Abstract
Atmospheric CO2 concentrations are predicted to double within the next century. Despite this trend, the extent and mechanisms through which elevated CO2 affects grass-endophyte symbionts remain uncertain. In the present study, the growth, chemical composition and pathogen resistance of endophyte-infected (E+) and uninfected (E−) tall fescue were compared under elevated CO2 conditions. The results showed that the effect of endophyte infection on the growth of tall fescue was significantly affected by elevated CO2. Significant advantage of E+ over E− tall fescue in tiller number, maximum net photosynthetic rate and shoot biomass occurred only under ambient CO2. With CO2 concentration elevated, the beneficial effect of endophyte infection on the growth disappeared. Similarly, endophyte infection reduced lesion number and spore concentration of Curvularia lunata only under ambient CO2. These results suggest that the beneficial effect of endophyte infection on the growth and pathogen resistance of tall fescue could be counteracted by elevated CO2. An explanation for the counteraction may be found in a change in photosynthesis and nutritive quality of leaf tissue.
Introduction
Atmospheric carbon dioxide (CO2) is expected to rise from a current ambient concentration of 390 ppm to between 550 and 1000 ppm by the year 21001. CO2 enrichment in the atmosphere in general stimulates photosynthetic activity and growth of C3 plants2. CO2 enrichment has also been shown to change plant resource allocation, especially plant C:N ratio3. The carbon-nutrient balance (CNB) hypothesis predicts that carbon products in excess of those needed for primary metabolic functions will result in increased carbon-based secondary metabolites and subsequent decreased N-based secondary metabolites4. Such alterations in plant primary and secondary metabolism are expected to alter the availability of photosynthates and defensive compounds for plant-associated microbes5, modifying plant-microbial interactions such as plant-rhizobial symbiosis6, mycorrhizae7, and plant-endophyte complexes8, 9.
Endophytic fungi within asymptomatic aerial tissues of plants represent a ubiquitous component of terrestrial plant communities10. Among them, the symbiosis between cool season grasses and Epichloë endophytes is most common and considered to have important ecological implications11. In the symbiosis, the host grasses provide photosynthates and nutrients to the endophytes12, and in turn, the endophytes can benefit grasses through increased growth13, 14, and by providing tolerance to abiotic and biotic stresses11, 15–20. Increasing evidence shows that the endophyte-host interaction depends in many cases, on resource availability9. In fact, many of the studies that have found improved growth and resistance in endophyte-infected grasses were done under benign conditions of moderate to high soil nutrient availability15, 21–23.
Many studies have shown that elevated CO2 increases mycorrhizal colonization of roots and alters plant – mycorrizae interactions24–27. Similar to mycorrhiza, fungal endophytes depend on carbon and energy provided by their host plants. However, up to now, studies examining responses of grass-endophyte associations to elevated CO2 are limited8, 9, 28–30. In the pioneering study, Marks and Clay29 found in perennial ryegrass (Lolium perenne, a C3 plant) and purpletop grass (Tridens flavus, a C4 plant), the growth of endophyte-infected (E+) and uninfected (E−) plants responded similarly to CO2 enrichment. Also in perennial ryegrass, Hunt, et al.8 reported that E+ biomass tended to be greater than E− plants only at elevated CO2, and they further found that E− plants had 40% lower concentrations of soluble protein under elevated CO2 than under ambient CO2, but this CO2 effect on soluble protein was absent in E+ plants. In tall fescue (Lolium arundinaceum), Newman, et al.9 did not find interaction between CO2 concentration and endophyte infection in the growth, but they found soluble crude protein concentration increased under elevated CO2 for E− plants but not for E+ plants. Ryan, et al.30 reported that endophyte-derived alkaloids decreased in response to elevated CO2. Taken together, the effects of endophyte infection on herbage quality as well as defensive chemistry can be affected by elevated CO2. Therefore, the endophyte-induced herbivore11 and pathogen resistance18, 31 of the host are likely to be impacted by elevated CO2 in the atmosphere.
Recently, the effect of Epichloë endophyte infection on pathogen resistance has been extensively investigated. The pioneering research by Shimanuki and Sato32 demonstrated that timothy plants (Phleum pratense) infected by Epichloë typhina were resistant to the fungus Cladosporium phlei. In in vitro investigations, White and Cole33, Siegel and Latch34 and Christensen35 found that Epichloë isolates inhibited the growth of pathogenic fungi, only the antifungal activity of endophytes differed between the isolates. In in planta investigations, the positive effect of endophyte infection on pathogen resistance of the host grass has been observed in tall fescue36, 37, ryegrass38–41 and other native grasses42–44. Certainly, endophytes do not always improve disease resistance of the host. Negative45, 46 and neutral47, 48 effects have also been reported. In our previous study44, we found that endophyte could enhance pathogen resistance of Leymus chinensis, and this endophytic benefit was strengthened by drought treatment. These different reports suggest that the interactions between endophytes and pathogens are complex, and may be affected by species difference as well as environmental factors31 such as elevated CO2 concentration in the atmosphere.
In the present study, E+ and E− tall fescue were planted under contrasting CO2 availability regimes to test the effect of the endophyte infection and CO2 concentration on the performance in terms of growth, chemical composition and pathogen resistance of tall fescue. Specifically, we addressed the following questions: (1) does endophyte infection improve growth and pathogen resistance of the grass host? (2) does elevated CO2 affect growth and pathogen resistance pattern of tall fescue – endophyte associate? If this is the case, (3) what is the mechanism involved might be?
Results
Plant height, leaf number and tiller number
Plant height was only significantly affected by N availability (Table 1), and N supply increased plant height (HN = 54.19 ± 4.80; LN = 31.61 ± 2.15; cm). Leaf number was significantly increased by elevated CO2 concentration, N supply as well as endophyte infection (EC = 72 ± 28.6, AC = 63 ± 22.4; HN = 90 ± 15.3; LN = 45 ± 8.3; E+ = 75 ± 25.1, E− = 60 ± 24.7). Tiller number was significantly affected by interaction between CO2 concentration and endophyte infection (Table 1, Fig. 1). Under ambient CO2 condition, tiller number of E+ was significantly more than that of E−, but under elevated CO2 condition, no significant difference occurred.
Table 1.
Three-way ANOVA for growth characters of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under various CO2 and nitrogen conditions.
| Plant height | Tiller number | Leaf number | Maximum net photosynthetic rate | Shoot biomass | Root biomass | Root:Shoot ratio | Leaf carbon concentration | Leaf nitrogen concentration | C:N ratio | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Endophyte (E) | 0.10 | 0.76 | 39.94 | 0.00 | 33.76 | 0.00 | 3.25 | 0.08 | 24.27 | 0.00 | 2.59 | 0.12 | 0.16 | 0.69 | 4.22 | 0.05 | 12.84 | 0.00 | 11.97 | 0.00 |
| CO2 (C) | 0.96 | 0.34 | 15.96 | 0.00 | 11.19 | 0.00 | 347.42 | 0.00 | 433.83 | 0.00 | 11.03 | 0.00 | 2.03 | 0.16 | 0.13 | 0.72 | 148.34 | 0.00 | 177.49 | 0.00 |
| Nitrogen (N) | 329.10 | 0.00 | 453.03 | 0.00 | 307.93 | 0.00 | 276.61 | 0.00 | 4722.89 | 0.00 | 216.40 | 0.00 | 0.50 | 0.48 | 143.99 | 0.00 | 143.33 | 0.00 | 117.76 | 0.00 |
| E × C | 0.50 | 0.48 | 5.75 | 0.02 | 1.52 | 0.23 | 6.65 | 0.02 | 13.55 | 0.00 | 0.16 | 0.69 | 0.21 | 0.65 | 0.14 | 0.71 | 3.27 | 0.08 | 0.21 | 0.65 |
| E × N | 0.04 | 0.85 | 0.05 | 0.83 | 0.28 | 0.60 | 13.16 | 0.00 | 8.72 | 0.01 | 2.38 | 0.13 | 0.90 | 0.35 | 1.71 | 0.20 | 0.67 | 0.42 | 2.99 | 0.09 |
| C × N | 0.31 | 0.58 | 14.82 | 0.00 | 9.43 | 0.00 | 0.03 | 0.87 | 269.27 | 0.00 | 8.33 | 0.01 | 0.03 | 0.86 | 0.51 | 0.48 | 33.33 | 0.00 | 0.01 | 0.91 |
| E × C × N | 0.11 | 0.75 | 1.19 | 0.28 | 0.52 | 0.47 | 4.12 | 0.05 | 7.65 | 0.01 | 0.84 | 0.37 | 1.58 | 0.22 | 0.94 | 0.34 | 4.74 | 0.04 | 2.65 | 0.11 |
Significant P-values are in bold.
Figure 1.
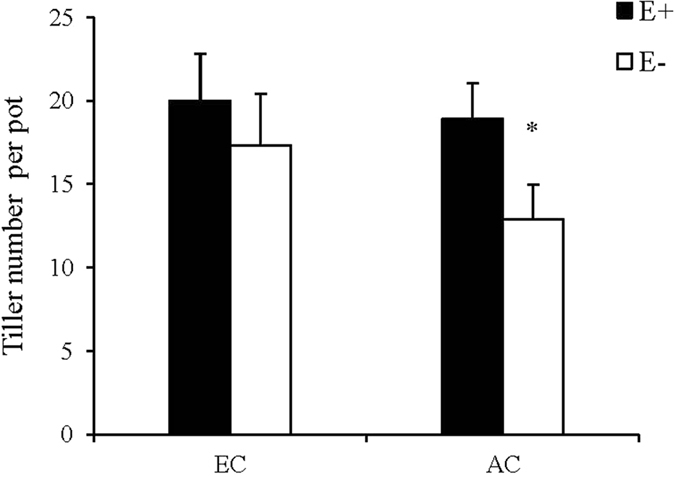
Comparison of tiller number of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under elevated CO2 (EC) and ambient CO2 (AC) conditions. *Meant significant difference at 0.05 level.
Maximum net photosynthetic rate and biomass
Maximum net photosynthetic rate and shoot biomass were significantly affected by three-way interaction among CO2 concentration, N supply and endophyte infection (Table 1). Only under ambient CO2 and high N condition, both maximum net photosynthetic rate and shoot biomass were greater in E+ than in E− plants (Fig. 2).
Figure 2.
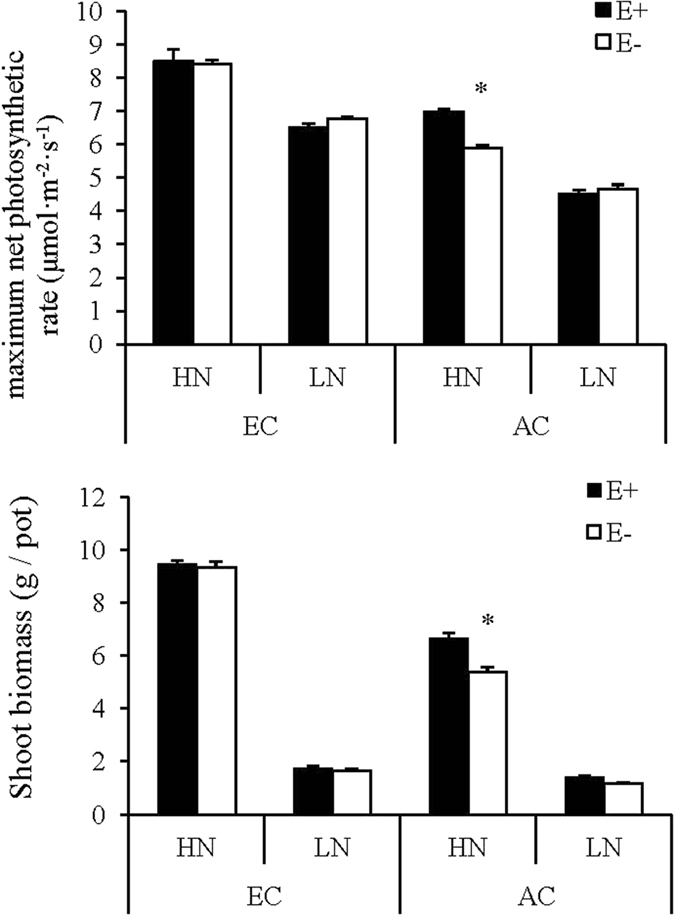
Comparison of maximum photosynthetic rate and shoot biomass of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under different CO2 and nitrogen levels. *Meant significant difference at 0.05 level.
Leaf carbon, nitrogen and C:N ratio
Leaf C concentration was significantly affected by N supply and endophyte infection (Table 1). Leaf N concentration was significantly affected by three-way interaction among CO2 concentration, N supply and endophyte infection. Leaf N concentration of E+ plants was lower than that of E− plants only under ambient CO2 and high N condition (Fig. 3a). Both elevated CO2 concentration and endophyte infection significantly improved leaf C:N ratio (Fig. 3b,c).
Figure 3.
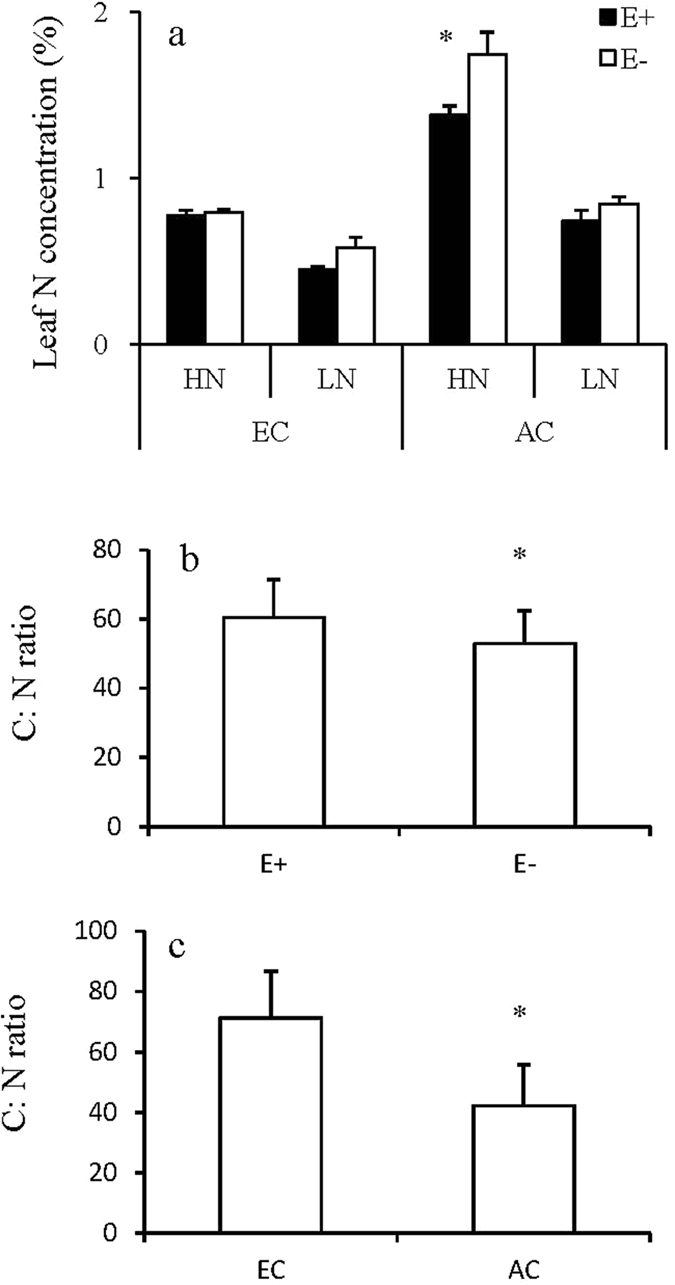
Leaf N concentration of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under different CO2 and nitrogen levels (a). Leaf C:N ratio of Festuca arundinacea under different endophyte (b) and CO2 treatments (c). *Meant significant difference at 0.05 level.
Lesion number and spore concentration of the pathogen
Both lesion number and pathogen spore concentration were significantly affected by the interaction between CO2 concentration and endophyte infection (Table 2). Under ambient CO2 concentration, endophyte infection reduced lesion number and pathogen spore concentration of the host leaves when exposed to Curvularia lunata. Elevated CO2 significantly improved pathogen resistance of both E+ and E− plants. However, no difference occurred in either lesion number or pathogen spore concentration between E+ and E− plants under elevated CO2 (Fig. 4). That is to say, the advantage in pathogen resistance of E+ over E− plants was alleviated by elevated CO2.
Table 2.
Three-way ANOVA for pathogen and physiological indices of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under various CO2 and pathogen conditions.
| Lesion number | Spore concentration | Soluble sugar concentration | Lignin concentration | RF1 | RF2 | RF3 | RF4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Endophyte (E) | 10.52 | 0.01 | 137.45 | 0.00 | 13.60 | 0.00 | 1.47 | 0.23 | 0.00 | 0.99 | 1.23 | 0.28 | 0.81 | 0.38 | 4.19 | 0.05 |
| CO2 (C) | 176.09 | 0.00 | 1662.69 | 0.00 | 16.44 | 0.00 | 14.40 | 0.00 | 12.07 | 0.00 | 28.94 | 0.00 | 0.93 | 0.34 | 0.00 | 0.96 |
| Pathogen (P) | — | — | — | — | 1.03 | 0.32 | 282.45 | 0.00 | 0.17 | 0.68 | 0.61 | 0.44 | 2.08 | 0.16 | 1.03 | 0.32 |
| E × C | 19.57 | 0.00 | 157.03 | 0.00 | 0.09 | 0.76 | 1.79 | 0.19 | 0.11 | 0.75 | 0.11 | 0.74 | 0.23 | 0.64 | 3.57 | 0.07 |
| E × P | — | — | — | — | 0.01 | 0.93 | 0.88 | 0.36 | 0.03 | 0.88 | 0.09 | 0.77 | 0.26 | 0.61 | 0.39 | 0.54 |
| C × P | — | — | — | — | 0.00 | 0.98 | 14.30 | 0.00 | 6.04 | 0.02 | 12.10 | 0.00 | 8.35 | 0.01 | 0.00 | 0.98 |
| E × C × P | — | — | — | — | 0.45 | 0.51 | 3.91 | 0.05 | 0.02 | 0.89 | 0.05 | 0.83 | 0.69 | 0.41 | 0.56 | 0.46 |
Signiflicant P-values are in bold.
Figure 4.
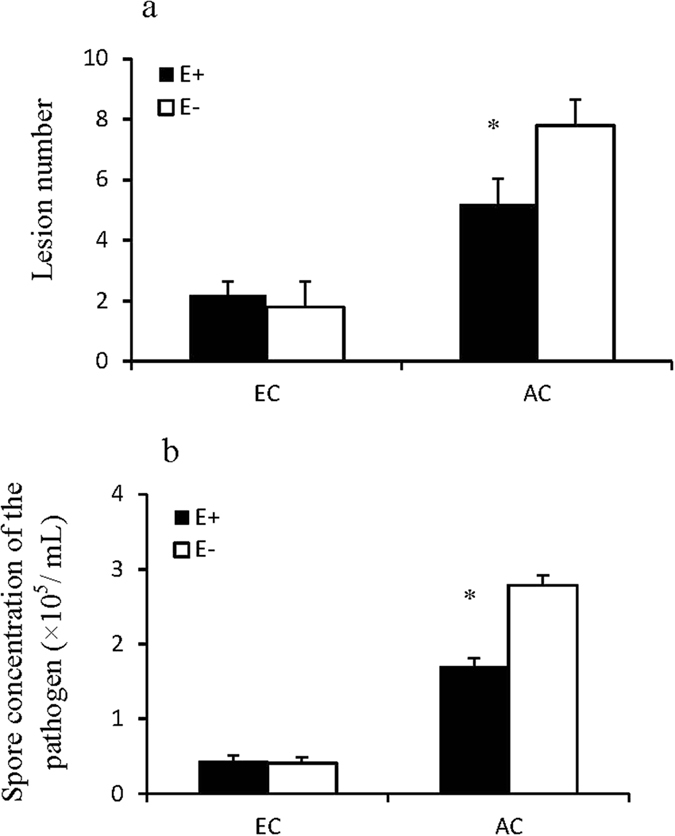
Lesion number (a) and pathogen spore concentration (b) of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under elevated CO2 (EC) and ambient CO2 (AC) treatments.*Meant significant difference at 0.05 level.
Soluble sugar and amino acids
Soluble sugar concentration was significantly affected by CO2 concentration and endophyte infection (Table 2). Elevated CO2 significantly increased soluble sugar concentration while endophyte infection significantly decreased soluble sugar concentration of tall fescue (Fig. 5).
Figure 5.
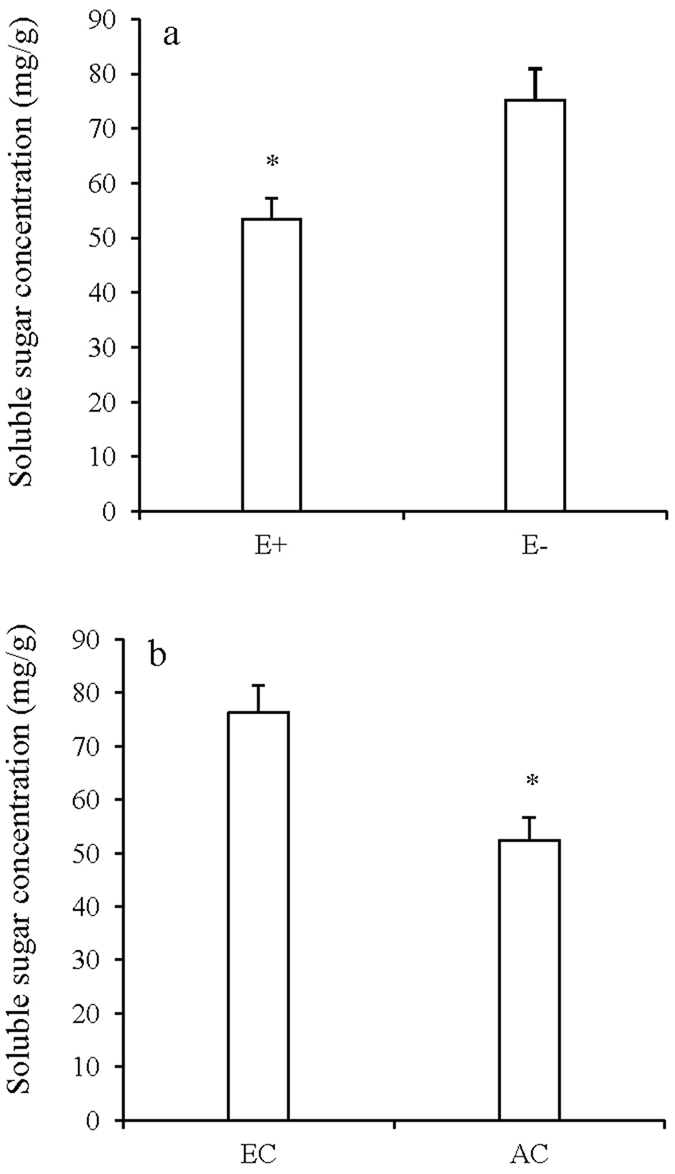
Soluble sugar concentration of Festuca arundinacea under different endophyte (a) and CO2 treatments (b). *Meant significant difference at 0.05 level.
Because the responses of the 17 amino acids that were measured were not independent, after measurement, we used a PCA to reduce the number of amino acid response variables to a new set of composite variables. To facilitate interpretation of the principal components, we subjected the first four principal components to factor rotation and retained four rotated factors (RF1, RF2, RF3, and RF4, which accounted for 82.61% of the total variance) (Fig. 6). As the values of the rotated factor increased, the variables that load heavily and positively (loading ≥ +0.5) also increased, while the variables that load heavily but negatively (loading ≤ −0.5) decreased. The standardized univariate responses of these variables are shown in Fig. 7 to facilitate the interpretation of the multivariate responses and to allow a closer inspection of the variables loading heavily onto RF1, RF2, RF3, and RF4.
Figure 6.
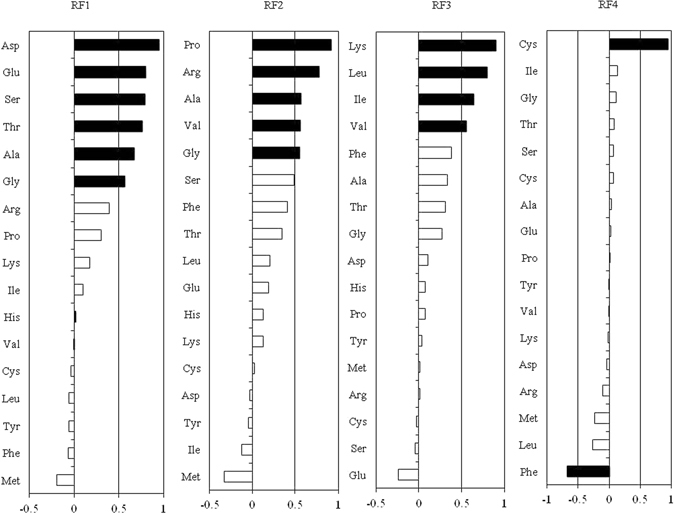
Loadings for each individual amino acid of Festuca arundinacea onto the first four rotated factors (RF). The individual amino acids loading heavily either positively (loading ≥ +0.5) or negatively (loading ≤ −0.5) are highlighted in black.
Figure 7.
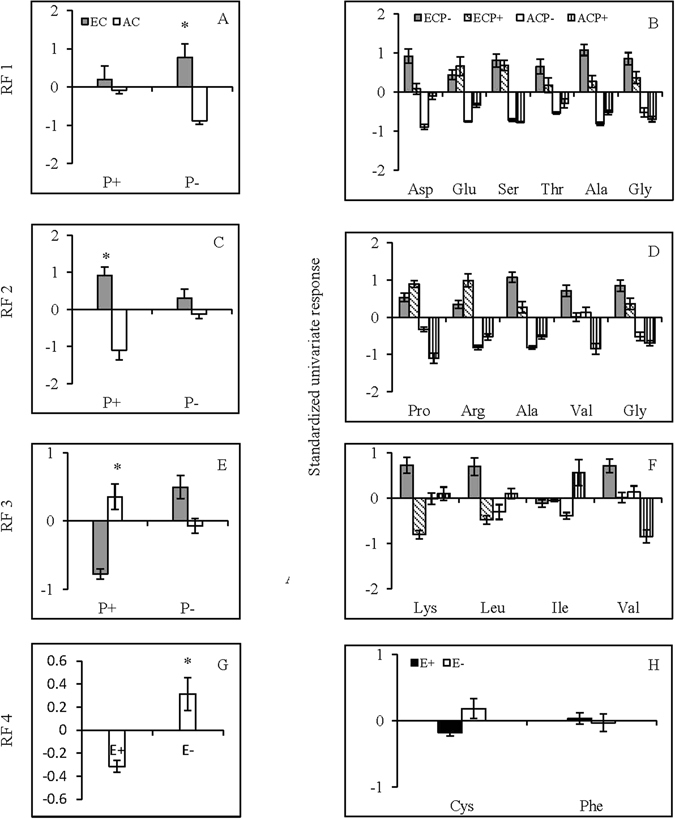
Mean response of rotated factors (RF1–4, A,C,E,G) and the standardized univariate response (B,D,F,H) of individual amino acids in Festuca arundinacea under different endophyte status (E+, endophyte-infected; E−, uninfected), CO2 concentration (EC, 800 ppm; AC, 400 ppm), and pathogen inoculation (P+, inoculated by Curvularia lunata; P−, uninoculated control). *Meant significant difference at 0.05 level.
Six amino acids, Asp, Glu, Ser, Thr, Ala and Gly loaded heavily and positively onto RF1; five amino acids, Pro, Arg, Ala, Val and Gly loaded positively onto RF2; four amino acids, Lys, Leu, Ile and Val loaded positively onto RF3 (Fig. 6). The interaction between elevated CO2 and pathogen inoculation significantly affected RF1, RF2 and RF3 (Table 2). In P- group, elevated CO2 tended to enhance RF1, RF2 and RF3, but significant effect only occurred on RF1. In P+ group, elevated CO2 increased RF2, decreased RF3 but had no effect on RF1 (Fig. 7). Cys loaded heavily and positively onto RF4, and Phe loaded heavily but negatively onto RF4 (Fig. 6). Endophyte infection significantly decreased RF4 (Table 2, Fig. 7).
Lignin accumulation
Lignin concentration was significantly affected by interaction between CO2 concentration and pathogen inoculation (Table 2). Pathogen inoculation resulted in lignin accumulation in the leaf of tall fescue under ambient CO2 concentration, and this trend was further strengthened by elevated CO2 concentration (Fig. 8). Lignin concentration was significantly affected by interactions among CO2 concentration, endophyte infection and pathogen inoculation. Only under ambient CO2 and pathogen inoculation condition, lignin concentration of the leaf was greater in E+ than in E− plants (Fig. 8).
Figure 8.
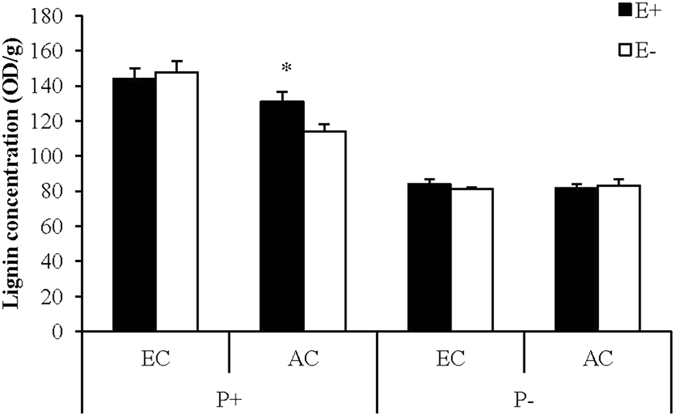
Lignin concentration of endophyte-infected (E+) or uninfected (E−) Festuca arundinacea under different CO2 concentration (EC, 800 ppm; AC, 400 ppm), and pathogen inoculation (P+, inoculated by Curvularia lunata; P−, uninoculated control). *Meant significant difference at 0.05 level.
Discussion
Plant growth response
The effects of elevated CO2 on growth of plants, especially C3 plants, have been widely studied, but most published papers on plant response to elevated CO2 fail to even state the endophyte status of their plant material. In the pioneering study, Marks and Clay29 found no significant interactions between CO2 enrichment and endophyte infection on the growth of perennial ryegrass. Similar results have been reported by Newman, et al.9 in tall fescue. In contrast, in the present study, we found a significant endophyte infection × CO2 interaction for tiller number, maximum net photosynthetic rate and shoot biomass. We found that growth advantage of E+ over E− plants occurred only under high N and ambient CO2 conditions. Under high N conditions, elevated CO2 improved shoot growth of both E+ and E− plants, but the growth advantage of E+ disappeared under elevated CO2. That is to say, elevated CO2 counteracted the beneficial effect of endophyte infection on the growth of the host. Although significant endophyte infection × N supply × CO2 interaction for growth response has not been reported, this result is consistent with most published reports in that growth advantage of E+ plants occurred under high N conditions9, 23, 30, 49, and consist with the results in tall fescue that no significant difference appeared in growth between E+ and E− plants under elevated CO2 9, 50.
In tall fescue, Brosi, et al.51 found that endophyte infection frequency was significantly higher under elevated CO2 compared to ambient; and Ryan, et al.30 found that endophyte concentration increased under elevated CO2. If fungal concentration was correlated with vegetative vigor of the host plant directly28, elevated CO2 may promote the plant-fungal endophyte mutualism. In the present study, we did find elevated CO2 improve the growth of E+ plants, but elevated CO2 improve the growth of E− plants in a higher degree, and thus a significant growth difference between E+ and E− plants did not exist anymore under elevated CO2. This phenomenon might be related to photosynthetic ability of tall fescue. Tall fescue belongs to a C3 grass. Because of the lack of CO2 concentrating ability, at ambient CO2, its carboxylation function of Rubisco is thought to be limited by CO2. With CO2 concentration in the air increasing, its photosynthetic rate will increase3, 52, 53. Under ambient CO2, photosynthetic ability of grasses can be improved by endophyte infection9, 54–56. Under elevated CO2, the carboxylation function of Rubisco in tall fescue might be near saturation, and the effect of endophyte infection on photosynthesis might be negligible. That is to say, elevated CO2 might counteract the beneficial effect of endophyte infection in photosynthesis and thus biomass to the host plants.
C and N metabolism
Independent of endophyte infection, elevated CO2 altered tall fescue tissue chemistry in some expected ways3, 57–59, such as increasing carbohydrates (here soluble sugar concentration), decreasing N concentration and thus increasing C:N ratio. As for amino acid concentrations, studies have reported both positive60, 61 and negative30, 62 effects of elevated CO2 on amino acids. In the present study, elevated CO2 tended to enhance the concentration of 12 out of 17 amino acids tested. From an herbivore perspective, increased concentrations of soluble sugar and amino acids would increase palatability63, 64. However, the subsequent decrease in the percentage of N and the increase in C:N ratio under elevated CO2 could offset this impact62, 65.
Endophyte infection has been described to result in a reduction of nitrogenous compounds in tall fescue66, 67 and ryegrass8. In the present study, we found that endophyte infection significantly decreased the soluble sugar concentration, leaf N and increased C:N ratio of the host grass. Endophyte infection had no effect on most amino acids tested, except decreased RF4. Although we found no interaction between CO2 and endophyte on soluble sugar, amino acids concentration and C:N ratio, similar to the results from Ryan, et al.30, we did find significant interaction between CO2 and endophyte infection on leaf N concentration. Under ambient CO2 and high N conditions, E+ plants had smaller leaf N concentration than E− plants. With CO2 elevated, however, no difference between E+ and E− plants occurred. Here, both elevated CO2 and endophyte infection can decrease leaf N concentration, but the decreasing degree resulted from CO2 was even larger. Under high N conditions, elevated CO2 resulted in 54.5% less while endophyte infection resulted in 20.8% less in leaf N concentration. Alkaloids are considered to contribute to defense. Although we did not measure alkaloids in the present study, both Ryan, et al.30 and Brosi, et al.51 in tall fescue found that alkaloid production decreased with CO2 concentration elevated. Ryan, et al.30 further suggested that plants where the C:N ratio was highest would have the lowest alkaloid per unit endophyte concentrations. All these results suggest that CO2 enrichment might buffer the effect of endophyte infection on the N-metabolism of host plants.
Pathogen resistance
In terms of the disease resistance of endophyte infection on the host, the beneficial effects of endophyte infection have been reported in perennial ryegrass, tall fescue and native grasses36, 39, 68. Pańka, et al.37 observed stronger susceptibility of E− tall fescue to Rhizoctonia zeae than E+ counterparts. A significant increase in resistance to dollar spot disease, caused by Sclerotinia homoeocarpa, has also been observed in Festuca rubra 42. In the present study, we found that endophyte infection improved pathogen resistance of tall fescue, but the significant effect occurred only under ambient CO2 concentration.
In studies examining plant response to fungal disease under elevated CO2, disease incidence and severity are variable, from decreased69, 70, unchanged71, 72 to increased73. When endophyte infection was considered, up to now, we found no report on its contribution to the host grasses under elevated CO2. In the present study, we found that disease severity of both E+ and E− plants decreased under elevated CO2.
The interesting result in the present study is that the advantage of E+ over E− plants in pathogen resistance under ambient CO2 disappeared with CO2 elevated. One possible explanation might be that the nutritive quality of leaves is responsible for pathogen development. Thompson and Drake74 found positive correlations existed between plant N concentration and disease severity. And this correlation has been proved by Mcelrone, et al.75 and Plessl, et al.70. In the present study, the main effects of elevated CO2 and endophyte infection were similar on reducing leaf N concentration and decreasing pathogen severity, but the contribution of elevated CO2 was even bigger. So it might be larger degree reduction of N concentration resulted from elevated CO2 that cover up the role of endophyte infection on N concentration and thus pathogen resistance. In the present study, we further found that both elevated CO2 and endophyte infection resulted in lignin accumulation in tall fescue after pathogen inoculation. Lignin is one of important phenolic compounds, whose deposition is believed to play a crucial role in barricading the pathogen from invading the plant through physical exclusion76. In the present study, lignin accumulation went along with a decrease in susceptibility and might be a factor contributing to pathogen resistance77, 78.
Our results shed some light of the effects of elevated CO2 on the mutualistic relationship between a grass and a fungus. Besides CO2 concentrations, other factors such as temperature and water availability are likely to be altered in coming years1. Therefore, the response of grass-endophyte symbiosis to pathogens will be more complex and depend largely on the specific environmental conditions encountered. Given the extensive acreage of tall fescue worldwide and the fact that the ecological effects of this grass–fungal endophyte symbiosis have been observed at population, community, and ecosystem-scales79, understanding the response of tall fescue and its endophytic fungi to climate change may be important in predicting not only the responses of pathogens, but also grazing herbivores and ecological processes such as litter decomposition and nutrient cycling.
Conclusions
Our experiments provided evidence that endophyte infection improved the growth of tall fescue, but this benefit was affected by elevated CO2 and N supply. Only under ambient CO2 and high N conditions, both maximum net photosynthetic rate and shoot biomass were greater in E+ than in E− plants. With CO2 concentration elevated, the beneficial effect of endophyte infection on the growth disappeared. Similarly, endophyte infection can enhance resistance of tall fescue towards Curvularia lunata only under ambient CO2. Elevated CO2 counteracted the beneficial effect of endophyte infection on the growth and pathogen resistance of the host grass.
Materials and Methods
Plant material
Endophyte-infected (E+) seeds of tall fescue (Lolium arundinaceum Darbyshire ex. Schreb., KY-31) were naturally infected with Epichloë coenophialum 80, 81, and uninfected (E−) seeds were acquired by eliminating the endophyte through the long-term storage of E+ seeds at room temperature. This procedure reduces the viability of the endophyte but not the seeds82. E+ and E− seeds were originally obtained from Professor Keith Clay at Indiana University, USA. The seeds used in this experiment were several generations distant from the storage treatment and came from freely cross-pollinated field-grown parents. To re-isolate the endophyte, 30 E+ and 30 E− plant individuals were randomly sampled, and the method described by Latch & Christensen83 was used with a slight modification that the time for sodium hypochlorite treatment was 8–10 min, and the petri plates containing potato dextrose agar (PDA) were incubated in the dark at 25 °C. Up to 4 weeks’ examination, only one species of endophyte, E. coenophialum, was isolated from E+ seedlings while no endophyte was found in E− seedlings. Meantime, seed germination rates for E+ and E− seeds were compared before the experiment. No significant differences were found between them, with regard to the number of days to first seedling emergence and germinations rates. Four weeks later, seven equally sized seedlings were transferred into each plastic pot (15 cm × 13.5 cm) filled with 1.4 kg of sterilized sand. After a week’s growth, they were differently treated and were placed into two separate growth chambers set at 400 or 800 ppm CO2. Plants were maintained at 30000 lux and a 12/12 h light/dark cycle at 25/20 °C, respectively. Endophyte status of the plants was checked both immediately before and after the experiment by microscopic examination from leaf sheaths stained with aniline blue described by Latch & Christensen83. We found that seedlings from E+ seeds were all infected (100%) while no seedling from E− seeds was infected (0%).
Experiment design
The present study included two experiments. In the first experiment, we addressed the questions: does endophyte improve growth of the grass host under elevated CO2 concentration? If this is the case, how does nitrogen (N) availability affect the symbiosis-dependent benefits? In the second experiment, we addressed the question: does elevated CO2 affect pathogen resistance of grass-endophyte symbiont? From the first experiment, we found that endophyte-associated benefit only occurred in high N condition. So in the second experiment, test was performed only in high N level.
Experiment 1
A three factors randomized block design was used in this experiment. The first factor was two CO2 concentrations with two levels: ambient CO2 (400 ppm, AC) and elevated CO2 (800 ppm, EC). The second factor was N availability with two levels: high N (HN) and low N (LN). The third factor was endophyte infection status: endophyte-infected (E+) and uninfected (E−). Each treatment was replicated five times, totally 40 pots.
The nutrients were supplied by the addition of a modified Hoagland nutrient solution. The composition of the nutrient solution was 5.0 mM CaCl2, 5.0 mM KCl, 2.5 mM MgSO4·7H2O, 2.0 mM KH2PO4, 29 μM Na2-EDTA, 20 μM FeSO4·7H2O, 45 μM H3BO3, 6.6 μM MnSO4, 0.8 μM ZnSO4·7H2O, 0.6 μM H2MoO4, 0.4 μM CuSO4·5H2O, and pH 6.0 ± 0.1. Nitrogen was added in the form of NH4NO3, which was delivered as 1 mM N (LN) or 10 mM N (HN), respectively. During the experiment, 100 ml of nutrient solution was added once a week to each pot, a total of 9 times. Plants were watered as necessary with deionized water. In each block, the positions of the pots were randomly rotated each week to minimize location effects. The experiment lasted for 63 days.
Experiment 2
A three factors randomized block design was used in this experiment. The first factor was two CO2 concentrations with two levels: ambient CO2 (400 ppm, AC) and elevated CO2 (800 ppm, EC). The second factor was pathogen inoculation with two levels: uninoculated control (P−) and inoculated by Curvularia lunata (P+). The third factor was endophyte infection status: E+ and E−. Each treatment was replicated five times, totally 40 pots. Pathogen inoculation was performed after 8 weeks’ growing in the growth chamber with different CO2 concentrations. All treatments were sampled at the 6th day after pathogen inoculation.
Response variables in Experiment 1
Photosynthesis parameters
At the end of experiment 1, gas exchange measurements were made on the youngest fully expanded attached leaf in a pot with a LI-COR 6400 infrared gas analyzer (LI-Cor, Lincoln, NE, USA). Under 400 μmol mol−1 or 800 μmol mol−1 CO2, net photosynthetic rate (Pn) was measured at 1,500, 1,200, 1,000, 800, 600, 400, 200, 150, 100, 50, 20 and 0 μmolm−2s−1 PPFD (photosynthetic photon flux density). According to Pn-PPFD curve, Pmax were determined.
Growth and biomass
At the end of experiment 1, regular measurement of tiller number, leaf number, and shoot height of the longest tiller were made on all ramets. Then, the shoot and the root were harvested separately. The harvested material was ven-dried at 80 °C for biomass measurement and C and N analyses.
Carbon (C) and nitrogen (N) concentration
C and N concentrations were determined using the dry combustion method with an Elemental Analyser (Vario EL/micro cube, Elementar, Hanau, Germany).
Response variables in Experiment 2
Pathogen inoculation and lesion index recorded
C. lunata was obtained from Grassland Protection Institute, Lanzhou University, China. It was originally isolated from Poa pratensis. For inoculum, the pathogen was cultured on PDA at 25 °C for 2 weeks. Spores were washed with sterile distilled water and filtered through two-layer sterile gauze. A haemocytometer was used to count the spores, and the spore concentration was 13.44 × 105/ml. Plants were inoculated by spraying the spore suspensions using a sprayer until small droplets were seen on the leaves84, and the control was sprayed with sterile distilled water. After inoculation, plants were immediately covered with a plastic bag for 36 h to maintain humidity.
Ten fully expanded mature leaves per pot were chosen for measuring the number and length of disease lesions. After measurement, pathogen spore concentration on the leaves was decided according to Nan & Li84.
Soluble sugar, amino acid and lignin
Soluble sugar content was analyzed using the phenol-sulphuric acid method according to Buysse and Merckx85. Amino acids were analyzed by reverse-phase high-performance liquid chromatography (HPLC, Waters 1500-series) with pre-column derivatization using dinitroflurobenzene (DNBF) according to Li and Sun86. Lignin measurement was according to the procedure of Reddy, et al.87.
Statistical analyses
For the amino acids, we performed a principal components analysis (PCA) on the correlations among the 17 response variables and then performed factor rotation using the varimax method63, 88. After varimax rotation, we retained four rotated factors (RF). The RF variables and all other indexes were subjected to three-way analyses of variance (ANOVA). Differences between the means were compared using Duncan’s multiple-range tests at P < 0.05. All statistical analyses were performed using SPSS 21.0 software.
Acknowledgements
This research was funded by National Key Research and Development Program (2016YFC0500702) and National Natural Science Foundation (31570433) of China.
Author Contributions
A.R. conceived and designed the experiments. W.C., H.L. and Wurihan performed the experiments. W.C. and A.R. analyzed the data. Y.G. contributed reagents, materials and analysis tools. The manuscript was written by A.R. and S.C., and reviewed by all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (ed. by T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley), pp. 148. Cambridge University Press, Cambridge, U. K. and New York, New York. doi:10.5061/dryad.56cn8015 10.1086/665032 (2013).
- 2.Ainsworth EA, Long SP. What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 3.Robinson EA, Ryan GD, Newman JA. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytologist. 2012;194:321–336. doi: 10.1111/j.1469-8137.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 4.Bryant JP, Chapin FS, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40:357–368. doi: 10.2307/3544308. [DOI] [Google Scholar]
- 5.Hu S, Firestone MK, Chapin FS. Soil microbial feedbacks to atmospheric CO2 enrichment. Trends in Ecology & Evolution. 1999;14:433–437. doi: 10.1016/S0169-5347(99)01682-1. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti S, et al. Stimulation of symbiotic N2 fixation in Trifolium repens L. under elevated atmospheric CO2 in a grassland ecosystem. Plant Physiology. 1996;112:575–583. doi: 10.1104/pp.112.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson NC, Wolf J, Koch G. Interactions among mycorrhizae, atmospheric CO2 and soil N impact plant community composition. Ecology Letters. 2003;6:532–540. doi: 10.1046/j.1461-0248.2003.00460.x. [DOI] [Google Scholar]
- 8.Hunt MG, Rasmussen SPCD, Parsons AJ, Newman JA. Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection on Lolium perenne L. growth, chemical composition and alkaloid production. Plant Cell & Environment. 2005;28:1345–1354. doi: 10.1111/j.1365-3040.2005.01367.x. [DOI] [Google Scholar]
- 9.Newman JA, et al. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Global Change Biology. 2003;9:425–437. doi: 10.1046/j.1365-2486.2003.00601.x. [DOI] [Google Scholar]
- 10.Arnold AE, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American Naturalist. 2002;160(Suppl 4):S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- 12.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- 13.Clay K. Fungal endophytes of grasses. Annual Review of Ecology & Systematics. 1990;21:275–297. doi: 10.1146/annurev.es.21.110190.001423. [DOI] [Google Scholar]
- 14.Saikkonen K, Wäli P, Helander M, Faeth SH. Evolution of endophyte–plant symbioses. Trends in Plant Science. 2004;9:275–280. doi: 10.1016/j.tplants.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hesse U, et al. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass & Forage Science. 2003;58:407–415. doi: 10.1111/j.1365-2494.2003.00393.x. [DOI] [Google Scholar]
- 16.Burns JC, Fisher DS. Intake and digestion of ‘Jesup’ tall fescue hays with a novel fungal endophyte, without an endophyte, or with a wild-type endophyte. Crop Science. 2006;46:216–223. doi: 10.2135/cropsci2005.04-0040. [DOI] [Google Scholar]
- 17.Gibert A, Hazard L. Endophyte infection of Festuca eskia enhances seedling survival to drought and cutting at the expense of clonal expansion. Journal of Plant Ecology. 2011;ume 4:201–208. doi: 10.1093/jpe/rtr009. [DOI] [Google Scholar]
- 18.Rúa MA, Mcculley RL, Mitchell CE. Fungal endophyte infection and host genetic background jointly modulate host response to an aphid-transmitted viral pathogen. Journal of Ecology. 2013;101:1007–1018. doi: 10.1111/1365-2745.12106. [DOI] [Google Scholar]
- 19.Worchel ER, Giauque HE, Kivlin SN. Fungal symbionts alter plant drought response. Microbial Ecology. 2013;65:671–678. doi: 10.1007/s00248-012-0151-6. [DOI] [PubMed] [Google Scholar]
- 20.Żurek G, Wiewióra B, Gozdowski D. Relations between bioclimatic variables and endophyte colonization of grasses in Poland. Fungal Ecology. 2013;6:554–556. doi: 10.1016/j.funeco.2013.09.001. [DOI] [Google Scholar]
- 21.Cheplick GP, Clay K, Marks S. Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytologist. 1989;111:89–97. doi: 10.1111/j.1469-8137.1989.tb04222.x. [DOI] [Google Scholar]
- 22.Lewis GC, Bakken AK, Macduff JH, Raistrick N. Effect of infection by the endophytic fungus Acremonium lolii on growth and nitrogen uptake by perennial ryegrass (Lolium perenne) in flowing solution culture. Annals of Applied Biology. 1996;129:451–460. doi: 10.1111/j.1744-7348.1996.tb05767.x. [DOI] [Google Scholar]
- 23.Ren AZ, Gao YB, Wang W, Wang JL, Zhao NX. Influence of nitrogen fertilizer and endophyte infection on ecophysiological parameters and mineral element content of perennial ryegrass. Journal of Integrative Plant Biology. 2009;51:75–83. doi: 10.1111/j.1744-7909.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 24.Rillig MC, Field CB, Allen MF. Fungal root colonization responses in natural grasslands after long-term exposure to elevated atmospheric CO2. Global Change Biology. 1999;5:577–585. doi: 10.1046/j.1365-2486.1999.00251.x. [DOI] [Google Scholar]
- 25.Hartwig UA, et al. Arbuscular mycorrhiza infection enhances the growth response of Lolium perenne to elevated atmospheric CO2. Journal of Experimental Botany. 2002;53:1207–1213. doi: 10.1093/jexbot/53.371.1207. [DOI] [PubMed] [Google Scholar]
- 26.Treseder KK. A meta‐analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytologist. 2004;164:347–355. doi: 10.1111/j.1469-8137.2004.01159.x. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Wu J, Burkey KO, Firestone MK. Plant and microbial N acquisition under elevated atmospheric CO2 in two mesocosm experiments with annual grasses. Global Change Biology. 2005;11:213–223. doi: 10.1111/j.1365-2486.2005.00905.x. [DOI] [Google Scholar]
- 28.Groppe K, et al. Interaction between the endophytic fungus Epichloë bromicola and the grass Bromus erectus: effects of endophyte infection, fungal concentration and environment on grass growth and flowering. Molecular Ecology. 1999;8:1827–1835. doi: 10.1046/j.1365-294x.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- 29.Marks S, Clay K. Effects of CO2 enrichment, nutrient addition, and fungal endophyte-infection on the growth of two grasses. Oecologia. 1990;84:207–214. doi: 10.1007/BF00318273. [DOI] [PubMed] [Google Scholar]
- 30.Ryan GD, Rasmussen S, Xue H, Parsons AJ, Newman JA. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant Cell & Environment. 2014;37:204–212. doi: 10.1111/pce.12146. [DOI] [PubMed] [Google Scholar]
- 31.Pérez LI, Gundel PE, Ghersa CM, Omacini M. Family issues: fungal endophyte protects host grass from the closely related pathogen Claviceps purpurea. Fungal Ecology. 2013;6:379–386. doi: 10.1016/j.funeco.2013.06.006. [DOI] [Google Scholar]
- 32.Shimanuki, T. & Sato, T. Studies on the mechanisms of the infection of timothy with purple spot disease caused by Cladosporium phlei (Gregory) de Vries. Research Bulletin of the Hokkaido National Agricultural Experiment Station, 1–56 (1984).
- 33.White JF, Cole GT. Endophyte-host associations in forage grasses. III. In vitro Inhibition of fungi by Acremonium coenophialum. Mycologia. 1985;77:487–489. doi: 10.2307/3793206. [DOI] [Google Scholar]
- 34.Siegel M, Latch G. Expression of antifungal activity in agar culture by isolates of grass endophytes. Mycologia. 1991;83:529–537. doi: 10.2307/3760368. [DOI] [Google Scholar]
- 35.Christensen MJ. Antifungal activity in grasses infected with Acremonium and Epichloë endophytes. Australasian Plant Pathology. 1996;25:186–191. doi: 10.1071/AP96032. [DOI] [Google Scholar]
- 36.Gwinn KD, Gavin AM. Relationship between endophyte infestation level of tall fescue seed lots and Rhizoctonia zeae seedling disease. Plant Disease. 1992;76:911–914. doi: 10.1094/PD-76-0911. [DOI] [Google Scholar]
- 37.Pańka D, West CP, Guerber CA, Richardson MD. Susceptibility of tall fescue to Rhizoctonia zeae infection as affected by endophyte symbiosis. Annals of Applied Biology. 2013;163:257–268. doi: 10.1111/aab.12051. [DOI] [Google Scholar]
- 38.Clay K. Clavicipitaceous endophytes of grasses: their potential as biocontrol agents. Mycological Research. 1989;92:1–12. doi: 10.1016/S0953-7562(89)80088-7. [DOI] [Google Scholar]
- 39.Tian P, Nan Z, Li C, German S. Effect of the endophyte Neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens. European Journal of Plant Pathology. 2008;122:593–602. doi: 10.1007/s10658-008-9329-7. [DOI] [Google Scholar]
- 40.Wiewióra B, Żurek G, Żurek M. Endophyte-mediated disease resistance in wild populations of perennial ryegrass (Lolium perenne) Fungal Ecology. 2015;15:1–8. doi: 10.1016/j.funeco.2015.01.004. [DOI] [Google Scholar]
- 41.Wheatley, W. M., Nicol, H. I., Hunt, E. R., Nikandrow, A. & Cother, N. An association between perennial ryegrass endophyte, a leaf-spot caused by Pyronophora semeniperda and preferential grazing by sheep. Proceedings of the 3rd International Conference on Harmful and Beneficial Microorganisms in Grassland, Pasture and Turf, 71–75, doi:10.1007/s00442-009-1345-6 (2000).
- 42.Clarke BB, et al. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Disease. 2006;90:994–998. doi: 10.1094/PD-90-0994. [DOI] [PubMed] [Google Scholar]
- 43.Reddy MN, Faeth S. Damping-off of Festuca arizonica caused by Fusarium. American Journal of Plant Sciences. 2010;1:104–105. doi: 10.4236/ajps.2010.12013. [DOI] [Google Scholar]
- 44.Wang X, et al. Pathogen resistant advantage of endophyte-infected over endophyte-free Leymus chinensis is strengthened by pre-drought treatment. European Journal of Plant Pathology. 2016;144:477–486. doi: 10.1007/s10658-015-0788-3. [DOI] [Google Scholar]
- 45.Krauss, J., Härri, S. A., Bush, L., Power, S. A. & Muller, C. B. Fungal grass endophytes, grass cultivars, nitrogen deposition and the associations with colonizing insects. In Proceedings of the 6th International Symposium on Fungal Endophytes of Grasses, 53–57 (2007).
- 46.Morgan-Jones G, Gams W. Notes on hyphomycetes. XLI. An endophyte of Festuca arundinacea and the anamorph of Epichloe typhina, new taxa in one of the two new sections of Acremonium. Mycotaxon. 1982;15:311–318. [Google Scholar]
- 47.Welty RE, Barker RE, Azevedo MD. Reaction of tall fescue infected and noninfected by Acremonium coenophialum to Puccinia graminis subsp. graminicola. Plant Disease. 1991;75:883–886. doi: 10.1094/PD-75-0883. [DOI] [Google Scholar]
- 48.Burpee LL, Bouton JH. Effect of eradication of the endophyte Acremonium coenophialum on epidemics of Rhizoctonia blight in tall fescue. Plant Disease. 1993;77:157–159. doi: 10.1094/PD-77-0157. [DOI] [Google Scholar]
- 49.Arachevaleta M, Bacon CW, Hoveland CS, Radcliffe DE. Effect of the tall fescue endophyte on plant response to environmental stress. Agronomy Journal. 1989;81:83–90. doi: 10.2134/agronj1989.00021962008100010015x. [DOI] [Google Scholar]
- 50.Chen X, et al. Plant nitrogen acquisition and interactions under elevated carbon dioxide: impact of endophytes and mycorrhizae. Global Change Biology. 2007;13:1238–1249. doi: 10.1111/j.1365-2486.2007.01347.x. [DOI] [Google Scholar]
- 51.Brosi GB, et al. Effects of multiple climate change factors on the tall fescue-fungal endophyte symbiosis: infection frequency and tissue chemistry. New Phytologist. 2011;189:797–805. doi: 10.1111/j.1469-8137.2010.03532.x. [DOI] [PubMed] [Google Scholar]
- 52.Poorter H, Navas ML. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist. 2003;157:175–198. doi: 10.1046/j.1469-8137.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 53.Sage RF, Kubien DS. Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynthesis Research. 2003;77:209–225. doi: 10.1023/A:1025882003661. [DOI] [PubMed] [Google Scholar]
- 54.Marks S, Clay K. Physiological responses of Festuca arundinacea to fungal endophyte infection. New Phytologist. 1996;133:727–733. doi: 10.1111/j.1469-8137.1996.tb01941.x. [DOI] [Google Scholar]
- 55.Ren AZ, et al. Benefits of a symbiotic association with endophytic fungi are subject to water and nutrient availability in Achnatherum sibiricum. Plant and Soil. 2011;346:363–373. doi: 10.1007/s11104-011-0824-9. [DOI] [Google Scholar]
- 56.Rozpądek P, et al. The fungal endophyte Epichloë typhina improves photosynthesis efficiency of its host orchard grass (Dactylis glomerata) Planta. 2015;242:1025–1035. doi: 10.1007/s00425-015-2337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettersson R, Mcdonald AJS, Stadenberg I. Response of small birch plants (Betula pendula Roth.) to elevated CO2 and nitrogen supply. Plant Cell & Environment. 1993;16:1115–1121. doi: 10.1111/j.1365-3040.1996.tb02069.x. [DOI] [Google Scholar]
- 58.Curtis PS, et al. Gas exchange, leaf nitrogen, and growth efficiency of Populus tremuloides in a CO2-enriched atmosphere. Ecological Applications. 2000;10:3–17. [Google Scholar]
- 59.Leakey AD, et al. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- 60.Geiger M, et al. Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell & Environment. 1998;21:253–268. doi: 10.1046/j.1365-3040.1998.00277.x. [DOI] [Google Scholar]
- 61.Guo H, et al. Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Global Change Biology. 2013;19:3210–3223. doi: 10.1111/gcb.12260. [DOI] [PubMed] [Google Scholar]
- 62.Sun Y, Ge F. How do aphids respond to elevated CO2? Journal of Asia-Pacific Entomology. 2011;14:217–220. doi: 10.1016/j.aspen.2010.08.001. [DOI] [Google Scholar]
- 63.Guo H, et al. Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytologist. 2014;201:279–291. doi: 10.1111/nph.12484. [DOI] [PubMed] [Google Scholar]
- 64.Johnson SN, Ryalls JMW, Karley AJ. Global climate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Annals of Applied Biology. 2014;165:62–72. doi: 10.1111/aab.12115. [DOI] [Google Scholar]
- 65.Nowak H, Komor E. How aphids decide what is good for them: experiments to test aphid feeding behaviour on Tanacetum vulgare (L.) using different nitrogen regimes. Oecologia. 2010;163:973–984. doi: 10.1007/s00442-010-1652-y. [DOI] [PubMed] [Google Scholar]
- 66.Belesky DP, Fedders JM. Does endophyte influence regrowth of tall fescue? Annals of Botany. 1996;78:499–505. doi: 10.1006/anbo.1996.0147. [DOI] [Google Scholar]
- 67.Sullivan TJ, et al. Symbiont-mediated changes in Lolium arundinaceum inducible defenses: evidence from changes in gene expression and leaf composition. New Phytologist. 2007;176:673–679. doi: 10.1111/j.1469-8137.2007.02201.x. [DOI] [PubMed] [Google Scholar]
- 68.Ma M, Christensen MJ, Nan Z. Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. European Journal of Plant Pathology. 2015;141:571–583. doi: 10.1007/s10658-014-0563-x. [DOI] [Google Scholar]
- 69.Pangga IB, Chakraborty S, Yates D. Canopy size and induced resistance in Stylosanthes scabra determine Anthracnose severity at high CO2. Phytopathology. 2004;94:221–227. doi: 10.1094/PHYTO.2004.94.3.221. [DOI] [PubMed] [Google Scholar]
- 70.Plessl M, Elstner EF, Rennenberg H, Habermeyer J, Heiser I. Influence of elevated CO2 and ozone concentrations on late blight resistance and growth of potato plants. Environmental & Experimental Botany. 2007;60:447–457. doi: 10.1016/j.envexpbot.2007.01.003. [DOI] [Google Scholar]
- 71.Percy KE, et al. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature. 2002;420:403–407. doi: 10.1038/nature01028. [DOI] [PubMed] [Google Scholar]
- 72.Pugliese M, Liu J, Titone P, Garibaldi A, Gullino ML. Effects of elevated CO2 and temperature on interactions of zucchini and powdery mildew. Phytopathologia Mediterranea. 2012;51:480–487. [Google Scholar]
- 73.Mitchell CE, Reich PB, Tilman D, Groth JV. Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Global Change Biology. 2003;9:438–451. doi: 10.1046/j.1365-2486.2003.00602.x. [DOI] [Google Scholar]
- 74.Thompson GB, Drake BG. Insects and fungi on a C3 sedge and a C4 grass exposed to elevated atmospheric CO2 concentrations in open-top chambers in the field. Plant Cell & Environment. 1994;17:1161–1167. doi: 10.1111/j.1365-3040.1994.tb02014.x. [DOI] [Google Scholar]
- 75.Mcelrone A, Reid C, Ka, Hart E, Jackson R. Elevated CO2 reduces disease incidence and severity of a red maple fungal pathogen via changes in host physiology and leaf chemistry. Global Change Biology. 2005;11:1828–1836. doi: 10.1111/j.1365-2486.2005.001015.x. [DOI] [Google Scholar]
- 76.Hammond-Kosack KE, Jones JD. Resistance gene-dependent plant defense responses. Plant Cell. 1996;8:1773–1791. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Funnell-Harris D, Jeffreyf P, Scotte S. Alteration in lignin biosynthesis restricts growth of Fusarium spp. in brown midrib sorghum. Phytopathology. 2010;100:671–681. doi: 10.1094/PHYTO-100-7-0671. [DOI] [PubMed] [Google Scholar]
- 78.Qawasmeh A, Obied HK, Raman A, Wheatley W. Influence of fungal endophyte infection on phenolic content and antioxidant activity in grasses: interaction between Lolium perenne and different strains of Neotyphodium lolii. Journal of Agricultural and Food Chemistry. 2012;60:3381–3388. doi: 10.1021/jf204105k. [DOI] [PubMed] [Google Scholar]
- 79.Omacini, M., Chaneton, E. J. & Ghersa, C. M. A framework for understanding the ecosystem consequences of endophyte grass symbioses. Neotyphodium in Cool-Season Grasses: Current Research and Applications (eds C. Roberts, C. P. West & D. Spiers), pp. 141–161. Blackwell Scientific, Oxford, UK., doi:10.1111/mec.12625 (2005).
- 80.Morganjones G, Gams W. Notes on Hyphomycetes. XLI. An endophyte of Festuca arundinacea and the anamorph of Epichloë typhina, new taxa in one of two new sections of Acremonium. Mycotaxon. 1982;50:113–117. [Google Scholar]
- 81.Leuchtmann A, Bacon CW, Schardl CL, White JF, Tadych M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia. 2014;106:202–215. doi: 10.3852/13-251. [DOI] [PubMed] [Google Scholar]
- 82.Clay K, Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science. 1999;285:1742–1745. doi: 10.1126/science.285.5434.1742. [DOI] [PubMed] [Google Scholar]
- 83.Latch GCM, Christensen MJ. Artificial infection of grasses with endophytes. Annals of Applied Biology. 1985;107:17–24. doi: 10.1111/j.1744-7348.1985.tb01543.x. [DOI] [Google Scholar]
- 84.Nan, Z. B. & Li, C. J. Neotyphodium in native grasses in China and observations on endophyte / host interactions. Proceedings of the 4th international Neotyphodium-grass interactions symposium, 41–50, doi:10.1111/mec.12451 (2000).
- 85.Buysse J, Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. Journal of Experimental Botany. 1993;44:1627–1629. doi: 10.1093/jxb/44.10.1627. [DOI] [Google Scholar]
- 86.Li D, Sun J. Determination of 18 kinds of amevo acids by HPLC with precolumn 2,4-dinitrofluorobenzene derivatization. Chemical Analysis & Meterage. 2004;13:18–20. [Google Scholar]
- 87.Reddy MVB, Arul J, Angers P, Couture L. Chitosan treatment of wheat seeds induces resistance to Fusarium graminearum and improves seed quality. Journal of Agricultural & Food Chemistry. 1999;47:1208–1216. doi: 10.1021/jf981225k. [DOI] [PubMed] [Google Scholar]
- 88.Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology. 2008;146:1440–1453. doi: 10.1104/pp.107.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]


