Abstract.
The first-line pharmacological treatment for patients with maturity-onset diabetes of the young type 1 (MODY1) and maturity-onset diabetes of the young type 3 (MODY3) are sulfonylureas (SUs) or insulin. However, several reports have suggested the possibility of using incretin-associated drugs, including dipeptidyl-peptidase-4 (DPP-4) inhibitors, for the treatment of patients with these types of MODY. Here we report a case of a pediatric patient with MODY1 who was successfully treated with a DPP-4 inhibitor, alogliptin. A 13-yr-old Japanese girl with diabetes was initially treated with insulin for 5 mo. After diagnosis of MODY1, confirmed via a genetic analysis, treatment was changed from insulin to alogliptin. SUs were prescribed temporarily, but monotherapy with alogliptin finally resulted in good glycemic control. After changing to alogliptin, the patient maintained optimal glycemic control with glycated hemoglobin levels of 6.3–7.0% while maintaining substantial β-cell function. No adverse events associated with alogliptin were observed. These results suggest that DPP-4 inhibitors may be a potential treatment for patients with MODY1 at the early stage of the disease when residual insulin secretion is still being sustained.
Keywords: alogliptin, glycemic control, MODY1, pediatric patient
Introduction
Maturity-onset diabetes of the young (MODY) involves 1–2% of all diabetes cases. MODY is often diagnosed at a young age, and is characterized by autosomal dominant inheritance, non-obesity, sustained endogenous insulin secretion, and lack of β-cell autoimmunity (1,2,3). Among all MODY cases, MODY type 1 (MODY1) is rare and is caused by mutations of hepatocyte nuclear factor-4α (HNF-4α), which is a transcription factor expressed primarily in the liver, but also in the pancreas and kidneys, affecting glucose metabolism through a variety of routes (4). Fetal heterozygosity results in significant macrosomia due to increased insulin secretion in utero and subsequent neonatal transient or prolonged hypoglycemia (5). Glycosuria is not a feature of MODY1, and carriers are recognized to have decreased apolipoprotein levels (6). Endogenous insulin secretion is maintained in an early stage, and sulfonylureas (SUs) are recommended as the first-line treatment for MODY1 patients. However, insulin production is progressively reduced because of a continuous loss of β-cell function, and eventually patients with MODY 1 require insulin treatment.
Dipeptidyl peptidase-4 (DPP-4) inhibitors augment glucose-dependent insulin secretion and suppress glucagon levels through enhancement of the action of endogenous incretin by inhibiting DPP-4, an incretin-degrading enzyme. DPP-4 inhibitors are generally well tolerated because of their low risk of hypoglycemia and other adverse events. Moreover, with their potential to improve β-cell function, a core defect of type 2 diabetes, DPP-4 inhibitors are becoming a major component of the treatment of type 2 diabetes in adults (7). Alogliptin (Takeda Inc., Osaka, Japan) is a highly selective DPP-4 inhibitor, and a once daily oral administration of alogliptin has a potential glucose-lowering effect, which is similar to that of other DPP-4 inhibitors, with a low risk of hypoglycemia and of weight gain (8).
We encountered a 13-yr-old Japanese girl with diabetes who had marked hyperglycemia and who was initially treated with insulin. She was finally diagnosed as having MODY1 via gene analysis, and her treatment was then changed to the DPP-4 inhibitor alogliptin. Monotherapy with alogliptin was successful for glycemic control. We hereby report the efficacy and safety of alogliptin for the treatment of MODY1 in a pediatric patient.
Case Report
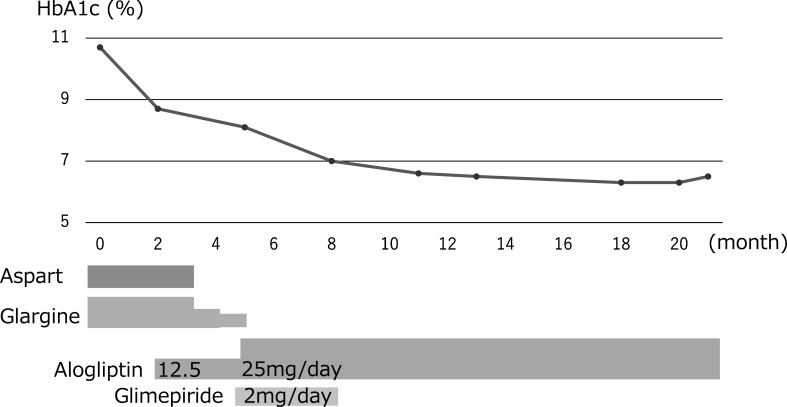
A 13-yr-old Japanese girl was referred to our department following a positive finding on a urine glucose test conducted as part of a school screening program for detecting childhood diabetes (9) in 2014. Her gestational age and weight at birth was 39 wk 0 d and 3422 g, respectively, indicating a large gestational birthweight. She had an episode of hypoglycemia at birth and received an intravenous infusion of glucose. Her maternal grandmother and paternal aunt had been diagnosed with diabetes. The patient was underweight with a body mass index of 17.5 kg/m2 and had no clinical signs indicating insulin resistance, such as acanthosis nigricans. Her urine test revealed a positive result for urine glucose and a negative result for ketonuria. She had a fasting plasma glucose (FPG) level of 315 mg/dL and a glycated hemoglobin (HbA1c) (National Glycohemoglobin Standardization Program value) level of 10.7%, which was consistent with the criteria for diagnosing diabetes. Islet autoantibodies, including antibodies against insulin, glutamic acid decarboxylase, zinc transporter 8 and insulinoma-associated protein-2, were all negative. After hospitalization, she showed preprandial plasma glucose (PG) levels > 300 mg/dL. Accordingly, we started insulin treatment to prevent progression to ketoacidosis and to achieve adequate glycemic control. She received basal-bolus insulin therapy using insulin aspart (Asp, Novo Nordisk, Bagsvaerd, Denmark) as bolus insulin, and insulin glargine (Glar, Sanofi, France) as basal insulin. The hyperglycemia improved following the introduction of insulin treatment, and then we evaluated β-cell function status following the elimination of glucotoxicity. The results were as follows: the peak value of C-peptide (CPR) on a glucagon-loading test was 3.2 ng/mL and 24-h urinary excretion of CPR was 77.7 µg. These results indicated that endogenous insulin secretion was maintained.
Based on the patient’s clinical characteristics (i.e., non-obesity, an episode of hypoglycemia at birth, testing as negative for islet autoantibodies), her suspected diagnosis was MODY. We obtained a written informed consent from the patient and her parents for MODY gene analysis. A heterozygous mutation of c.940C>T on exon 8 in the HNF-4α gene was identified, thereby confirming the diagnosis of MODY1.
After confirmation of the diagnosis of MODY1, insulin treatment was changed to another pharmacological treatment at the early stage of the disease while residual β-cell function was still sustained. The DPP-4 inhibitor alogliptin was used in addition to insulin after obtaining a written informed consent from the patient and her parents and explaining the potent glucose lowering effect and possible adverse events associated with alogliptin treatment. First, the Asp injections were eliminated before each meal with the initiation of alogliptin at the low dose of 12.5 mg daily. Continuous glucose monitoring records showed high PG levels continually after each meal, whereas the FPG level was almost normal even after eliminating the Asp injections. We increased the dose of alogliptin by 25.0 mg daily, and subsequently discontinued the Glar injection at bedtime. An SU, glimepiride, was then transiently added, at a dose of 2 mg daily in addition to alogliptin for 3 mo. Subsequently, she maintained comparatively optimal PG levels, after which glimepiride was eliminated. She then showed continued adequate glycemic control, with HbA1c levels between 6.3–7.0%, using alogliptin monotherapy for a further treatment period lasting 18 mo (Fig. 1). No adverse events associated with alogliptin, including skin rash, hepatotoxicity, pancreatitis, and hypoglycemia, were observed.
Fig. 1.
Change in HbA1c values during treatment with alogliptin.
Mutation Analysis of the MODY Gene
All coding exons, exon-intron boundaries, and promoter regions of the HNF1-α, glucokinase, HNF-4α, and HNF-1β genes were amplified from the genomic DNA of the patient. The amplified products were purified using a Wizard PCR Preps DNA Purification Kit (Promega, Madison, WI, USA) and directly sequenced using a BIGDYE TERMINATION V3.1 Cycle Sequencing Kit (Roche, Basel, Switzerland). They were then analyzed with an ABI PRISM 3100Xl automated sequencer (Applied Biosystems, Foster City, CA, USA) (10). The MODY gene analysis was approved by the Institutional Review Board at Osaka City General Hospital.
Discussion
Frequency of mutations in HNF-4α represent only 10% of all MODY cases; less than 50 mutations have been reported so far (3). The mutation of c.940C>T on exon 8 in HNF-4α, which has been not reported in the past, was identified in the patient as a nonsense mutation; thus, the protein activity is thought to be reduced. Homozygous loss of functional HNF-4α protein was found to be lethal in utero, while heterozygous loss of functional HNF-4α protein causes impaired expression of the genes involved in glucose transport and glycolysis (4). Besides, HNF-4α is an upstream regulator of transcription factor 1 (TCF1) (2), and a deficit in TCF1 results in impaired adenosine triphosphate (ATP) production in mitochondria in response to glucose (11). In MODY1 patients, insulin response to glucose stimuli progressively deteriorates, and eventually insulin treatment is required for glycemic control (1,2,3).
SUs are recommended as a first-line pharmacological treatment for patients with MODY1 and MODY3 caused by mutations of HNF-1α, which is a key gene related to TCF1. Various reports have demonstrated a good response to SUs in both patients with MODY3 (12) and MODY1 (13). The use of SUs in patients with both types of MODY is considered to represent a good example of pharmacogenomics (3). However, SUs carry a risk of hypoglycemia, which can result in a reluctance to continue long-term treatment with SUs, and weight gain is also unfavorable with using SUs. Another problem with long-term treatment with SUs is the deterioration of β-cell function, ultimately resulting in the need for insulin treatment in some cases. Østoft et al. (14) reported that the GLP-1 receptor agonist, liraglutide, has a glucose-lowering effect similar to that of the SU glimepiride, without a risk of hypoglycemia in a double-blind, randomized, crossover trial. They demonstrated that FPG, postprandial PG, and HbA1c levels significantly improved from baseline after using both glimepiride and liraglutide in adult patients with MODY3, whereas hypoglycemia occurred at a lower frequency in patients using liraglutide. Docena et al. (15) also reported the benefits of treatment with liraglutide in addition to an SU in a young adult case. Moreover, the authors previously reported a case of a 12-yr-old girl who showed a good response to liraglutide as monotherapy after being changed from insulin, and who sustained adequate glycemic control for 3 yr (16). Substantial β-cell function was maintained during the treatment period. However, she eventually required insulin treatment after progressing on to impaired endogenous insulin secretion.
In the present study, the DPP-4 inhibitor, alogliptin, was used to treat a 13-yr-old girl with MODY1. The mechanism of deterioration of glucose metabolism in MODY1 is similar to that in MODY3, but the decrease in endogenous insulin secretion could be faster in MODY1 (1,2,3). Several studies have demonstrated that GLP-1 receptor agonists have a greater PG lowering effect, with more stimulation of endogenous insulin secretion and greater inhibition of glucagon release than DPP-4 inhibitors (17,18,19). However, the injection of GLP-1 agonists on a regular basis is a burden for most patients, and weight reduction, which is a particular effect of GLP-1 receptor agonists, will not occur for non-obese patients. In the present study, we demonstrated the efficacy of monotherapy with a DPP-4 inhibitor, alogliptin, was demonstrated in a pediatric patient with MODY1. This treatment achieved sustained adequate glycemic control without the occurrence of hypoglycemia for at least 18 mo. There could be some advantages to using DPP-4 inhibitors instead of SUs in patients with MODY1. First, DPP-4 inhibitors provide a glucose-lowering effect and thereby stimulate endogenous insulin secretion in a glucose-dependent manner. This effect is not caused by ATP production, but rather occurs via a sub-pathway of insulin secretion in response to increased c-AMP production. Besides, patients with MODY1, as well as patients with MODY3, lack TCF1, resulting in impaired ATP production in mitochondria in response to glucose (11). Therefore, insulin secretion via a sub-pathway in response to increased c-AMP production by DPP-4 inhibitors, rather than due to increased ATP production, could be more effective in stimulating endogenous insulin secretion in patients with MODY1. Secondly, β-cell function in MODY1 progressively deteriorates during the course of the disease. DPP-4 inhibitors possibly have a modest effect on the improvement of β-cell function as monotherapy or combination therapy with other oral hypoglycemic drugs (20,21,22). Thirdly, DPP-4 inhibitors agonists carry a low risk of hypoglycemia, which is a major adverse event and burden in pediatric patients, in particular. For these reasons, DPP-4 inhibitors could be the optional medication for patients with MODY 1 and possibly for those with MODY3.
The limitation of the present study is that the efficacy and safety of the DPP-4 inhibitor was evaluated only in one patient with MODY1. Patients with MODY1 are rare, and it would be difficult to compare the effectiveness of DPP-4 inhibitors and SUs between the two treatment groups. Therefore, it is necessary to confirm the efficacy and safety of DPP-4 inhibitors in a larger number of patients with MODY1 and MODY3.
In conclusion, 18 months of treatment with the DPP-4 inhibitor alogliptin provided adequate glycemic control with no safety concerns in a pediatric patient with MODY1. β-cell function was also maintained with this therapy for at least 18 months. DPP-4 inhibitors may be a potential treatment for patients with MODY1 at the early stage of the disease when residual insulin secretion is still being sustained.
Conflict of interest: TU received honoraria from Novo Nordisk and Sanofi as a speaker and for attendance at advisory boards. None of the other authors have any conflicts of interest.
Acknowledgement
We thank Dr. Toru Yorifuji, Osaka City General Hospital, for mutation screening of MODY genes in the patient.
References
- 1.Andersen G, Hansen T, Pedersen O. Genetics of common forms of glycaemia with pathological impact on vascular biology: are we on the right track? Curr Mol Med 2005;5: 261–74. doi: 10.2174/1566524053766077 [DOI] [PubMed] [Google Scholar]
- 2.Gat-Yablonski G, Shalitin S, Phillip M. Maturity onset diabetes of the youngreview. Pediatr Endocrinol Rev 2006;3(Suppl 3): 514–20. [PubMed] [Google Scholar]
- 3.Kavvoura FK, Owen KR. Maturity onset diabetes of the young: clinical characteristics, diagnosis and management. Pediatr Endocrinol Rev 2012;10: 234–42. [PubMed] [Google Scholar]
- 4.Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci USA 1997;94: 13209–14. doi: 10.1073/pnas.94.24.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 2007;4: e118. doi: 10.1371/journal.pmed.0040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehto M, Bitzén PO, Isomaa B, Wipemo C, Wessman Y, Forsblom C, et al. Mutation in the HNF-4alpha gene affects insulin secretion and triglyceride metabolism. Diabetes 1999;48: 423–5. doi: 10.2337/diabetes.48.2.423 [DOI] [PubMed] [Google Scholar]
- 7.Saisho Y. Alogliptin benzoate for management of type 2 diabetes. Vasc Health Risk Manag 2015;11: 229–43. doi: 10.2147/VHRM.S68564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 2012;344: e1369. doi: 10.1136/bmj.e1369 [DOI] [PubMed] [Google Scholar]
- 9.Urakami T, Morimoto S, Nitadori Y, Harada K, Owada M, Kitagawa T. Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome-incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res 2007;61: 141–5. doi: 10.1203/pdr.0b013e31802d8a69 [DOI] [PubMed] [Google Scholar]
- 10.Yorifuji T, Fujimaru R, Hosokawa Y, Tamagawa N, Shiozaki M, Aizu K, et al. Comprehensive molecular analysis of Japanese patients with pediatric-onset MODY-type diabetes mellitus. Pediatr Diabetes 2012;13: 26–32. doi: 10.1111/j.1399-5448.2011.00827.x [DOI] [PubMed] [Google Scholar]
- 11.Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, et al. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem 1998;273: 24457–64. doi: 10.1074/jbc.273.38.24457 [DOI] [PubMed] [Google Scholar]
- 12.Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362: 1275–81. doi: 10.1016/S0140-6736(03)14571-0 [DOI] [PubMed] [Google Scholar]
- 13.Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia 2005;48: 878–85. doi: 10.1007/s00125-005-1738-y [DOI] [PubMed] [Google Scholar]
- 14.Østoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care 2014;37: 1797–805. [DOI] [PubMed] [Google Scholar]
- 15.Docena MK, Faiman C, Stanley CM, Pantalone KM. Mody-3: novel HNF1A mutation and the utility of glucagon-like peptide (GLP)-1 receptor agonist therapy. Endocr Pract 2014;20: 107–11. doi: 10.4158/EP13254.OR [DOI] [PubMed] [Google Scholar]
- 16.Urakami T, Habu M, Okuno M, Suzuki J, Takahashi S, Yorifuji T. Three years of liraglutide treatment offers continuously optimal glycemic control in a pediatric patient with maturity-onset diabetes of the young type 3. J Pediatr Endocrinol Metab 2015;28: 327–31. [DOI] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376: 431–9. doi: 10.1016/S0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- 18.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. 1860-LIRA-DPP-4 Study Group. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375: 1447–56. doi: 10.1016/S0140-6736(10)60307-8 [DOI] [PubMed] [Google Scholar]
- 19.Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. 1860-LIRA-DPP-4 Study Group. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011;65: 397–407. doi: 10.1111/j.1742-1241.2011.02656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 010 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 2008;31: 2315–7. doi: 10.2337/dc08-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q, Alogliptin Study 007 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab 2009;11: 167–76. doi: 10.1111/j.1463-1326.2008.01016.x [DOI] [PubMed] [Google Scholar]
- 22.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q, Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract 2009;63: 46–55. doi: 10.1111/j.1742-1241.2008.01933.x [DOI] [PubMed] [Google Scholar]