Abstract
Amiodarone-induced pulmonary toxicity (APT) is a severe side effect that can lead to lung fibrosis or fatal respiratory failure. Usually APT occurs during long term therapy after administration of prolonged loading doses or high cumulative doses. We present the case of a 58 year old woman who underwent thoracic surgery with lobe resection. She developed atrial fibrillation with hemodynamic-instability on the first post-operative day. We initiated amiodarone therapy and four days later she developed respiratory failure. The pulmonary function further deteriorated showing signs of an acute respiratory distress syndrome (ARDS). We therefore started mechanical ventilation, but still the gas exchange did not improve. A computer tomography-(CT)-scan presented bilateral interstitial and alveolar infiltrations. The patient also presented with leukocytosis, elevated C-reactive protein (CRP) levels however without elevated procalcitonin (PCT) concentrations. In the tracheal secretion we only harvested foam cells, but got no evidence for pathogens causing pneumonia. We immediately started glucocorticoid therapy with prednisolone 50 mg/d for five days. Almost instantaneously the gas exchange ameliorated. We were able to wean the patient from the respirator within five days. Pulmonary infiltrations were nearly vanished in a CT-scan few days later and completely disappeared in follow up examinations. This case demonstrates a per-acute onset of APT caused by a low loading dose in association with thoracic surgery. The initiation of glucocorticoid therapy in parallel to amiodarone withdrawal led to full recovery of the patient. One should consider APT when signs of pulmonary failure occur during brief periods of amiodarone therapy especially after thoracic surgery.
Keywords: Amiodarone, Pulmonary toxicity, Thoracic surgery, Glucocorticoid therapy, Inflammation, Lung fibrosis
Highlights
-
•
Amiodarone-induced pulmonary toxicity (APT) can develop after low dose and short term amiodarone therapy.
-
•
The early generation of APT is supported by thoracic surgery.
-
•
APT can cause severe adult respiratory distress syndrome, leading to respiratory failure.
-
•
Glucocorticoid therapy ameliorates APT symptoms and can restore respiratory failure in early state.
1. Introduction
Amiodarone is frequently used to treat effectively a broad variety of arrhythmias including atrial fibrillation. However it has a wide range of side effects among these APT occurs in approximately 2–10% of treated patients and it is the most severe side effect due to its association to amiodarone induced death [1]. Usually APT correlates with high cumulative doses that have been administered over months to years and with high daily doses above 400 mg [2]. The earliest appearance of APT has been reported to occur within few weeks after initiation of amiodarone treatment. The mechanisms underlying APT are rather complex and are not fully understood. It is assumed that amiodarone has either a direct toxic effect onto lung cells or via an indirect immunological pathway [3], [4]. Both processes are supported by the fact that amiodarone and its metabolite desathylamiodarone accumulate in the lung where they reach concentrations that exceed serum levels by 100–500 times [5]. The cell injury induces a persistent inflammation causing a chronic pneumonitis eventually leading to lung fibrosis [6]. Main clinical diagnostic features are newly occurring dyspnea and decreased carbon monoxide diffusion capacity [7]. In conventional X-ray images APT displays mainly the pattern of regional to diffuse alveolar or interstitial opacities and can also present as a mixture of both [8], [9]. However radiologic patterns can exist without clinical symptoms. So far we do not know any report about acute pulmonary toxicity that develops within few days after initiation of a low-dose amiodarone therapy. The following case report follows the SCARE criteria for surgical case reports [10].
2. Presentation of case
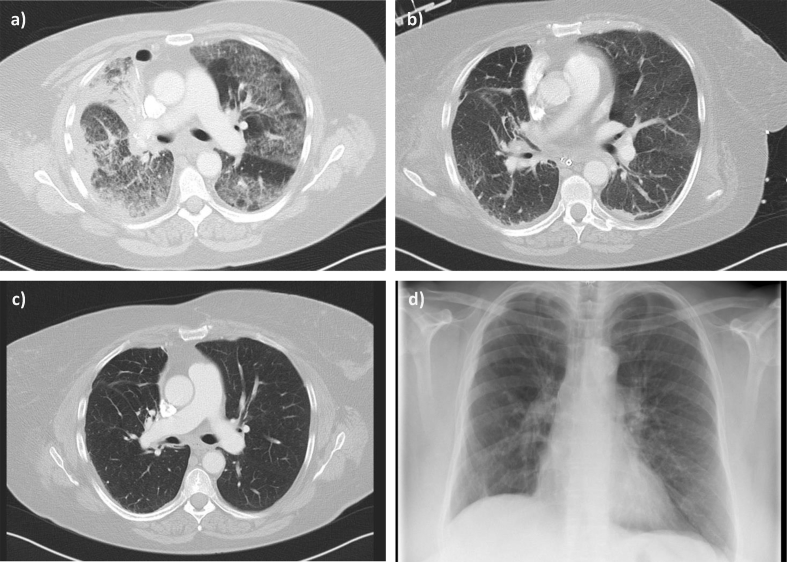
A 58 year old female patient underwent a right upper lobe resection because of an adenocarcinoma. Her medical history reported of preexisting chronic obstructive pulmonary disease, smoking, arterial hypertension, paroxysmal atrial fibrillation and diabetes mellitus type II. The lobe resection was performed via a thoracotomy under general anesthesia. The blood loss during this surgery was estimated at 800 ml, the patient received no transfusion. Two chest drains were inserted in “Bülau position” with negative pressure adjusted at 15 mmHg. Following the surgical procedure she was extubated and carried to the intensive care unit (ICU) breathing spontaneously. On the first postoperative day she developed atrial fibrillation with hemodynamic instability. According to the guidelines a biphasic cardioversion was applied three times (120 J each) without success. Heart frequency control was achieved with digitonin and metoprolol. In parallel we started oral amiodarone therapy with a loading dose of 3 times 200 mg/d. The maintenance dose of 200 mg/d was planned for further three months. Five days after starting the amiodarone therapy the patient developed dyspnea under light physical stress. A subsequent diuretic treatment did not improve the clinical aspect. Soon she developed an acute respiratory failure accompanied with leukocytosis of 32/nl but without fever. In several bronchoscopic lavages (BAL) we harvested specimen for microbiologic diagnostic. But we gained no evidence for an infectious pneumonia, however we started an empirical antibiotic therapy using piperacillin/tazobactam. In a subsequent CT-scan we detected diffuse bilateral opal consolidations (Fig. 1), pleural effusion with atelectasis of the middle lobe and enlarged pulmonary lymph nodes. A pulmonary arterial embolism was excluded. The respiratory situation further deteriorated showing the signs of a severe ARDS so we started invasive pressure controlled ventilation (FiO2: 0.9, PEEP: 14 cm H2O, PaO2/FiO2: 89). In repeated bronchoscopies we found clear mucus and physiological mucosa; in a further BAL we only harvested foam cells. A transthoracic echocardiography diagnosed normal global ventricular function. Using the pulse contour cardiac output technology we estimated slightly enhanced extravascular lung water index of 11.9 ml/kg. Histo-pathologic investigation of the excised right upper lobe obtained evidence of a adenocarcinoma with low grade differentiation. This pathological investigation provided no signs of any infection or other inflammatory or rheumatic process. Since we had no evidence for an infection we suspected an APT, only CRP levels were increased while PCT serum levels were unaltered (Table 1). We immediately stopped the amiodarone therapy and started prednisolone therapy with 50 mg/d. Several hours after the first prednisolone dose was administered the respiratory situation improved and we weaned the patient from the respirator. She was extubated three days later, still requiring non-invasive ventilation. She was discharged from the ICU to a low care ward two days later. Additionally the radiological aspect improved as demonstrated in a further CT-scan performed at the day she left the ICU (Fig. 1). The prednisolone therapy was maintained and slowly tapered. The patient fully recovered without any sign of recurrent symptoms after complete withdrawal of the glucocorticoid therapy. The Patient was routinely re-evaluated during the follow-up care period of two years. She still presents in a good condition with no further restrictions in her daily life. Consecutive CT-scans demonstrated no lung infiltrations or fibrotic remodelling one year after the APT incidence (Fig. 1c). Also in conventional thoracic radiograph taken two years later we found no structural residues of the APT (Fig. 1d).
Fig. 1.
CT-image of patient with amiodarone-induced pulmonary toxicity. a) Five days after starting amiodarone therapy bilateral alveolar and interstitial infiltrations became obvious. Apparently only little pleural effusion was found in the right cavity, following surgery on this side. b) Almost complete recovery from pneumonitic infiltrations after withdrawal of amiodarone therapy and treatment with glucocorticoids for five days. c) CT-scan recorded one year after the APT incidence displays complete remission of pulmonary infiltrates. d) A conventional anterior-posterior radiograph taken twenty-two months after APT demonstrates almost normal pulmonary structures without pulmonary residues.
Table 1.
Serological findings after initiating amiodarone therapy.
| Day 1 | Day 2 | Reference | |
|---|---|---|---|
| leukocytes | 25,8 | 26,2 | 4,0–10,0/nl |
| hemoglobin | 9,1 | 8,6 | 12–16 g/dl |
| platelets | 575 | 571 | 150–450/nl |
| creatinine | 0,57 | 0,6 | <1,1 mg/dl |
| CRP | 39,2 | 41,4 | <1,0 mg/dl |
| PCT | <0,1 | 0,17 | <0,1 μg/l |
| TSH | 1,0 | 0,46–4,6 mU/l | |
| Troponin I | 0,03 | <0,034 μg/l |
3. Discussion
APT has initially been reported with varying incidences reaching 61% of treated patients [11]. Albeit in recent studies the incidence of APT ranges between 1.6% and 2.9%. These reduced APT incidences are nowadays achieved by using lower loading doses over brief periods followed by low maintenance doses [7], [12].
However, APT may still occur after several months of amiodarone treatment and can also remain undetected or underestimated especially in intensive care patients [3], [12], [13]. The present case shows an unusual early development of APT. So far it has been reported, that APT follows after high dose amiodarone application for more than a week [14]. Thus we observed severe respiratory failure following five days after initiation of a low dose amiodarone therapy. So far this seems to be uncommon, in the current literature the period until respiration is being impaired is more prolonged ranging up to several months [15], [16], [17]. In few case reports APT became apparent only after the application of high cumulative amiodarone doses above 12.5 g which often lead to fatal outcome yet that is in contrast to this case [18], [19], [20]. Fatal outcomes may also occur long after the initiation of amiodarone therapy and too independently from the cumulative dose after the therapy had been finished. Overall mortality rates of APT range between 1 and 33% depending on the respiratory situation [2], [19]. Further, thoracic surgery promotes the onset of APT, this observation is supported by the assumption that inflammatory processes are responsible for the generation of APT. The latter predominantly causes fatal outcome [14], [21], [22]. But there exists a high inter-individual diversity of amiodarone susceptibility ranging from early toxicity already induced after short term treatment <14 d to cumulative doses <10 g accompanied with low serum levels [19], [23]. It has also been reported that low maintenance doses <305 mg/d do not prevent APT. According to the current data it is recommended to apply 200 mg/d as a maintenance dose to keep the probability for APT as low as possible [19].
Pivotal for APT generation is the amiodarone accumulation and its major metabolite desethylamiodarone in the lung where both reach concentrations of 100–500 fold compared to serum levels [5]. These high pulmonary concentrations also exceed concentrations in the heart, the classical amiodarone target. Further, amiodarone is compartimentalized mainly in pneumocytes type II. This accumulation accompanied with low tissue clearance rates further the theory of a direct toxicity [2], [14]. However immunologic processes may also be relevant for the induction of long term lung damage or fibrosis [24]. The findings of the present case support an immunologic process in the generation of APT. We observed high serum concentrations of CRP and leukocytes but unaltered PCT levels and several sterile BAL probes. In the lung lobe that was excised before initiating the amiodarone therapy we found no evidence of inflammation or fibrosis, indicating that amiodarone was the sole inducer of the inflammation. Typical for APT seems to be the infiltration of CD8 T lymphocytes that can be found via BAL or in tissue biopsies [25]. Here we mainly observed neutrophil infiltration, small numbers of lymphocytes and foam cells.
APT can occur in treated patients with different manifestation forms. A chronic interstitial pneumonitis can be found most often that goes along with ARDS, organizing pneumonia and/or a solitary mass [1], [26]. In our patient we found a bilateral interstitial pneumonitis with clinical symptoms of an ARDS becoming already visible after brief therapy and with a low cumulative dose. We found no sign of lung fibrosis, which usually develops after long term exposure. The diagnosis criteria of APT are still not defined and too there exist no consistent monitoring mechanisms of this disease. Clinical findings are dyspnoea and unproductive cough thus these symptoms are rather unspecific. During APT these symptoms may exist over month with progressive character until a diagnosis has been found.
In patients that are believed to suffer from APT several different diagnostic methods should be performed to exclude other pathologic pulmonary functions. Before starting an amiodarone therapy it is recommended to X-ray the lung [27]. In our patient we already had several X-ray images before and after der thoracic surgery. Additionally when the first symptoms occurred we immediately performed a CT-scan. This allowed us to evaluate morphological changes in detail. The distribution pattern of the pulmonary alterations was typically bilateral, with interstitial and alveolar infiltrations displaying the characteristics for alveolar proteinosis [28], [29]. The asymmetrical or patchy infiltrations found in the CT-scan seem to be common in APT. Conventional chest X-ray images may underestimate the extent of the APT thus the CT-images are superior to detect early infiltrations or changes in lung structure especially in prone position [30]. The impaired diffusion capacity of carbon monoxide (>20%) can be used to strengthen the diagnosis, however it is recommended to measure carbon monoxide diffusion capacity already before initiating amiodarone therapy [31], [32]. So far serological findings are non-specific for APT they only can indicate an inflammatory process [3], [33].
To minimize the likelihood of APT it is recommended to induce the therapy for supraventricular arrhythmias with low amiodarone loading doses of 600 mg/d for four weeks or 1000 mg/g for seven days. The maintenance dose should range between 100 and 400 mg/d [33].
When there exists strong suspicion for APT it is of paramount importance to permanently interrupt the amiodarone administration, thus this may only be sufficient in some situations. However, often the withdrawal of amiodarone does not improve the respiratory function and in long term observations it has been noticed that symptoms of APT may return more than eight months after amiodarone therapy has been finished [1], [34]. The latter we did not observe in the follow up period, whether in radiologic scans nor in clinical investigations. The administration of glucocorticoids can reduce the inflammatory reaction and improve clinical symptoms. It may be necessary to extend the corticoid therapy for several months to attain complete recovery [19]. A continuation of the amiodarone therapy has to be avoided while the patient still receives glucocorticoids, since recurrent symptoms with progressive impaired lung function have been observed under these conditions. It is difficult to recommend a certain corticoid dosage because no controlled clinical trials exist investigating this issue. So far doses of prednisolone between 40 mg and 60 mg per day are recommended over periods of days to weeks [19], [23]. Glucocorticoid therapy of more than six months is required to avoid any return of the symptoms then it should be reduced slowly. In our patient we observed a full recovery within few days, clinical symptoms improved immediately accompanied by normalised CT-scan. The procedure of short term glucocorticoid therapy may be insufficient in patients receiving high doses amiodarone over longer periods. Thus the approach in glucocorticoid therapy of APT in our patient can't be transferred in general to all other cases. In follow up investigations of two years we observed a full recovery of the radiologic scans and of clinical signs. Many patients who received high doses of amiodarone present in long term investigations structural changes in terms of lung fibrosis and too they permanently suffer from impaired gas exchange. In the case of returning symptoms or impairment of pulmonary function the corticoid therapy has to be continued. A recurring APT may be more severe and can lead to fatal outcome. The lung-fibrosis induced by amiodarone is an irreversible process and glucocorticoids may only briefly improve clinical aspects. Patients developing ARDS often require mechanical ventilation, because respiratory failure is rapidly progressing and it responds rarely to glucocorticoid treatment.
4. Conclusion
In contrast to our existing knowledge APT can be triggered by low cumulative doses during short therapeutic periods. This issue has to be considered especially in cases with pre-existing lung disease and/or in combination with thoracic surgery. One should be aware of sudden appearing symptoms that indicate respiratory insufficiency without any evidence for infection. However, infections have to be excluded by microbiological monitoring in combination with control of serum parameters that indicate inflammation or infection. Glucocorticoid therapy should be started after confirmation of APT. When clinical symptoms improve very soon the patient will almost completely recover.
Ethical approval
The patient was informed that the data concerning her case would be submitted for publication. The patient gave written consent to publish her case.
Sources of funding
Heiko Baumann: none.
Phillip Fichtenkamm: none.
Thomas Schneider: none.
Jürgen Biscoping: none.
Michael Henrich: none.
Author contribution
Heiko Baumann: data collection, data analysis, writing.
Phillip Fichtenkamm: data collection, data analysis.
Thomas Schneider: data collection, data analysis.
Jürgen Biscoping: data analysis, writing.
Michael Henrich: data collection, data analysis, writing.
Conflicts of interest
Heiko Baumann: none.
Phillip Fichtenkamm: none.
Thomas Schneider: none.
Jürgen Biscoping: none.
Michael Henrich: none.
Guarantor
Heiko Baumann.
Michael Henrich.
References
- 1.Schwaiblmair M., Berghaus T., Haeckel T. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clin. Res. Cardiol. 2010;99:693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 2.Hughes M., Binning A. Intravenous amiodarone in intensive care. Time for a reappraisal? Intensive Care Med. 2000;26:1730–1739. doi: 10.1007/s001340000668. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafian H., Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120:275–282. doi: 10.1378/chest.120.1.275. [DOI] [PubMed] [Google Scholar]
- 4.Ott M.C., Khoor A., Leventhal J.P. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646–651. doi: 10.1378/chest.123.2.646. [DOI] [PubMed] [Google Scholar]
- 5.Brien J.F., Jimmo S., Brennan F.J. Distribution of amiodarone and its metabolite, desethylamiodarone, in human tissues. Can. J. Physiol. Pharmacol. 1987;65:360–364. doi: 10.1139/y87-062. [DOI] [PubMed] [Google Scholar]
- 6.Beasley M.B. The pathologist's approach to acute lung injury. Arch. Pathol. Lab. Med. 2010;134:719–727. doi: 10.5858/134.5.719. [DOI] [PubMed] [Google Scholar]
- 7.Dusman R.E., Stanton M.S., Miles W.M. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–59. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Erasmus J.J., McAdams H.P., Rossi S.E. High-resolution CT of drug-induced lung disease. Radiol. Clin. North Am. 2002;40:61–72. doi: 10.1016/s0033-8389(03)00109-x. [DOI] [PubMed] [Google Scholar]
- 9.Siniakowicz R.M., Narula D., Suster B. Diagnosis of amiodarone pulmonary toxicity with high-resolution computerized tomographic scan. J. Cardiovasc Electrophysiol. 2001;12:431–436. doi: 10.1046/j.1540-8167.2001.00431.x. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Fowler A.J., Saeta A. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Jessurun G.A., Boersma W.G., Crijns H.J. Amiodarone-induced pulmonary toxicity. Predisposing factors, clinical symptoms and treatment. Drug Saf. 1998;18:339–344. doi: 10.2165/00002018-199818050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Piccini J.P., Berger J.S., O'Connor C.M. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur. Heart J. 2009;30:1245–1253. doi: 10.1093/eurheartj/ehp100. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J.S., Podrid P.J. Side effects from amiodarone. Am. Heart J. 1991;121:158–171. doi: 10.1016/0002-8703(91)90969-o. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein I., Topilsky M., Segev D. Very early onset of acute amiodarone pulmonary toxicity presenting with hemoptysis. Chest. 1997;111:1446–1447. doi: 10.1378/chest.111.5.1446. [DOI] [PubMed] [Google Scholar]
- 15.Ernawati D.K., Stafford L., Hughes J.D. Amiodarone-induced pulmonary toxicity. Br. J. Clin. Pharmacol. 2008;66:82–87. doi: 10.1111/j.1365-2125.2008.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung R.C., Chan W.K., Chu C.M. Low dose amiodarone-induced lung injury. Int. J. Cardiol. 2006;113:144–145. doi: 10.1016/j.ijcard.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 17.Vorperian V.R., Havighurst T.C., Miller S. Adverse effects of low dose amiodarone: a meta-analysis. J. Am. Coll. Cardiol. 1997;30:791–798. doi: 10.1016/s0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 18.Adams G.D., Kehoe R., Lesch M. Amiodarone-induced pneumonitis. Assessment of risk factors and possible risk reduction. Chest. 1988;93:254–263. doi: 10.1378/chest.93.2.254. [DOI] [PubMed] [Google Scholar]
- 19.Goldschlager N., Epstein A.E., Naccarelli G.V. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Viswam D., Nair S.G., Patel V. Ultra-short course of low-dose amiodarone-induced post-operative fatal pulmonary toxicity. J. Assoc. Physicians India. 2011;59:443–447. [PubMed] [Google Scholar]
- 21.Ghezel-Ahmadi V., Kurschner V.C., Fisseler-Eckhoff A. Amiodarone-induced pneumonitis. Lethal complication in a patient after thoracic surgery. Anaesthesist. 2008;57:982–987. doi: 10.1007/s00101-008-1407-x. [DOI] [PubMed] [Google Scholar]
- 22.Handschin A.E., Lardinois D., Schneiter D. Acute amiodarone-induced pulmonary toxicity following lung resection. Respiration. 2003;70:310–312. doi: 10.1159/000072016. [DOI] [PubMed] [Google Scholar]
- 23.Kharabsheh S., Abendroth C.S., Kozak M. Fatal pulmonary toxicity occurring within two weeks of initiation of amiodarone. Am. J. Cardiol. 2002;89:896–898. doi: 10.1016/s0002-9149(02)02213-0. [DOI] [PubMed] [Google Scholar]
- 24.Nagata N., Suematsu R., Yoshii C. Characterization of amiodarone pneumonitis as related to inflammatory cells and surfactant apoprotein. Chest. 1997;112:1068–1074. doi: 10.1378/chest.112.4.1068. [DOI] [PubMed] [Google Scholar]
- 25.Coudert B., Bailly F., Lombard J.N. Amiodarone pneumonitis. Bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102:1005–1012. doi: 10.1378/chest.102.4.1005. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S.E., Erasmus J.J., McAdams H.P. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20:1245–1259. doi: 10.1148/radiographics.20.5.g00se081245. [DOI] [PubMed] [Google Scholar]
- 27.Siddoway L.A. Amiodarone: guidelines for use and monitoring. Am. Fam. Physician. 2003;68:2189–2196. [PubMed] [Google Scholar]
- 28.De Wever W., Meersschaert J., Coolen J. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging. 2011;2:117–132. doi: 10.1007/s13244-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza C.A., Marchiori E., Goncalves L.P. Comparative study of clinical, pathological and HRCT findings of primary alveolar proteinosis and silicoproteinosis. Eur. J. Radiol. 2012;81:371–378. doi: 10.1016/j.ejrad.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Oyama N., Yokoshiki H., Kamishima T. Detection of amiodarone-induced pulmonary toxicity in supine and prone positions: high-resolution computed tomography study. Circ. J. 2005;69:466–470. doi: 10.1253/circj.69.466. [DOI] [PubMed] [Google Scholar]
- 31.Magro S.A., Lawrence E.C., Wheeler S.H. Amiodarone pulmonary toxicity: prospective evaluation of serial pulmonary function tests. J. Am. Coll. Cardiol. 1988;12:781–788. doi: 10.1016/0735-1097(88)90321-x. [DOI] [PubMed] [Google Scholar]
- 32.Ohar J.A., Jackson F., Redd R.M. Usefulness of serial pulmonary function testing as an indicator of amiodarone toxicity. Am. J. Cardiol. 1989;64:1322–1326. doi: 10.1016/0002-9149(89)90575-4. [DOI] [PubMed] [Google Scholar]
- 33.Fuster V., Ryden L.E., Cannom D.S. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Papiris S.A., Triantafillidou C., Kolilekas L. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010;33:539–558. doi: 10.2165/11532320-000000000-00000. [DOI] [PubMed] [Google Scholar]