage of the mutant mice cause disc degeneration and the resultant early-onset severe disc herniation.
ACAN Mutations in Humans
Autosomal-dominant spondyloepiphyseal dysplasia Kimberley type (SEDK) is characterized by short stature, shortening of the trunk and limbs, and early-onset osteoarthritis (OA) (15). In 2002, a linkage study identified a novel locus responsible for this disorder on chromosome 15q26 in a multigenerational South African family (16). ACAN was considered a strong candidate gene for SEDK because it maps to the same region and because an Acan mutation results in a form of chondrodysplasia in a mouse model (14). In a family with SEDK reported by Anderson et al. (1990), Gleghorn et al. (2005) screened for ACAN mutations and identified heterozygosity for a single-base pair insertion within the variable repeat region of exon 12 (OMIN 155760.0001) in affected individuals (8, 15).
The autosomal recessive ACAN mutation has only been reported in a single family (3). The disorder, termed spondyloepimetapheseal dysplasia aggrecan type, was characterized with extreme short stature (a final height of 66–71 cm) with a short neck, a barrel chest, brachydactyly, broad thumbs, and craniofacial abnormalities including macrocephaly, low-set rotated ears, prognathism, and severe mid-face hypoplasia. Genetic studies of the affected family members revealed a homozygous missense mutation (p.Asp2381Asn) in the G3 C-type lectin domain of ACAN (Fig. 1). Heterozygous carriers of the mutation in the family exhibited a mild proportionate short stature phenotype (mean adult height between –2 and –4 SDs).
The heterozygous ACAN mutations exhibit a broad phenotypic spectrum of non-syndromic short stature associated with advanced bone maturation, osteochondritis dissecans (OCD), early-onset OA, and mild dysmorphic features including mid-facial hypoplasia, brachydactyly, broad great toes, and lumbar lordosis, with no genotype-phenotype correlations (4,5,6,7,8,9,10,11). The phenotype is highly variable, even in patients from the same family.
At least 25 pathological ACAN mutations, consisting of 10 nonsense mutations, 6 frameshift mutations, 8 missense mutations, and 1 splice exon-skipping mutation have been reported (3,4,5,6,7,8,9,10,11). The locations and types of the mutations in literature are shown in Table 1 and Fig. 1, which are modified from those of Dateki et al. (11). Seventeen (68%) of the 25 mutations lead to premature stop codons, which result in early truncation of the aggrecan protein and probably nonsense mediated mRNA decay, implying that haploinsufficiency of ACAN is the main mechanism underlying the heterozygous aggrecan-related diseases (17).
Clinical Manifestations in Patients with Heterozygous ACAN Mutations
Short stature
The short stature in patients with ACAN mutations is typically associated with an advanced bone age and early cessation of growth. Individuals with ACAN mutations have mild short stature with advanced bone age at a pre-pubertal stage that leads to premature growth cessation after the start of puberty, resulting in a severely short adult height (4,5,6,7). Children with ACAN mutations are mostly proportionate, though the arm span is longer than the height in some patients (11). Premature hypertrophic chondrocyte maturation and early invasion of blood vessels and osteoblasts in the growth plate have been proposed as the mechanism for the advanced bone age, early epiphyseal fusion, and premature growth cessation in patients with ACAN mutations (12, 13). In general, a short stature is most commonly associated with a delayed bone age in endocrine disorders (GH deficiency, hypothyroidism, and Cushing syndrome), malnutrition, chronic diseases, and idiopathic short stature (1). Conversely, the combination of short stature and advanced bone age is much less common. Thus, when clinicians see patients with such a combination, an ACAN mutation may be the most likely cause of pathogenesis.
Early-onset lumbar disc herniation
We recently reported a female Japanese patient with a heterozygous ACAN mutation (p.Phe582fs*69) who had early-onset herniation of multiple lumbar discs from L1/2 to L5/S1 (Fig. 2) (11). In addition, Gkourogianni et al. reported several patients with ACAN mutations suffering from back pain and intervertebral disc disease (7). In this regard, the aggrecan-deficient cmd mice exhibit degeneration of vertebral discs leading to misalignment of the vertebral spine and early disc herniation (14). The spinal phenotype of aggrecan-deficient mice implies that ACAN mutations, which lead to reduced aggrecan levels in the cartilage and degeneration of disc cartilage, can cause early-onset and multiple spinal disc herniation and may be involved in the genetic predisposition to disc herniation in humans.
Fig. 2.

Spinal phenotype of an ACAN mutation. The radiographic image shows vertebral spicules and severe lumbar deformity (A), and the T2-weighted magnetic resonance image shows multiple lumbar disc herniation (white arrows) (B) in a female patient with a heterozygous ACAN mutation (11).
OCD and early-onset OA
ACAN mutations can affect not only the growth plate cartilage but also the articular cartilage. The related articular cartilage phenotypes have been reported as OCD and early-onset OA. OCD is characterized by the separation of an articular cartilage and subchondral bone fragment from the articular surface. To date, only two mutations in ACAN (p.Leu2355Pro and p.Val2417Met) have been associated with familial OCD (4, 9). Since both missense mutations were located at the C-terminal C-type lectin domain in ACAN, dominant negative effects of the mutant proteins have been proposed for the specific articular phenotype.
Early-onset OA has also been reported in patients with ACAN mutations (6, 7, 9). The phenotype is associated with missense, truncating, and nonsense mutations located in various regions of ACAN, indicating that there is no genotypic correlation for the phenotype.
Other facial and skeletal features
ACAN mutations have been associated with mild dysmorphologic findings including mid-facial hypoplasia, flat nasal bridge, relative macrocephaly, frontal bossing, brachydactyly, broad thumbs, and lordosis (7). The facial and skeletal phenotypes are variable, even in the same family. This may be explained by the notion that haploinsufficiency of developmental genes is usually associated with a wide range of penetrance and expressivity depending on other genetic and environmental factors (18), though the actual underlying factors remain to be identified.
Treatment of Patients with ACAN mutations
An advanced bone age at the pre-pubertal stage and premature growth cessation after the start of puberty has been noted in patients with ACAN mutations (4,5,6,7, 11). In this regard, blocking puberty through means such as GnRH analog therapy might be an option for patients with ACAN mutations. Recently, the effectiveness of combined GH and GnRH analog treatment for achieving an appropriate adult height has been reported in several cases with ACAN mutations (4, 6, 11). Gkourogianni et al. found that the average height SD score of GH-treated adult individuals with ACAN mutations (n = 5) was –2.5, while that of untreated adult individuals (n = 65) was –3.0 (7). Furthermore, Van der Steen et al. reported that patients with ACAN mutations who received GH treatment in combination with GnRH analog treatment for 2 yr from the onset of puberty were 5–8 cm taller at their adult height than their same-sex family members with the same ACAN mutation (6). In our study, the estimated final height of the elder brother who received combined GH and GnRH analog treatment was higher than that of the younger brother, who only received the GnRH analog (158.5 cm vs. 145.6 cm) (11). Collectively, these observations suggest a modest response to GH and GnRH analog treatment for adult height in patients with ACAN mutations.
Conclusion
We reviewed current knowledge regarding aggrecan-associated diseases. ACAN haploinsufficiency is a newly discovered cause of short stature with accelerated bone age. Further studies are needed to determine the incidence of ACAN mutations in patients with idiopathic short stature and to clarify the effectiveness and safety of GH and GnRH analog treatment for patients with ACAN mutations.
Conflicts of Interest: The authors declare no conflicts of interest in association with this study.
Acknowledgements
We thank Koh-ichiro Yoshiura, Hiroyuki Moriuchi, Akiko Nakatomi, Satoshi Watanabe, Hitomi Shimizu, Kei Izumi, Eiichi Kinoshita, and Hideo Baba for their fruitful discussion about this review article.
References
- 1.Baron J, Sävendahl L, De Luca F, Dauber A, Phillip M, Wit JM, et al. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol 2015;11: 735–46. doi: 10.1038/nrendo.2015.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop 2014;1: 8. doi: 10.1186/s40634-014-0008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, et al. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet 2009;84: 72–9. doi: 10.1016/j.ajhg.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson O, Guo MH, Dunbar N, Popovic J, Flynn D, Jacobsen C, et al. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab 2014;99: E1510–8. doi: 10.1210/jc.2014-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintos JB, Guo MH, Dauber A. Idiopathic short stature due to novel heterozygous mutation of the aggrecan gene. J Pediatr Endocrinol Metab 2015;28: 927–32. doi: 10.1515/jpem-2014-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Steen M, Pfundt R, Maas SJ, Bakker-van Waarde WM, Odink RJ, Hokken-Koelega AC. ACAN gene mutations in short children born SGA and response to growth hormone treatment. J Clin Endocrinol Metab 2016. (e-pub ahead of print 6 Oct 2016;doi: ). [DOI] [PubMed]
- 7.Gkourogianni A, Andrew M, Tyzinski L, Crocker M, Douglas J, Dunbar N, et al. Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. J Clin Endocrinol Metab 2016. (e-pub ahead of print 21 Nov 2016;doi: ). [DOI] [PMC free article] [PubMed]
- 8.Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet 2005;77: 484–90. doi: 10.1086/444401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, et al. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet 2010;86: 126–37. doi: 10.1016/j.ajhg.2009.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stattin EL, Tegner Y, Domellöf M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage 2008;16: 890–6. doi: 10.1016/j.joca.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Dateki S, Nakatomi A, Watanabe S, Shimizu H, Inoue Y, Baba H, et al. Identification of a novel heterozygous mutation of the Aggrecan gene in a family with idiopathic short stature and multiple intervertebral disc herniation. J Hum Genet 2017. (e-pub ahead of print 23 Mar 2017;doi: ). [DOI] [PubMed]
- 12.Domowicz MS, Cortes M, Henry JG, Schwartz NB. Aggrecan modulation of growth plate morphogenesis. Dev Biol 2009;329: 242–57. doi: 10.1016/j.ydbio.2009.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauing KL, Cortes M, Domowicz MS, Henry JG, Baria AT, Schwartz NB. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev Biol 2014;396: 224–36. doi: 10.1016/j.ydbio.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe H, Nakata K, Kimata K, Nakanishi I, Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci USA 1997;94: 6943–7. doi: 10.1073/pnas.94.13.6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson IJ, Tsipouras P, Scher C, Ramesar RS, Martell RW, Beighton P. Spondyloepiphyseal dysplasia, mild autosomal dominant type is not due to primary defects of type II collagen. Am J Med Genet 1990;37: 272–6. doi: 10.1002/ajmg.1320370223 [DOI] [PubMed] [Google Scholar]
- 16.Eyre S, Roby P, Wolstencroft K, Spreckley K, Aspinwall R, Bayoumi R, et al. Identification of a locus for a form of spondyloepiphyseal dysplasia on chromosome 15q26.1: exclusion of aggrecan as a candidate gene. J Med Genet 2002;39: 634–8. doi: 10.1136/jmg.39.9.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holbrook JA, Neu-Yilik G, Hentze MW, Kulozik AE. Nonsense-mediated decay approaches the clinic. Nat Genet 2004;36: 801–8. doi: 10.1038/ng1403 [DOI] [PubMed] [Google Scholar]
- 18.Anderson IJ, Tsipouras P, Scher C, Ramesar RS, Martell RW, Beighton P. Spondyloepiphyseal dysplasia, mild autosomal dominant type is not due to primary defects of type II collagen. Am J Med Genet 1990;37: 272–6. doi: 10.1002/ajmg.1320370223 [DOI] [PubMed] [Google Scholar]