Abstract
Mutations inTFAP2B has been reported in patients with isolated patent ductus arteriosus (PDA) and Char syndrome. We performed mutation analysis of TFAP2B in 43 patients with isolated PDA, 7 patients with PDA with other congenital heart defects and 286 patients with isolated tooth agenesis with or without other dental anomalies. The heterozygous c.1006G>A mutation was identified in 20 individuals. Those mutation carriers consisted of 1 patient with term PDA (1/43), 16 patients with isolated tooth agenesis with or without other dental anomalies (16/286; 5.6%), 1 patient with PDA and severe valvular aortic stenosis and tooth agenesis (1/4) and 2 normal controls (2/100; 1%). The mutation is predicted to cause an amino-acid substitution p.Val336Ile in the TFAP2B protein. Tfap2b expression during early mouse tooth development supports the association of TFAP2B mutation and dental anomalies. It is hypothesized that this incidence might have been the result of founder effect. Here we report for the first time that TFAP2B mutation is associated with tooth agenesis, microdontia, supernumerary tooth and root maldevelopment. In addition, we also found that TFAP2B mutations, the common causes of PDA in Caucasian, are not the common cause of PDA in Thai population.
Introduction
Patent ductus arteriosus (PDA) is a clinically significant congenital heart disease that accounts for 5–10% of all congenital heart disease.1 It has been reported that mutations in TFAP2B gene cause both isolated (nonsyndromic) PDA2, 3 and syndromic PDA, Char Syndrome.4, 5, 6 Char syndrome is characterized by PDA, dysmorphic facial appearances and aplasia or hypoplasia of the middle phalanges of the fifth fingers.7 The TFAP2B gene is located on chromosome 6p12, consisting of seven exons with a 28.8 kb span and coding for 460 amino acids.8 This gene encodes transcription factor AP-2 beta (TFAP2B), which is important for cardiac morphogenesis, by regulating cell proliferation and development.9 Until now, 11 TFAP2B mutations have been reported in patients with isolated PDA and Char syndrome.2, 3, 4, 5, 6, 10 Here we report for the first time that a TFAP2B mutation is associated with isolated tooth agenesis, microdontia, supernumerary tooth and tooth root maldevelopment.
Materials and methods
Ethic statement
The study was conducted with informed consent from all patients and their parents. The study was approved by the Human Experimentation Committees of the Faculty of Medicine and Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Subjects
The familial cases (Patients 1–8) are described below. The clinical findings and TFAP2B mutation of all 18 patients from 14 families are present in Table 1.
Table 1. Patients with TFAP2B mutation.
| Family | Patient | Gender | Ethnic | Cardiac findings | Dental anomalies | TFAP2B mutation | WNT10A mutation |
|---|---|---|---|---|---|---|---|
| 1 | 1 | M | Karen | PDA, AS | Microdontia of perm. max. canines. Short ant. roots. Taurodontism of max. molars | p.Val336Ile | No |
| 2 (Mother of Pt. 1) | F | Karen | None | Hypodontia of Rt mand. perm. lateral incisor. Short ant. max. and mand. roots. Tapered mand. premolar and molar roots | p.Val336Ile | No | |
| 2 | 3 | F | Karen | PDA | None | p.Val336Ile | No |
| 4 (Brother of Pt. 3) | M | Karen | None | Supernumerary tooth | p.Val336Ile | No | |
| 3 | 5 | F | Thai | None | Hypodontia of Lt mand. perm. Lateral incisor and Lt mand. deciduous lateral incisor | p.Val336Ile | No |
| 6 (Brother of Pt. 5) | M | Thai | None | Microdontia of Lt perm. max. second premolar | p.Val336Ile | p.Ser292Gly | |
| 4 | 7 | M | Northern Thai | None | Microdontia of Lt and Rt perm. max. lateral incisors. Taurodontism in Rt and Lt perm. mand. second molars and max. Lt perm. second molar | p.Val336Ile | No |
| 8 (Mother of Pt. 7) | F | Northern Thai | None | Taurodontism of Rt and Lt perm. mand. second molars and max. Rt perm. second molar | p.Val336Ile (Homozygous) | No | |
| 5 | 9 | F | Thai | None | Hypodontia of Rt and Lt perm. max. second molars | p.Val336Ile | No |
| 6 | 10 | F | Thai | None | Hypodontia of Lt and Rt perm. mand. central incisors | p.Val336Ile | No |
| 7 | 11 | F | Thai | None | Hypodontia of Lt perm. mand. lateral incisor | p.Val336Ile | No |
| 8 | 12 | F | Thai | None | Hypodontia of Rt and Lt perm. max. canines | p.Val336Ile | No |
| 9 | 13 | F | Thai | None | Microdontia of Rt and Lt perm. max. lateral incisor | p.Val336Ile | No |
| 10 | 14 | F | Thai | None | Hypodontia of Rt and Lt perm. mand. lateral incisor | p.Val336Ile | No |
| 11 | 15 | F | Thai | None | Hypodontia of Rt perm mand. lateral incisor | p.Val336Ile | No |
| 12 | 16 | F | Thai | None | Hypodontia of Lt perm. mand. lateral incisor and Rt perm. mand. central incisor | p.Val336Ile | No |
| 13 | 17 | M | Karen | None | Hypodontia of Rt perm. mand. second premolar | p.Val336Ile | No |
| 14 | 18 | F | Thai | None | Hypodontia of all third molars | p.Val336Ile | No |
Abbreviations: ant., anterior; AS, aortic stenosis; F, female; Lt, left; M, male; mand., mandibular; max., maxillary; PDA, patent ductus arteriosus; perm., permanent; Pt, patient; Rt, right.
Patient 1
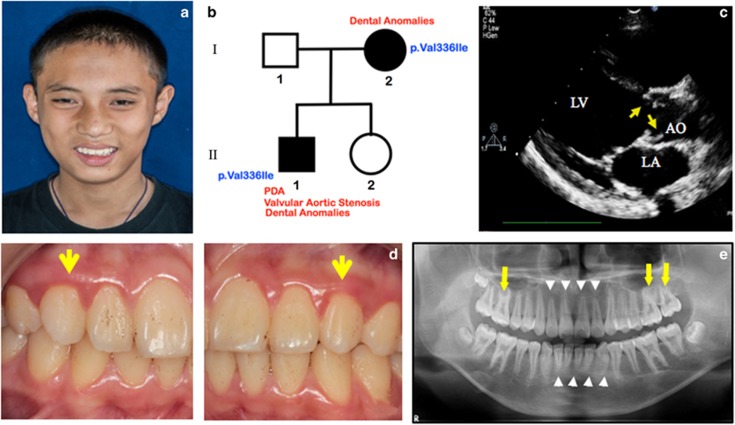
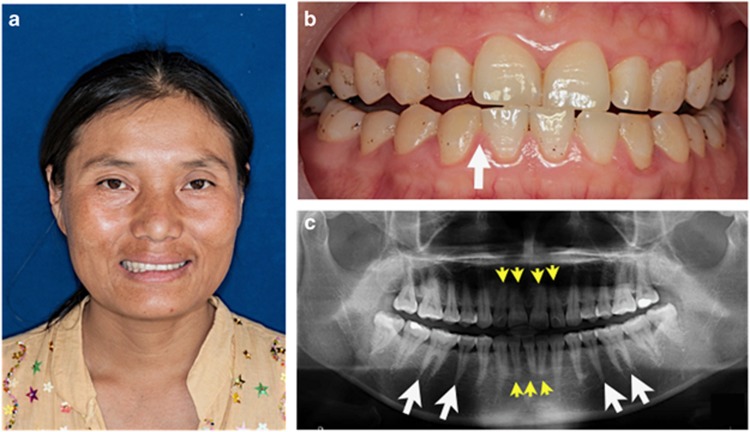
A 13-year-old Karen male was the first child (II-1) of healthy nonconsanguineous parents (Figures 1a and b). The parents and his younger sister were healthy. He was delivered at 32 weeks of gestation after an uneventful pregnancy with a birth weight of 1700 g (25th–50th percentile). He has been diagnosed with severe valvular aortic stenosis (Figure 1c) and a small PDA (2 mm in diameter) at birth. Oral examination showed microdontia of both maxillary permanent canines (Figure 1d). A panoramic radiograph showed taurodontism (the body of the tooth and pulp chamber is enlarged vertically at the expense of the roots) in the maxillary permanent first and second molars and short roots of the maxillary and mandibular incisors (Figure 1e). His mother (Patient 2; I-2) was a 35-year-old Karen whose health was unremarkable (Figures 1b and 2a). Her echocardiography confirmed the absence of any cardiac abnormalities. The oral examination revealed tooth agenesis of the right permanent mandibular lateral incisor (Figure 2b). The panoramic radiograph revealed shortening of both maxillary and mandibular incisors. The roots of the mandibular premolars and permanent molars were tapered (Figure 2c). The oral examination and echocardiographs of other members of the family appeared to be normal.
Figure 1.
Patient 1. (a) 13-year-old boy with PDA and valvular aortic stenosis. Normal facial appearance. (b) Pedigree of Patients 1 (II-1) and 2 (I-2). (c) Echocardiography shows valvular aortic stenosis. Yellow arrows indicate thickening and doming of aortic valves. LV, Left ventricle, AO, aorta, LA, left atrium. (d) Microdontia of the maxillary permanent canines (yellow arrows) (e) Panoramic radiograph shows short anterior tooth roots (white arrow heads) and taurodontism in the maxillary molars (yellow arrows).
Figure 2.
Patient 2. Mother of Patient 1. (a) Normal facial appearance. (b) Missing right mandibular permanent lateral incisor (white arrow). (c) Panoramic radiograph shows the absence of right mandibular permanent lateral incisor, short roots of the maxillary and mandibular incisors (yellow arrow heads) and tapered roots of the premolars and molars (white arrows).
Patient 3
A 3-year-old Karen girl was born to nonconsanguineous, healthy parents. She was the youngest child in a family of four children. She was delivered at 40 weeks of gestation after an uneventful pregnancy. Her birth weight was 3700 g (50th–75th percentile). She had been diagnosed with term isolated PDA since she was born. The diameter of the PDA was 7 mm. Echocardiographs of other members of the family showed no other heart abnormalities. A panoramic radiograph of her older brother (Patient 4) showed a supernumerary maxillary incisor (mesiodens). Oral examination of Patient 3 and other members of this family showed them to be normal.
Patient 5
A 10-year-old girl was the third child of nonconsanguineous Thai parents of Chinese ethnic background. The first daughter of this family had severe developmental delay since birth with no congenital heart defects. However, no definitive diagnosis has been made. The other members of the family were healthy with no history of congenital heart defects. Oral examination of Patient 5 at age 10 years showed tooth agenesis of the left mandibular permanent lateral incisor. The left mandibular deciduous lateral incisor was reported to be missing. Oral examination of her elder brother (Patient 6) showed microdontia in the left permanent maxillary second premolar. Her parents and the youngest daughter had normal teeth.
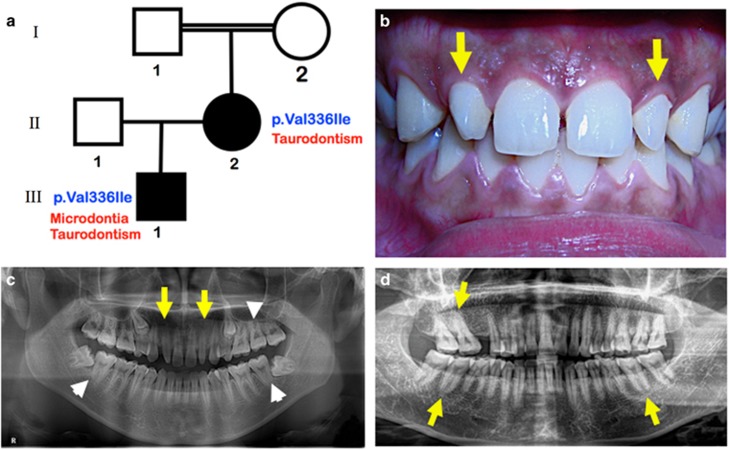
Patient 7
A 20-year-old Thai male (II-1) was born to nonconsanguineous, healthy parents. His mother’s parents were reported to be consanguineous (Figure 3a). The general health status of all members of this family appeared to be normal. Oral examination of Patient 7 revealed microdontia of the right and left permanent maxillary lateral incisors (Figure 3b). A supernumerary maxillary incisor (mesiodens) was extracted at age 10 years. A panoramic radiograph showed microdontia of both permanent maxillary lateral incisors and taurodontism in the right and left permanent mandibular second molars and the maxillary left permanent second molar (Figure 3c). The oral and radiographic examination of his mother (Patient 8) showed taurodontism in the right and left permanent mandibular second molars and the maxillary right permanent second molar. The maxillary right permanent first molar was extracted as a result of dental infection (Figure 3d).
Figure 3.
Patients 7 (III-1) and 8 (II-2). (a) Pedigree demonstrates consanguineous marriage. (b) Patient 7. Microdontia of the right and left maxillary permanent lateral incisors (yellow arrows). (c) Panoramic radiograph of Patient 7 shows microdontia of the right and left permanent maxillary lateral incisors (yellow arrow) and taurodontism in the right and left permanent mandibular second molars and the maxillary left permanent second molar (white arrow heads). (d) Panoramic radiograph of Patient 8 (Patient 7’s mother) shows taurodontism in the right and left permanent mandibular second molars and the maxillary right permanent second molar (yellow arrows).
Mutation analysis
One milliliter of saliva was collected using an Oragene Saliva Collecting Kit (OG-575) (DNA Genotek Inc., Ottawa, ON, Canada). Genomic DNA was extracted from the saliva according to the prepIT L2P protocol for the purification of genomic DNA from the Oragene Collecting Kit (DNA Genotek Inc.). Sequencing data were analyzed using the Sequencher 4.8 Sequence analysis software (Genecodes, Ann Arbor, MI, USA). Mutation analysis of TFAP2B was performed in 50 patients who were diagnosed with isolated PDA or PDA with other congenital heart defects, and 286 patients who were affected with isolated tooth agenesis. DNA sequencing was carried out in both directions using PCR primers. Sequencing reactions were carried out using an ABI PRISM BigDye Terminator v3.1 and analyzed on a ABI 3730xl sequencer at Functional Biosciences (Madison, WI, USA). As WNT10A mutations have been reported to be the most common cause of isolated tooth agenesis,11 we performed mutation analysis of WNT10A in all patients with the TFAP2B variant who had tooth agenesis in order to rule out WNT10A mutation-associated tooth agenesis.
In situ hybridization of Tfap2b during tooth development
Radioactive in situ hybridization with 35S-UTP-radiolabelled riboprobes was performed as described previously by Ohazama et al.12 Briefly, the slides were pretreated with 5 mg ml−1 proteinase K and 0.25% acetic anhydride to reduce background. Hybridization was carried out overnight at 55 °C. The slides were then washed twice at high stringency at 65 °C and treated with 40 mg ml−1 RNAse A for 30 min at 37 °C to remove any nonspecifically bound probe. The slides were air-dried and dipped in Ilford K.5 photographic emulsion. Autoradiography was performed by exposing the sections in a light-tight box at 4 °C for 10 to 14 days. Slides were developed, fixed and counterstained with hematoxylin. To make a Tfap2b probe, reverse transcriptase-PCR of total RNA from E13.5 embryo bodies with primers (forward 5′-CCAAGAAGTGGGCTCAGAAG-3′ and reverse 5′-ACGTGACATTTGCTGCTTTG-3′) was carried out, and reverse transcriptase-PCR products were cloned into pGEM-T Easy vector (Promega, Madison, WI, USA) and subjected to automated DNA sequence analysis.
Statistical analysis
The associations between PDA, dental anomalies and the p.Val336Ile variant were assessed by calculating odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) by using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
We performed mutation analysis of TFAP2B in 43 patients with isolated term PDA, 4 patients with PDA and ventricular septal defect, 1 patient with PDA with coarctation of aorta, 1 patient with PDA and atrial septal defect, 1 patient with preterm PDA and valvular aortic stenosis and tooth agenesis (patient 1) and 286 patients with isolated dental anomalies. The heterozygous single base substitution in exon 6 (c.1006G>A) was identified in 20 individuals. (Supplementary Figure S1). Those variant carriers consisted of 1 patient with term PDA (1/43; 2.3%), 16 patients with isolated tooth agenesis with or without other dental anomalies (16/286; 5.6%), 1 patient with PDA and severe valvular aortic stenosis and dental anomalies (patient 1), 1 patient with term PDA (patient 3) and 2 normal controls (2/100; 1%). Homozygous single base substitution (c.1006G>A) was identified in one patient (Patient 8) (Supplementary Figure S2). The variant is predicted to cause an amino-acid substitution p.Val336Ile in the TFAP2B protein. This variant was found in 2 out of the 100 normal controls of the same ethnic background, which accounted for 1% of the 200 alleles. The protein alignment sequence of TFAP2B protein, using the Clustal X 2.1 multiple sequence alignment program, has shown that the amino-acid valine at position 336 is highly conserved throughout evolution (Supplementary Figure S3). This variant is predicted to be disease causing by the MutationTaster2, PolyPhen-2 and HANSA programs.
The clinical findings of the patients and their family members who carried the TFAP2B variant varied from isolated term PDA (Patient 3), preterm PDA with valvular aortic stenosis and dental anomalies (Patient 1), isolated dental anomalies to being unaffected. Patient 6, who had microdontia of the maxillary left second premolar, carried digenic heterozygous missense variants of WNT10A and TFAP2B. The clinical findings and the variants of all patients are presented in Table 1.
Mutation analysis of WNT10A in patients who carried the TFAP2B mutation and had tooth agenesis showed a heterozygous single base substitution in exon 4 (c.874>G) in Patient 6 (Supplementary Figure S4). This variant is predicted to cause an amino-acid substitution p.Ser292Gly in WNT10A protein. This variant was not present in the 200 chromosomes of 100 normal Thai controls who were healthy and had normal teeth. ExAC browser reports its frequency of 0.0084 in East asian population and zero in all others (overall frequency=0.0004665). The protein alignment sequence of WNT10A protein, using the Clustal X 2.1 multiple sequence alignment program, has shown that the amino-acid serine at position 292 is not conserved in the house mouse, Norway rat, chicken or Florida lancelet (Supplementary Figure S5). The mutation prediction of this variant is predicted to be disease causing by the HANSA and SIFT programs; however, it is predicted to be single polymorphism by the MutationTaster2 and PolyPhen-2 programs.
Statistical analysis
The OR is a measure of association that compares the odds of finding the variant in the PDA or dental anomaly patients to the odds of finding the variant in the control or normal population. There is no data of the allele frequencies in Southeast Asian in any public databases, therefore we calculated the ORs using the ExAC database of South Asia and East Asia to estimate the odds of variant for Southeast Asia where Thailand is located. The ORs between PDA and the p.Val336Ile variant were significant only in South Asian (OR=244.6, 95% CI=28.8–2076.1, P<0.001) while that of East Asian showed no significance (OR=2.0, 95% CI=0.3–14.5, P=0.495). This means that the chance of finding the p.Val336Ile variant in patients with PDA is 244.6 times higher than in the normal population of South Asia. While the ORs between dental anomalies and the p.Val336Ile variant were 4.9 in South Asia and 608.8 in East Asia (P<0.001), which showed a significant association between dental anomalies and the p.Val336Ile variant. This means that the chances of finding this variant in the patients with dental anomalies are 4.9 and 608.8 times higher in the South Asia and East Asia populations, respectively. We found that 2 out of the 100 normal controls or 200 alleles (1%) in our study carried this variant. The result from our study showed a significant association between dental anomalies and the p.Val336Ile variant (OR=5.9, 95% CI=1.3–25.8, P=0.008). This means that the chance of finding this variant in the patients with dental anomalies in our cohort is 5.9 times higher than in the normal control group.
In situ hybridization of Tfap2b
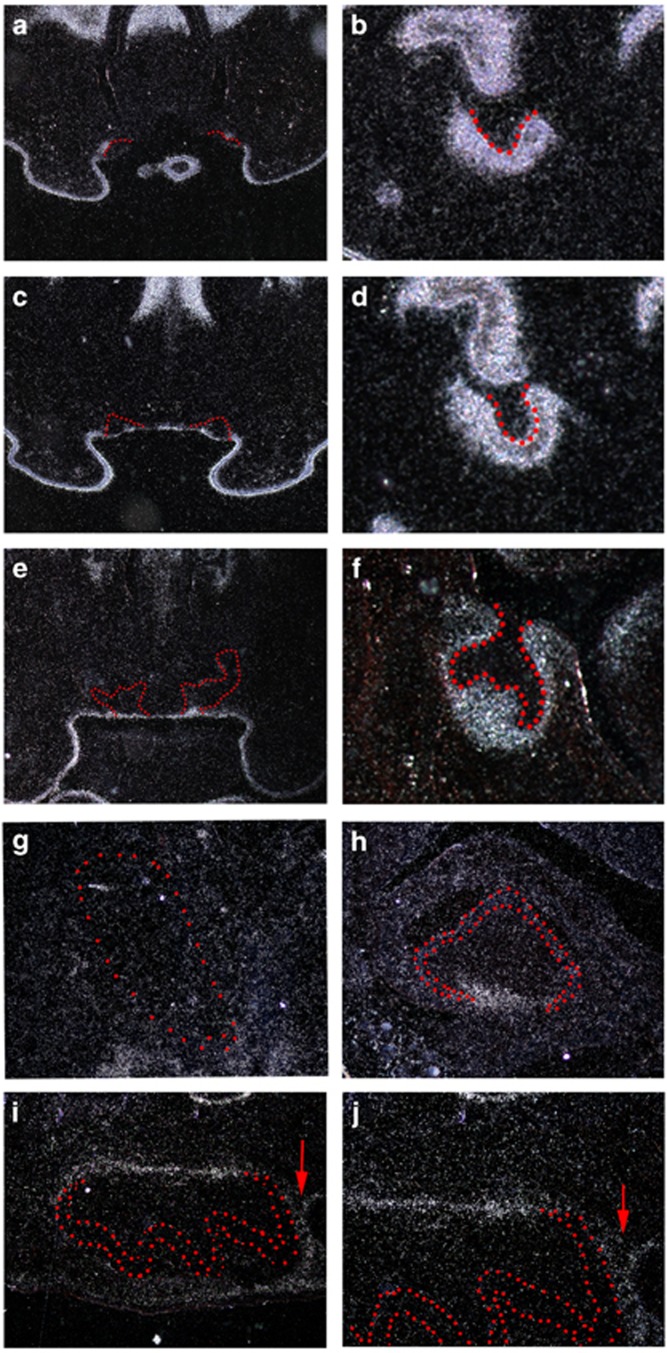
Tfap2b showed no expression in any tooth germs at the initiation stage (E10.5 and E11.5; data not shown). At E12.5, Tfap2b was expressed in the entire molar tooth mesenchyme, while Tfap2b expression was restricted to incisor mesenchymal cells facing tooth epithelium (Figures 4a and b). At the bud stage (E13.5) and the cap stage (E14.5), Tfap2b expression was observed in molar mesenchyme, whereas the expression of Tfap2b was restricted to a single-cell layer facing the oral cavity in the incisor epithelium (Figures 4c–f). No expression of Tfap2b could be detected in incisor tooth germs at birth (Figure 4g). On the other hand,Tfap2b showed restricted expression in caudal dental papillae of newborn molar tooth germ (Figure 4h). In addition to caudal dental papillae, Tfap2b was weakly expressed in the dental follicle at postnatal day 5 (Figures 4i and j).
Figure 4.
The expression of Tfap2b during rodent tooth development. Radioactive in situ hybridization of Tfap2b expression on frontal (a–h) and sagittal (i, j) head sections. (a, b) At E12.5, Tfap2b is expressed in the entire molar tooth mesenchyme, whereas its expression is restricted to incisor mesenchymal cells facing tooth epithelium. (c, d) At bud stage E13.5 and (e, f) cap stage E14.5, Tfap2b expression is observed in molar mesenchyme, whereas its expression is restricted to a single-cell layer facing the oral cavity in the incisor epithelium. (g, h). Newborn. No expression of Tfap2b is found in the incisor tooth germ, whereas Tfap2b is expressed in the oral epithelium of the incisor region. In the molar tooth germ, Tfap2b shows restricted expression in caudal dental papillae facing the cervical loop. (i, j). At postnatal day 5, Tfap2b is expressed in caudal dental papillae and the dental follicle (arrows). Upper incisor (a, c, e, g) and molar (b, d, f, h, i, j) tooth germ epithelium is outlined in red dots.
Discussion
We have found heterozygous a missense variant (c.1006G>A; p.Val336Ile) in patients with isolated term PDA (1/43; 2.3%), preterm PDA with severe valvular aortic stenosis and dental anomalies (1/1) and isolated dental anomalies (16/286; 5.6%). This variant was found in 1% of our 200 normal alleles. Our study shows that the p.Val336Ile variant is associated with isolated dental anomalies. The variant is strongly associated with PDA only if the prevalence of this variant in South Asia population was used (244.6 times higher) but it is not associated if the prevalence of East Asia population was used.
Our study has expanded the phenotypic spectrum of TFAB2B-associated clinical findings. Isolated tooth agenesis, microdontia, supernumerary teeth (mesiodens) and tooth root maldevelopment have not been reported before in patients with TFAP2B mutations. Tooth root maldevelopment found in this study consisted of taurodontism, short tooth roots and tapered tooth roots. The prevalence of taurodontism in the Thai population is unknown, but it has been reported to be 2.8% in a North Indian population13 and 2.5% in adult Caucasians.14 The prevalences of short tooth roots and tapered tooth roots have not been reported. Mesiodens has been reported to be 0.15–1.9% in the general population.15 The prevalences of isolated term PDA and congenital aortic stenosis in the Thai population are unknown, but in the general population, they have been reported to be 0.31 and 0.27 per 1000 live births, respectively.16
The p.Val336Ile variant was also found in 3 of the 100 normal controls in a study of Southern Chinese population.10 The overall allele frequencies reported by ExAC, 1000 Genome and GO-ESp are 0.0009, 0.0022 and 0.0008, respectively. The presence of this variant in our normal control (1%), and three normal controls in the study of Xiong et al.,10 suggests that the TFAP2B variant-associated phenotypes appear to have an autosomal-dominant mode of inheritance with incomplete penetrance,10 as 5.6% of our patients with dental anomalies carried this variant and most of them did not even know they had dental anomalies. It is possible that the variant carriers who were considered ‘normal’ in the studies of ExAC, 1000 Genome and GO-ESP might have undetected dental anomalies.
The heterozygous p.Val336Ile variant is predicted to affect the DNA-binding domain of TFAP2B and the DNA-binding properties of this domain is expected to be disrupted. As TFAP2B has an important role in a transcriptional network with endothelin-1, hypoxia-induced transcription factor and genes encoding proteins that are important for the oxygen-sensing mechanism during ductus arteriosus closure,9 the p.Val336Ile variant is hypothesized to cause disruption of the important pathway for the closure of ductus arteriosus. Although Patient 3 was affected with isolated term PDA, her heterozygous parents were normal. Her brother (Patient 4) who carried the same mutation had a supernumerary tooth. The valvular aortic stenosis found in Patient 1 might be coincidental.
Fourteen patients with TFAP2B mutations have previously been reported to have PDA and tooth agenesis.6 Interestingly, we have identified tooth agenesis, microdontia, taurodontism, short tooth roots, tapered tooth roots and supernumerary teeth (mesiodens) in 17 patients who carried the TFAP2B variant. In addition, we have demonstrated for the first time that Tfap2b is expressed during early tooth and root development, supporting the association of TFAP2B variant and dental anomalies in our patients. The fact that Tfap2b expression was not detected in developing ameloblasts or odontoblasts during the cytodifferentiation stage of tooth development suggests that TFAP2B does not have a role in the differentiation of ameloblasts and odontoblasts. The normal enamel and dentin in the patients who carried TFAP2B mutation supports this finding.
Mutations in WNT10A are the most common causes of isolated tooth agenesis.11 Except for the WNT10A variant found in Patient 5, we are convinced that the dental anomalies found in our patients were associated with the TFAP2B variant. The amino-acid serine at the position 292 of WNT10A protein found in Patient 6 is not highly conserved; it is hypothesized that the WNT10A variant may not be related to the microdontia of his maxillary left second premolar or this microdontia might have been the combined effects of WNT10A and TFAP2B mutations.
Patient 8, who had a homozygous TFAP2B variant, demonstrated taurodontism in the right and left permanent mandibular second molars and the right maxillary second molar, while her son (Patient 7) had a supernumerary tooth, taurodontism in the mandibular second molars, the left permanent maxillary second molar and microdontia of the maxillary lateral incisors. It is interesting to note that the homozygote was less severely affected than the heterozygote. It is noteworthy that four heterozygous carriers of the TFAP2B mutation were normal. This phenomenon suggests incomplete penetrance and variable expressivity of the TFAP2B mutation-associated phenotypes. Taurodontism, tapered tooth roots and short anterior tooth roots in Patients 1, 2, 7 and 8 might have been the consequences of TFAP2B variant that affected TFAP2B protein function and led to abnormal root development. This is supported by Tfap2b expression in the tooth germ at P5 at caudal dental papillae facing the cervical loop and the dental follicle, which is the area of early tooth root development.
It is noteworthy that the heterozygous c.1006G>A variant was the only TFAP2B mutation found in the study. Statistically this variant is associated with tooth agenesis but not PDA and other congenital heart defects in our study. According to the ExAc database and the result of our study, this TFAP2B variant has been reported in only those with Chinese background. The affected Thai or northern Thai patients in this study were of Chinese descent. Regarding the Karen tribe living in Thailand, anthropologically it has been reported that their ancestors were Chinese living in the southwestern and western China and later migrated through Myanmar to northern Thailand.17 It is possible that the shared variant (c.1006G>A; p.Val336Ile) might have been the consequence of the founder effect.
The c.1006G>A variant has been reported in six southern Chinese patients who were affected with tetralogy of Fallot (one patient), persistent truncus arteriosus (two patients) and PDA (three patients)10; our study shows statistical association between this variant and PDA when the allele frequency of South Asia was used but not when that of East Asia was used. This heterozygous missense variant that passed from parents to the probands in our study and the difference of phenotypes among all patients of the same families in this study support the inheritance being autosomal dominant with variable expressivity and incomplete penetrance. As this variant was also found in normal controls, this may imply that this variant is associated with the dysmorphogenesis of dental anomalies but does not have direct causal effect on the phenotypes. Here we demonstrate for the first time the role of Tfap2b during early tooth root development and show that TFAP2B mutations are not only associated with syndromic and non-syndromic PDA but also with isolated tooth agenesis, microdontia, taurodontism, short tooth roots, tapered tooth roots and supernumerary tooth (mesiodens).
Acknowledgments
We thank the patients and their families for their kind cooperation and allowing us to use their medical and dental information for publication. This research was supported by the Center of Excellence in Medical Genetics Research, Chiang Mai University, The Thailand Research Fund (TRF), Faculty of Dentistry, Chiang Mai University, and Dental Association of Thailand. We thank Dr M Kevin O Carroll, Professor Emeritus of the University of Mississippi School of Dentistry, USA and Faculty Consultant at Chiang Mai University Faculty of Dentistry, Thailand for his assistance in the preparation of the manuscript.
Footnotes
Supplementary Information accompanies the paper on Journal of Human Genetics website (http://www.nature.com/jhg)
The authors declare no conflict of interest.
Supplementary Material
References
- Schneider, D. J. & Moore, J. W. Patent ductus arteriosus. Circulation 114, 1873–1882 (2006). [DOI] [PubMed] [Google Scholar]
- Khetyar, M., Syrris, P., Tinworth, L., Abushaban, L. & Carter, N. Novel TFAP2B mutation in nonsyndromic patent ductus arteriosus. Genet. Test 12, 457–459 (2008). [DOI] [PubMed] [Google Scholar]
- Chen, Y. W., Zhao, W., Zhang, Z. F., Fu, Q., Shen, J., Zhang, Z. et al. Familial nonsyndromic patent ductus arteriosus caused by mutations in TFAP2B. Pediatr. Cardiol. 32, 958–965 (2011). [DOI] [PubMed] [Google Scholar]
- Satoda, M., Zhao, F., Diaz, G. A., Burn, J., Goodship, J., Davidson, H. R. et al. Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat. Genet. 25, 42–46 (2000). [DOI] [PubMed] [Google Scholar]
- Zhao, F., Weismann, C. G., Satoda, M., Pierpont, M. E., Sweeney, E., Thompson, E. M. et al. Novel TFAP2B mutations that cause Char syndrome provide a genotype-phenotype correlation. Am. J. Hum. Genet. 69, 695–703 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, A., Radhakrishnan, J., Farhi, A., Carew, K. S., Warnes, C. A., Nelson, W. C. et al. Syndromic patent ductus arteriosus: evidence for haploinsufficient TFAP2B mutations and identification of a linked sleep disorder. Proc. Natl Acad. Sci. USA 102, 2975–2979 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Char, F. Peculiar facies with short philtrum, duck-bill lips, ptosis and low-set ears—a new syndrome? Birth Defects Orig. Artic. Ser. 14, 303–305 (1978). [PubMed] [Google Scholar]
- Lingaiah, K., Sosalagere, D. M., Mysore, S. R., Krishnamurthy, B., Narayanappa, D. & Nallur, R. B. Mutations of TFAP2B in congenital heart disease patients in Mysore, South India. Indian J. Med. Res. 134, 621–626 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey, K. N., Sutcliffe, D., Richardson, J., Clyman, R. I., Garcia, J. A. & Srivastava, D. Transcriptional regulation during development of the ductus arteriosus. Circ. Res. 103, 388–395 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, F., Li, Q., Zhang, C., Chen, Y., Li, P., Wei, X. et al. Analyses of GATA4, NKX2.5, and TFAP2B genes in subjects from southern China with sporadic congenital heart disease. Cardiovasc. Pathol. 22, 141–145 (2013). [DOI] [PubMed] [Google Scholar]
- Van den Boogaard, M. J., Creton, M., Bronkhorst, Y., van der Hout, A., Hennekam, E., Lindhout, D. et al. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J. Med. Genet. 49, 327–331 (2012). [DOI] [PubMed] [Google Scholar]
- Ohazama, A., Johnson, E. B., Ota, M. S., Choi, H. Y., Porntaveetus, T., Oommen, S. et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE 3, e4092 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti, R., Chandra, A., Tikku, A. P. & Arya, D. Prevalence of Taurodont molars in a North Indian population. Indian J. Dent. 6, 27–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers, M. T. & Witkop, C. J. Jr Taurodontism, an isolated trait associated with syndromes and X-chromosomal aneuploidy. Am. J. Hum. Genet. 32, 396–413 (1980). [PMC free article] [PubMed] [Google Scholar]
- Van Buggenhout, G. & Bailleul-Forestier, I. Mesiodens. Eur. J. Med. Genet. 51, 178–181 (2008). [DOI] [PubMed] [Google Scholar]
- Marelli, A. J., Mackie, A. S., Ionescu-Ittu, R., Rahme, E. & Pilote, L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 115, 163–172 (2007). [DOI] [PubMed] [Google Scholar]
- LaPolla, R. J. in Areal Diffusion and Genetic Inheritance: Problems in Comparative Linguistics, 1st edn (eds Alexandra, Y. A. & Robert, M. W.) 225–234 (Oxford University Press, New York, NY, USA, 1999).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.