Introduction
Common cutaneous warts caused by human papillomavirus (HPV) strains 1, 2, and 4, can be challenging to treat. Immunosuppressed patients are especially vulnerable to this infection, and the warts may cause physical and psychologic distress. There are currently 3 commercially available vaccines in the United States that target HPV strains commonly associated with the development of anogenital warts, cervical dysplasia, and cervical, vulvar, vaginal, and anal cancers. We report a case of an immunosuppressed adult with eruptive cutaneous warts who responded well to treatment with the nonavalent HPV vaccine (targeted against HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58).1
Case report
A 77 year-old white man with a long-term history of immunosuppression presented with a 6-month history of pruritic and eruptive white papules on the trunk and extremities. His medical history was significant for ulcerative colitis treated with long-term 6-mercaptopurine, 75 mg daily, and mesalamine, up to 800 mg 3 times daily, and a history of occasional cutaneous warts, totaling less than 7 at any time at previous dermatologic visits in the preceding 5 years. His warts had been treated with liquid nitrogen and salicylic acid and occasionally shave removal. The patient had no risk factors for HIV.
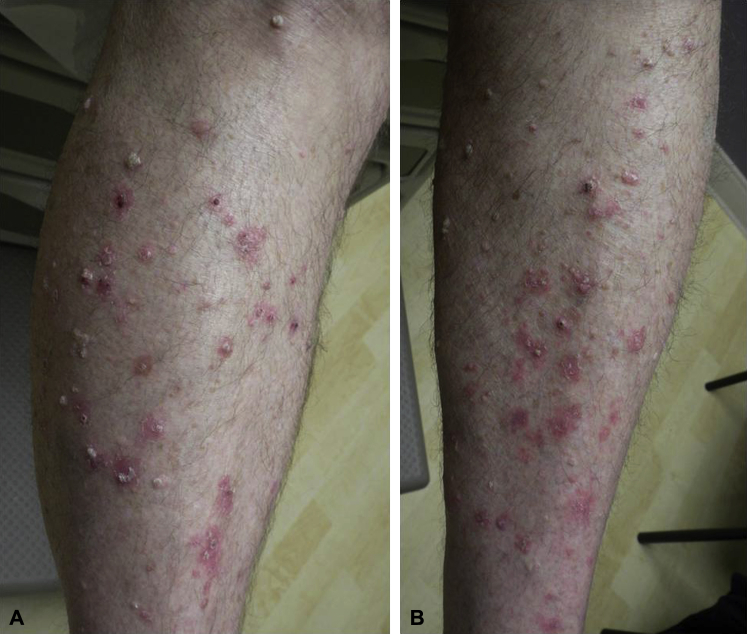
On examination recently, the patient had more than 70 inflamed thick, scaly white plaques ranging in size from 2 to 20 mm on the trunk and extremities clinically consistent with warts (Fig 1, A and B). Initial management included shave removal of 6 larger, more cumbersome lesions and freezing of more than 20 smaller areas with liquid nitrogen. Pathology findings for each sample were consistent with verruca. HPV typing was not performed because of cost.
Fig 1.
A and B, Lower legs at baseline highlighting many inflamed warts.
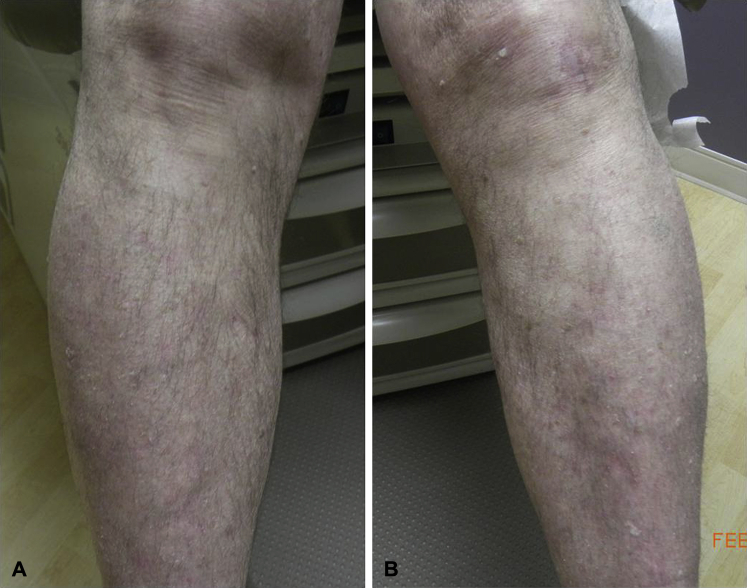
At follow-up 2 weeks later, the patient complained of continuing to develop new itchy white bumps. Different alternative treatments were discussed including the possibility of administering the HPV vaccine, based on several case reports of successful treatment of recalcitrant cutaneous warts.2, 3, 4, 5 The patient elected to try the nonavalent HPV vaccine and it was given at baseline and 2 and 6 months later. No additional treatments were performed during the vaccine period. The patient noticed a decrease in his warts and his level of pruritus within the first 3 weeks. Photos were obtained with the patient's permission at baseline and 6 months. The number of cutaneous warts dramatically decreased from the time of the initial vaccine administration to the 6-month time point (Fig 2, A and B). The warts did not fully clear, but the number of warts decreased over time without a change in his immunosuppressive medications, down to a low of 3 warts 15 months after the initial vaccine dose.
Fig 2.
A and B, Lower legs 6 months after the HPV vaccine series was initiated (seen at his appointment for the third and final vaccine administration).
Discussion
Routine vaccination against HPV has been available in most industrialized countries since 2006. First available as bivalent and quadrivalent vaccines, the nonavalent vaccine has been available since 2015.6 It is targeted against HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 and for those older than 15 years, the recommended dosing schedule is at zero, 2, and 6 months.6 It has been shown to produce a robust immune response with seroconversion rates approaching 100%.1 Studies on HPV vaccine administration in immunosuppressed populations are sparse, but a multicenter trial did show adequate immunogenic response to quadrivalent vaccine administration in immunosuppressed children regardless of age or cause of immunosuppression.7
Recalcitrant common warts, especially in the immunosuppressed population, can prove to be difficult to treat. Although many therapeutic options for warts exist, there is no definitive cure. Several case reports have described the successful use of quadrivalent HPV vaccine in treating recalcitrant warts.2, 3, 4, 5 Within the body of literature surrounding HPV vaccines, there is some indication for a cross-protection effect against HPV strains other than those targeted by the individual vaccine administered.8, 9, 10 Although the HPV strains that cause common warts may be less genetically related to those implicated in cervical, vulvar, and anal cancer, the possibility of cross-over effects with vaccines has been postulated previously.2, 3, 4 Interestingly, one case series reported success in treating children less than 14 years old with the quadrivalent vaccine, but less improvement was noted in teenagers (3 of 6 patients cleared) and in adults (1 of 4 patients cleared), perhaps indicating a more robust response to the vaccine in children.4
Our patient quickly experienced both a decrease in the number of warts and decreased irritation by the warts after the initial administration of the nonavalent HPV vaccine. His warts did not fully clear by 6 months, as has been reported when previously describing good responses to HPV vaccine.2, 3, 4, 5 This finding may indicate a cross-protection effect for certain but not all of his HPV strains. We did not perform HPV typing in this case because of patient cost; however, HPV status has been previously examined in a case report showing clearance of cutaneous warts negative for HPV 6, 11, 16, and 18 but positive for a general probe for HPV.3
Warts can spontaneously regress, and we cannot be certain that the vaccine played a role in the clearance of our patient's warts or in the previously reported cases. However, our patient saw a dramatic improvement in quality of life after administration of this vaccine. HPV vaccine administration should be considered a potential treatment alternative for recalcitrant warts in the immunosuppressed population. More specifically, our case provides evidence that the nonavalent vaccine may help decrease the morbidity associated with recalcitrant warts in this population.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Lopalco P.L. Spotlight on the 9-valent HPV vaccine. Drug Des Devel Ther. 2016;11:35–44. doi: 10.2147/DDDT.S91018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venugopal S.S., Murrell D.F. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccines ( types 6,11,16 and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146(5):475–477. doi: 10.1001/archdermatol.2010.71. [DOI] [PubMed] [Google Scholar]
- 3.Landis M.N., Lookingbill D.P., Sluzevich J.C. Recalcitrant plantar warts treated with recombinant quadrivalent human papillomavirus vaccine. J Am Acad Dermatol. 2012;67(2):e73–e74. doi: 10.1016/j.jaad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Abeck D., Folster-Holst R. Quadrivalent human papillomavirus vaccination: a promising treatment for recalcitrant cutaneous warts in children. Acta Derm Venereol. 2015;95(8):1017–1019. doi: 10.2340/00015555-2111. [DOI] [PubMed] [Google Scholar]
- 5.Daniel B.S., Murrell D.F. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149(3):370–372. doi: 10.1001/jamadermatol.2013.1463. [DOI] [PubMed] [Google Scholar]
- 6.CDC Vaccine Information Sheet. Human Papillomavirus. Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hpv.html.
- 7.MacIntyre C.R., Shaw P., Mackie F.E. Immunogenicity and persistence of immunity of a quadrivalent Human Papillomavirus (HPV) vaccine in immunocompromised children. Vaccine. 2016;34(36):4343–4350. doi: 10.1016/j.vaccine.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Ault K.A. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol Oncol. 2007 Nov;107:s31–s33. doi: 10.1016/j.ygyno.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 9.Nakalembe M., Banura C., Namujju P., Mirembe F. The levels of anti-HPV16/18 and anti- HPV 31/33/35/45/52/58 antibodies among AS04-adjuvanted HPV 16/18 vaccinated and non-vaccinated Ugandan girls aged 10-16 years. Infect Agent Cancer. 2014;9:29. doi: 10.1186/1750-9378-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler C.M., Kjaer S.K., Sigurdsson K. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199(7):936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]