Abstract
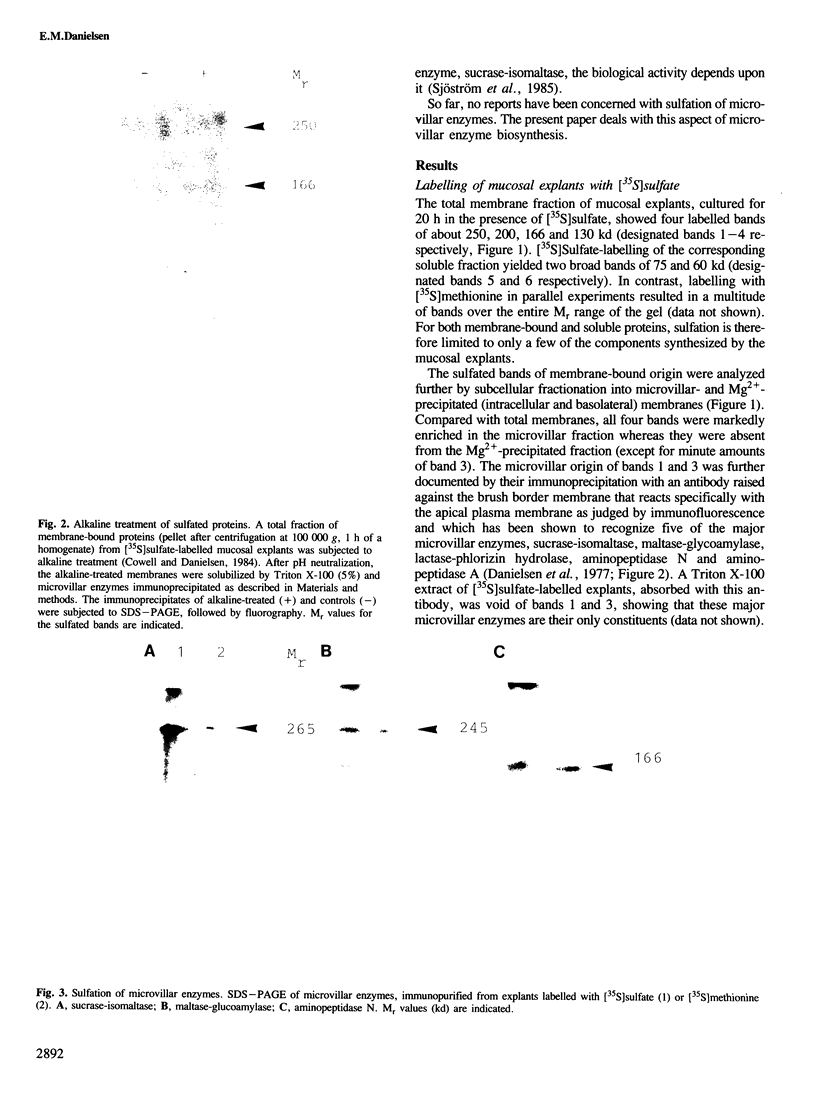
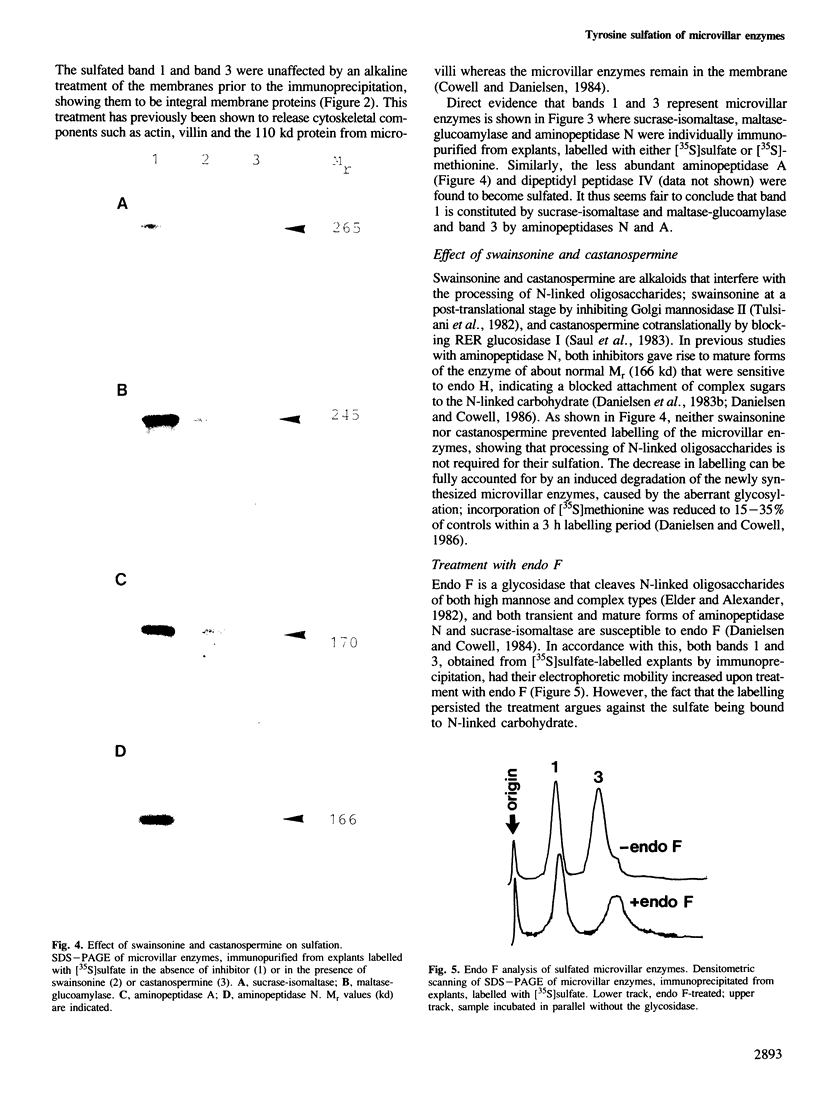
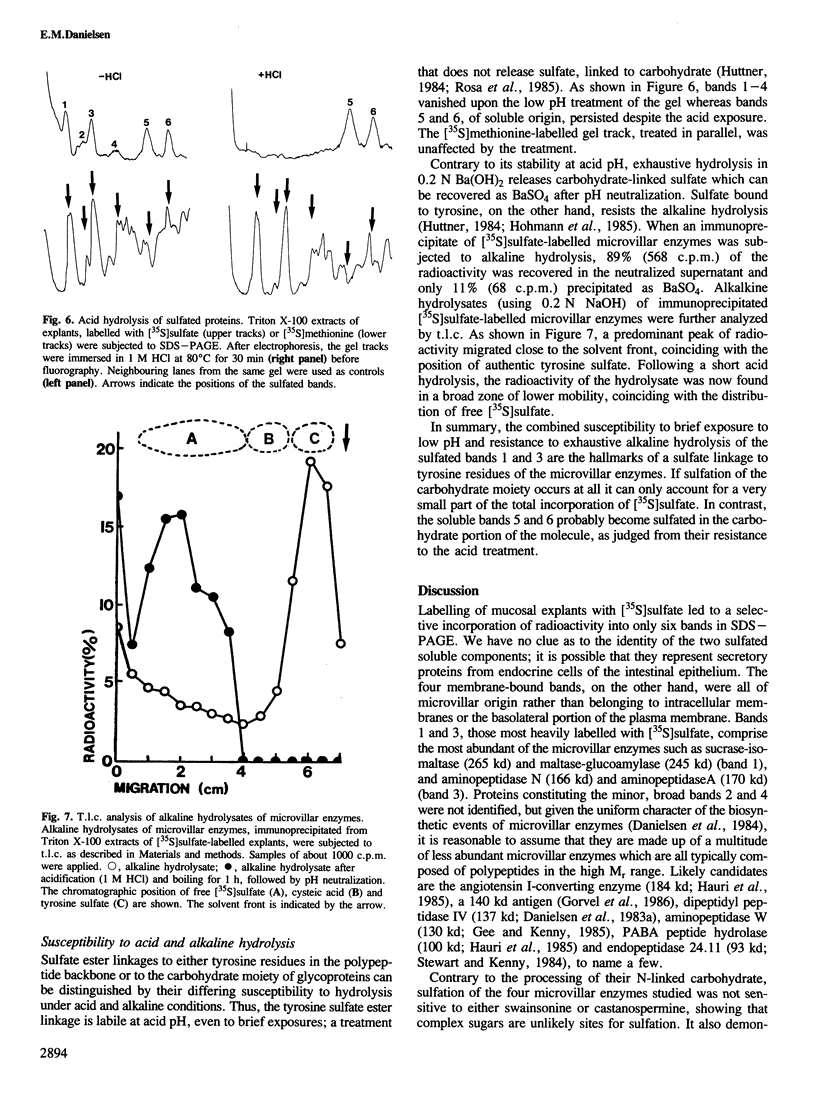
Protein sulfation in small intestinal epithelial cells was studied by labelling of organ cultured mucosal explants with [35S]-sulfate. Six bands in SDS-PAGE became selectively labelled; four, of 250, 200, 166 and 130 kd, were membrane-bound and two, of 75 and 60 kd, were soluble. The sulfated membrane-bound components were all enriched in the microvillar fraction but either absent or barely detectable in intracellular or basolateral membranes. Immunopurification of sucrase-isomaltase, maltase-glucoamylase, aminopeptidase N and aminopeptidase A showed that these microvillar enzymes become sulfated. Most if not all the sulfate was bound to tyrosine residues rather than to the carbohydrate of the microvillar enzymes, showing that this type of modification can occur on plasma membrane proteins as well as on secretory proteins.
Full text
PDF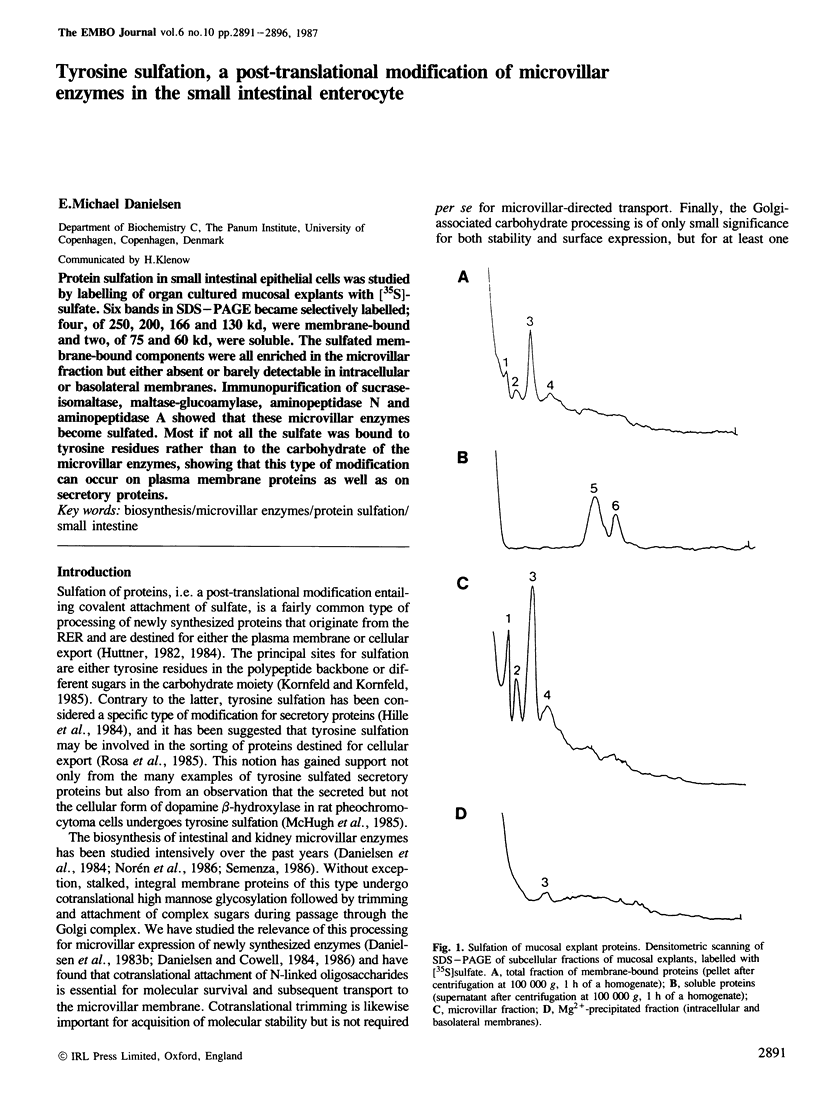


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell G. M., Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Rapid expression of cytoskeletal components in microvilli of pig small intestinal mucosal explants. FEBS Lett. 1984 Jul 9;172(2):309–314. doi: 10.1016/0014-5793(84)81147-3. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on aminopeptidase N and sucrase-isomaltase. Biochem J. 1982 Jun 15;204(3):639–645. doi: 10.1042/bj2040639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Biosynthesis of intestinal microvillar proteins. Further characterization of the intracellular processing and transport. FEBS Lett. 1984 Jan 23;166(1):28–32. doi: 10.1016/0014-5793(84)80038-1. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Biosynthesis of intestinal microvillar proteins. Processing of N-linked carbohydrate is not required for surface expression. Biochem J. 1986 Dec 15;240(3):777–782. doi: 10.1042/bj2400777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M. Microscale purification of proteins by line immunoelectrophoresis: application of the technique in protein biogenesis studies. J Biochem Biophys Methods. 1983 Aug;8(1):41–47. doi: 10.1016/0165-022x(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Norén O., Sjöström H. Biosynthesis of microvillar proteins. Biochem J. 1984 Jul 1;221(1):1–14. doi: 10.1042/bj2210001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Norén O., Sjöström H., Dorling P. R. Biosynthesis of intestinal microvillar proteins. The effect of swainsonine on post-translational processing of aminopeptidase N. Biochem J. 1983 Nov 15;216(2):325–331. doi: 10.1042/bj2160325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O. Biosynthesis of intestinal microvillar proteins. Pulse-chase labelling studies on maltase-glucoamylase, aminopeptidase A and dipeptidyl peptidase IV. Biochem J. 1983 Feb 15;210(2):389–393. doi: 10.1042/bj2100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Bro B., Dabelsteen E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J. 1982 Mar 15;202(3):647–654. doi: 10.1042/bj2020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Sjöström H., Norén O., Dabelsteen E. Immunoelectrophoretic studies on pig intestinal brush border proteins. Biochim Biophys Acta. 1977 Oct 26;494(2):332–342. doi: 10.1016/0005-2795(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Kenny A. J. Proteins of the kidney microvillar membrane. The 130 kDa protein in pig kidney, recognized by monoclonal antibody GK5C1, is an ectoenzyme with aminopeptidase activity. Biochem J. 1985 Sep 15;230(3):753–764. doi: 10.1042/bj2300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A., Rosa P., Huttner W. B. Tyrosine sulfation: a post-translational modification of proteins destined for secretion? FEBS Lett. 1984 Nov 5;177(1):129–134. doi: 10.1016/0014-5793(84)80996-5. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Gerisch G., Lee R. W., Huttner W. B. Cell-free sulfation of the contact site A glycoprotein of Dictyostelium discoideum and of a partially glycosylated precursor. J Biol Chem. 1985 Nov 5;260(25):13869–13878. [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Huttner W. B. (Glu62, Ala30, Tyr8)n serves as high-affinity substrate for tyrosylprotein sulfotransferase: a Golgi enzyme. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6143–6147. doi: 10.1073/pnas.82.18.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Baenziger J. U. In vivo and in vitro tyrosine sulfation of a membrane glycoprotein. J Biol Chem. 1986 Jan 15;261(2):856–861. [PubMed] [Google Scholar]
- McHugh E. M., McGee R., Jr, Fleming P. J. Sulfation and constitutive secretion of dopamine beta-hydroxylase from rat pheochromocytoma (PC12) cells. J Biol Chem. 1985 Apr 10;260(7):4409–4417. [PubMed] [Google Scholar]
- Rosa P., Fumagalli G., Zanini A., Huttner W. B. The major tyrosine-sulfated protein of the bovine anterior pituitary is a secretory protein present in gonadotrophs, thyrotrophs, mammotrophs, and corticotrophs. J Cell Biol. 1985 Mar;100(3):928–937. doi: 10.1083/jcb.100.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Danielsen E. M. Enzymatic activity of "high-mannose" glycosylated forms of intestinal microvillar hydrolases. J Pediatr Gastroenterol Nutr. 1985 Dec;4(6):980–983. doi: 10.1097/00005176-198512000-00021. [DOI] [PubMed] [Google Scholar]
- Stewart J. R., Kenny A. J. Proteins of the kidney microvillar membrane. Biosynthesis of endopeptidase-24.11, dipeptidylpeptidase IV and aminopeptidases N and A in pig kidney slices. Biochem J. 1984 Dec 1;224(2):549–558. doi: 10.1042/bj2240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]





