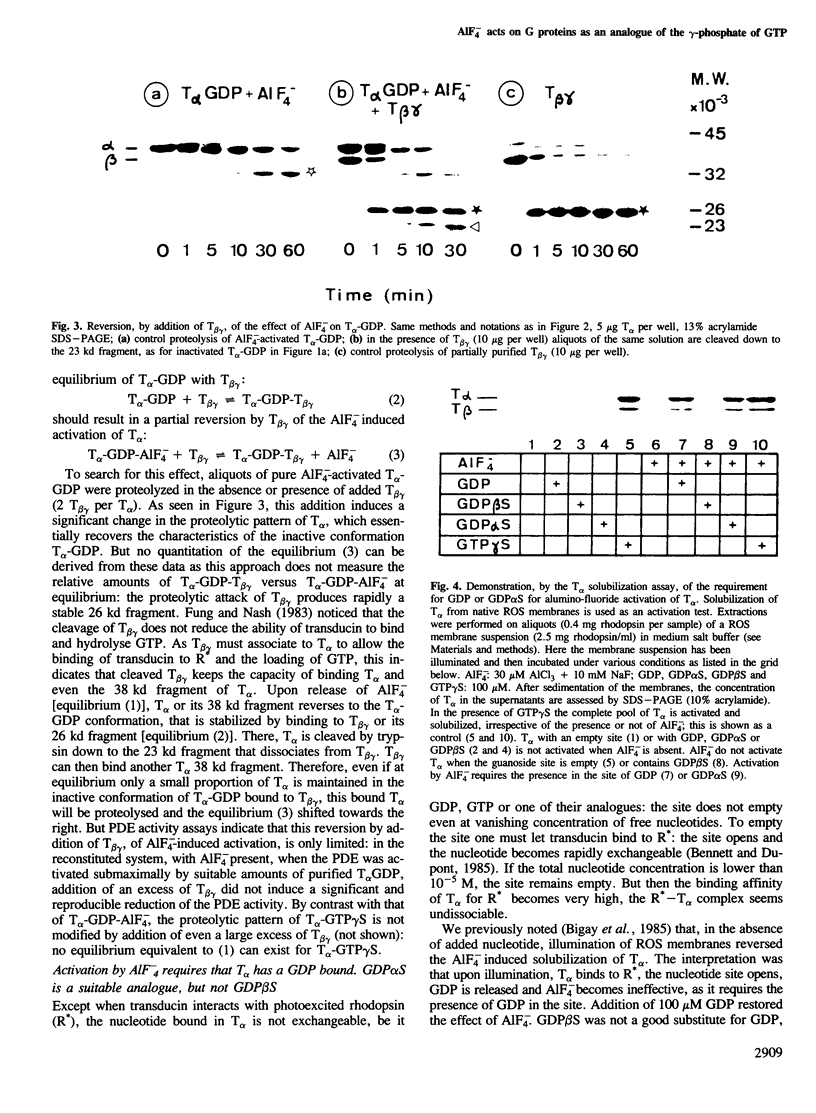
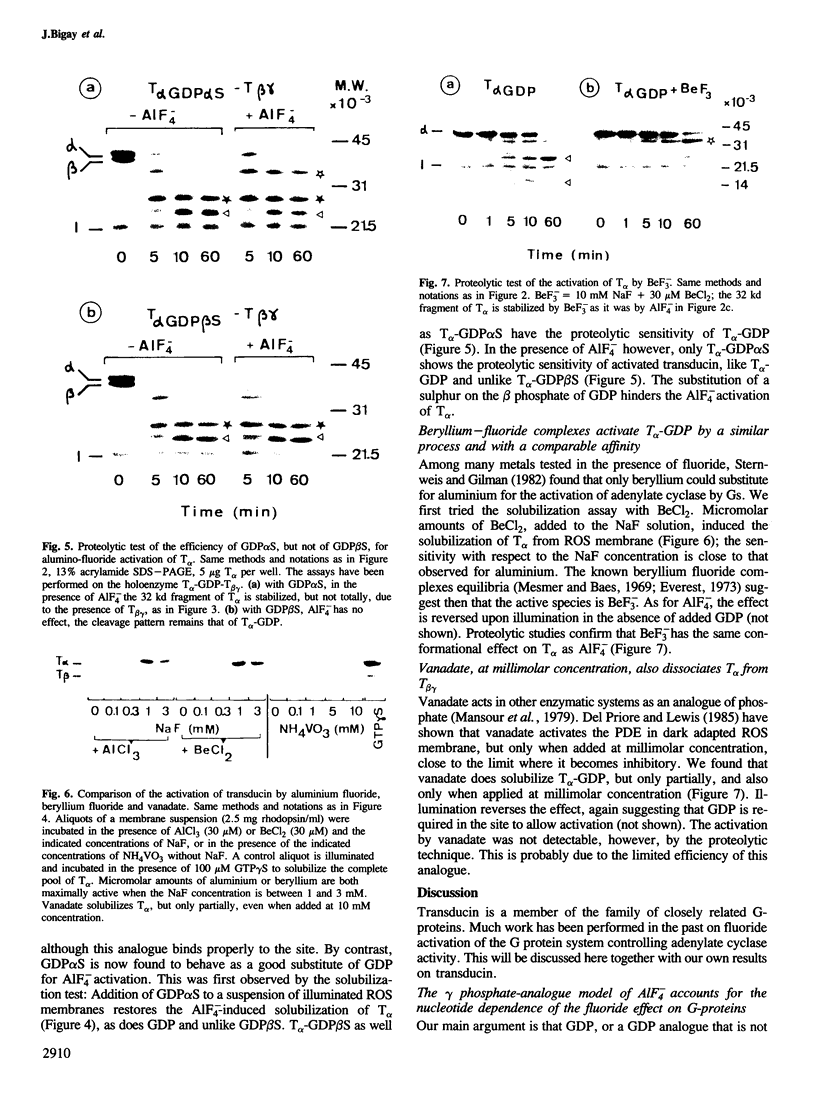
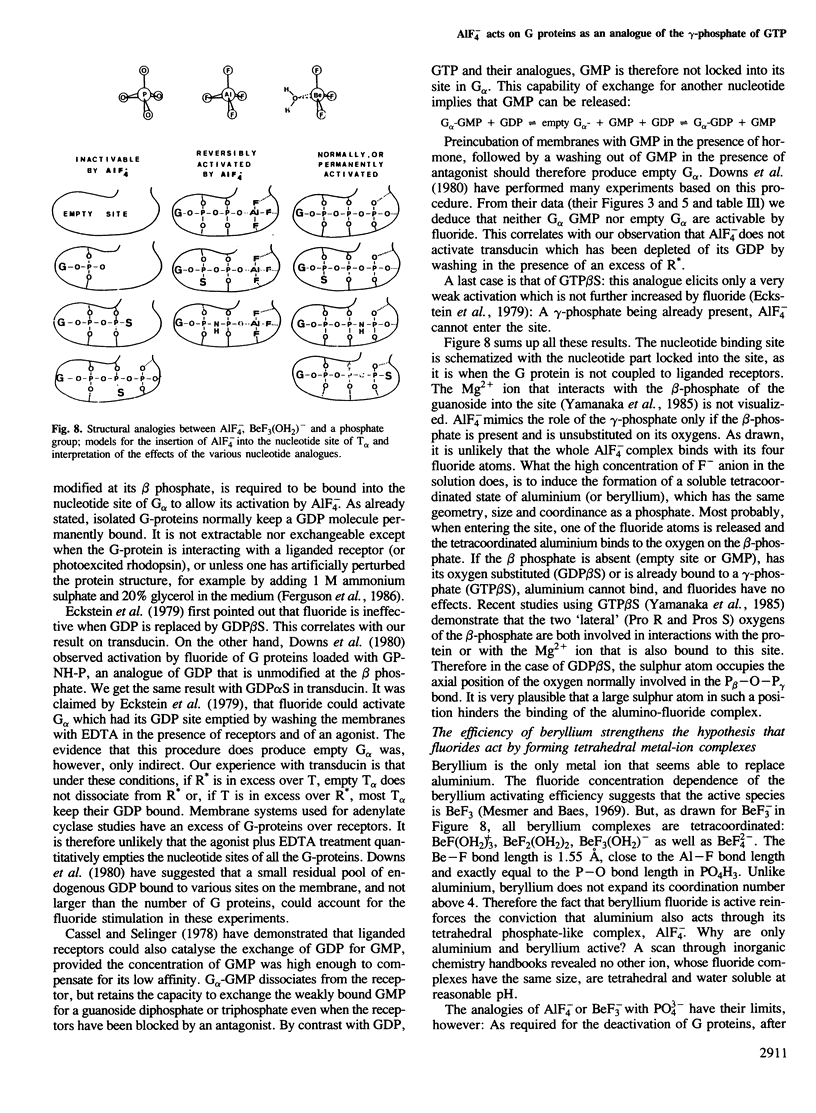
Abstract
Fluoride activation of G proteins requires the presence of aluminium or beryllium and it has been suggested that AIF4- acts as an analogue of the gamma-phosphate of GTP in the nucleotide site. We have investigated the action of AIF4- or of BeF3- on transducin (T), the G protein of the retinal rods, either indirectly through the activation of cGMP phosphodiesterase, or more directly through their effects on the conformation of transducin itself. In the presence of AIF4- or BeF3-, purified T alpha subunit of transducin activates purified cyclic GMP phosphodiesterase (PDE) in the absence of photoactivated rhodopsin. Activation is totally reversed by elution of fluoride or partially reversed by addition of excess T beta gamma. Activation requires that GDP or a suitable analogue be bound to T alpha: T alpha-GDP and T alpha-GDP alpha S are activable by fluorides, but not T alpha-GDP beta S, nor T alpha that has released its nucleotide upon binding to photoexcited rhodopsin. Analysis of previous works on other G proteins and with other nucleotide analogues confirm that in all cases fluoride activation requires that a GDP unsubstituted at its beta phosphate be bound in T alpha. By contrast with alumino-fluoride complexes, which can adopt various coordination geometries, all beryllium fluoride complexes are tetracoordinated, with a Be-F bond length of 1.55 A, and strictly isomorphous to a phosphate group. Our study confirms that fluoride activation of transducin results from a reversible binding of the metal-fluoride complex in the nucleotide site of T alpha, next to the beta phosphate of GDP, as an analogue of the gamma phosphate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Del Priore L. V., Lewis A. Vanadate, tungstate and molybdate activate rod outer segment phosphodiesterase in the dark. Biochim Biophys Acta. 1985 Apr 22;845(1):81–85. doi: 10.1016/0167-4889(85)90057-6. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Pfister C., Chabre M. Guanine nucleotides and magnesium dependence of the association states of the subunits of transducin. FEBS Lett. 1984 Dec 10;178(2):228–232. doi: 10.1016/0014-5793(84)80606-7. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Downs R. W., Jr, Spiegel A. M., Singer M., Reen S., Aurbach G. D. Fluoride stimulation of adenylate cyclase is dependent on the guanine nucleotide regulatory protein. J Biol Chem. 1980 Feb 10;255(3):949–954. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Ferguson K. M., Higashijima T., Smigel M. D., Gilman A. G. The influence of bound GDP on the kinetics of guanine nucleotide binding to G proteins. J Biol Chem. 1986 Jun 5;261(16):7393–7399. [PubMed] [Google Scholar]
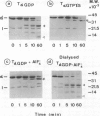
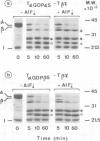
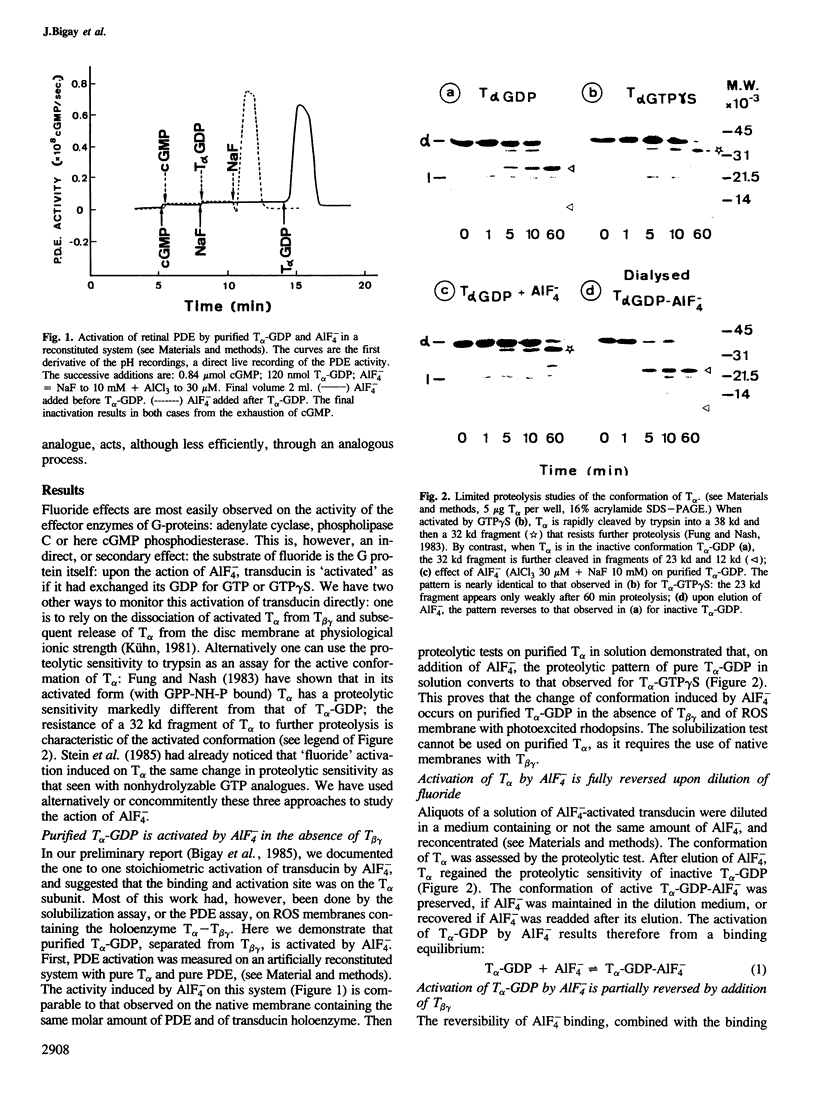
- Fung B. K., Nash C. R. Characterization of transducin from bovine retinal rod outer segments. II. Evidence for distinct binding sites and conformational changes revealed by limited proteolysis with trypsin. J Biol Chem. 1983 Sep 10;258(17):10503–10510. [PubMed] [Google Scholar]
- Guillon G., Mouillac B., Balestre M. N. Activation of polyphosphoinositide phospholipase C by fluoride in WRK1 cell membranes. FEBS Lett. 1986 Aug 18;204(2):183–188. doi: 10.1016/0014-5793(86)80808-0. [DOI] [PubMed] [Google Scholar]
- Howlett A. C., Sternweis P. C., Macik B. A., Van Arsdale P. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Association of a regulatory component of the enzyme with membranes containing the catalytic protein and beta-adrenergic receptors. J Biol Chem. 1979 Apr 10;254(7):2287–2295. [PubMed] [Google Scholar]
- Katada T., Northup J. K., Bokoch G. M., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Subunit dissociation and guanine nucleotide-dependent hormonal inhibition. J Biol Chem. 1984 Mar 25;259(6):3578–3585. [PubMed] [Google Scholar]
- Lange A. J., Arion W. J., Burchell A., Burchell B. Aluminum ions are required for stabilization and inhibition of hepatic microsomal glucose-6-phosphatase by sodium fluoride. J Biol Chem. 1986 Jan 5;261(1):101–107. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem. 1958 Jun;232(2):1065–1076. [PubMed] [Google Scholar]
- Robinson J. D., Davis R. L., Steinberg M. Fluoride and beryllium interact with the (Na + K)-dependent ATPase as analogs of phosphate. J Bioenerg Biomembr. 1986 Dec;18(6):521–531. doi: 10.1007/BF00743148. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Virmaux N., Mandel P. On the mechanism of light activation of retinal rod outer segments cyclic GMP phosphodiesterase (light activation-influence of bleached rhodopsin and KF-deinhibition). Exp Eye Res. 1977 Aug;25(2):163–169. doi: 10.1016/0014-4835(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Stein P. J., Halliday K. R., Rasenick M. M. Photoreceptor GTP binding protein mediates fluoride activation of phosphodiesterase. J Biol Chem. 1985 Aug 5;260(16):9081–9084. [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Aluminum: a requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Stereochemistry of the guanyl nucleotide binding site of transducin probed by phosphorothioate analogues of GTP and GDP. Biochemistry. 1985 Dec 31;24(27):8094–8101. doi: 10.1021/bi00348a039. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]